Abstract
Objective To assess the effects of different types of individualised risk communication for patients who are deciding whether to participate in screening.
Design Systematic review.
Data sources Specialist register of the Cochrane consumers and communication review group, scientific databases, and a manual follow up of references.
Selection of studies Studies were randomised controlled trials addressing decisions by patients whether or not to undergo screening and incorporating an intervention with an element of “individualised” risk communication—based on the individual's own risk factors for a condition (such as age or family history).
Outcome measures The principal outcome was uptake of screening tests; further cognitive and affective measures were also assessed to gauge informed decision making.
Results 13 studies were included, 10 of which addressed mammography programmes. Individualised risk communication was associated with an increased uptake of screening tests (odds ratio 1.5, 95% confidence interval 1.11 to 2.03). Few cognitive or affective outcomes were reported consistently, so it was not possible to conclude whether this increase in the uptake of tests was related to informed decision making by patients.
Conclusions Individualised risk estimates may be effective for purposes of population health, but their effects on increasing uptake of screening programmes may not be interpretable as evidence of informed decision making by patients. Greater attention is required to ways of developing interventions for screening programmes that can achieve this.
Introduction
Various tests and procedures can be used for screening—to identify individuals and groups at “high risk” of various diseases or conditions. Many of these tests and procedures form part of screening programmes whose purposes are usually to reduce morbidity in the population but which may not always be effective for individuals. At the individual level several issues are important. These include the chances of detecting and preventing disease, or of false positive or negative tests with their important consequences in terms of tests or treatments to be undergone, or morbidity “unnecessarily” suffered.1 These chances of the potential harms and benefits must be considered by individuals making informed decisions about whether or not to undergo screening tests in health care.2
Understanding of how best to present and discuss risks and benefits of health care in general, and screening in particular, for an individual is still limited. Some screening programmes provide information about population or “average” risks of contracting a disease; these often simply try to motivate people to attend for tests that are perceived by authorities to be in their own or the population's best interests.3 Other programmes attempt to provide information that is more personally relevant to the patient in question. We describe this as “individualised risk communication.”4 This may be based on the individual's own risk factors for a condition (such as age or family history). In some cases it is calculated from an individual's risk factors by using formulas derived from epidemiological data, such as the Gail formula for risk of breast cancer.5 The information itself may then be represented as an absolute risk or as a risk score, or categorised—for example, into high, medium, or low risk groups. It may be less detailed, entailing a listing—for example, of a patient's risk factors as a focus for discussion and intervention.
In these scenarios it is generally anticipated that because the information is thought to be more pertinent individualised risk communication is more likely to be useful in making a decision about whether or not to participate in screening. But awareness is growing that decisions about screening can be influenced by the way in which information on risk is presented and that this may not necessarily be evidence of informed decision making.6,7 Raffle warns that harm to uninformed participants leads to anger, bitterness, and potentially litigation.8 The social and psychological costs of screening therefore need to be assessed.9
Reviews of mass risk communication (to populations), primarily from the discipline of environmental health10-12 and from narrow clinical fields such as familial cancer, are available.13 The effects of communicating risk in screening are not well understood to date. We undertook a systematic review to evaluate these effects, in particular to assess the effects of providing individual risk estimates in comparison with more general information on risk, and to see whether there is evidence that these contribute to informed decision making by consumers. This report summarises a Cochrane review published earlier this year.14
Methods
Two reviewers assessed for inclusion and extracted data on trial quality, the intervention, and relevant outcome data. We undertook meta-analysis where feasible. This review used standard methods based on guidance from the Centre for Reviews and Dissemination (UK)15 and from the Cochrane Collaboration's consumers and communication review group.
Types of studies
We included only randomised controlled trials. We did not exclude studies that were not by intention to treat, but their lower methodological quality is reflected in their method scores and the weight attached to their findings tempered accordingly.16
Types of participants
We included studies of people facing real decisions (not hypothetical exercises) about whether to undergo screening (presumptive identification of unrecognised disease or defect by the application of tests or other procedures, which can be applied rapidly). This could therefore include individuals making decisions alone or on another's behalf (for a young child), or couples making decisions together.
The screening activities entailed investigations performed by or provided by health professionals. Examples could include mammography, cervical Pap smears, colorectal screening, screening for prostatic cancer (PSA test), antenatal screening, genetic screening (including breast cancer gene testing), screening for high cholesterol and cardiovascular risk, and neonatal screening.
Types of interventions
The interventions were providing information on individualised risk: firstly, individualised risk score or individual actual risk information (for example, calculated risk of breast cancer over the next 10 years17); secondly, categorisations of risk status based on these estimates (for example, high, medium, or low risk status, such as for colorectal cancer18); or, thirdly, discussion of personal risk factors relevant to the screening decision (the individual's own characteristics are taken into account in assessing their actual risk or heightened risk status relative to others—examples include risk factors for breast or prostate cancer19,20 that are relevant to the individual).
We compared interventions providing information on individualised risk with those providing information on generalised risk, including average or population risk estimates (such as risk of breast cancer or cervical cancer), general information on risk factors, and general encouragement to acknowledge risks or change risk behaviour. We rejected studies if they simply evaluated health education or promotion to reduce risk factors or increase adherence to screening, without discussing the risks and benefits of undergoing or not undergoing screening.
Types of outcome measures
We extracted outcome data if available in the following areas21,22): firstly, cognitive outcomes: knowledge of risk, accurate risk perception; secondly, affective outcomes: anxiety, satisfaction with decisions made, decisional conflict, anxiety, intention to take up screening; thirdly, behavioural outcomes: uptake of tests, adherence to choice regarding screening programme, “appropriate” uptake; and fourthly, health status outcomes—for example specific status measures or quality of life measures such as the short form questionnaire 36 (SF-36).
Search strategy for identification of studies
Two reviewers (SU and AE) searched the specialised register of the Cochrane consumers and communication review group, Medline, EMBASE, CancerLit, CINAHL, ClinPSYC, and the Science Citation Index Expanded. The search strategies included three layers of search terms—keywords and medical subject headings (MeSH)—to identify articles about screening that included counselling or education on risk specifically. Databases were searched in Ovid (www.ovid.com) from 1985 onwards, as preceding years have low yields in this field.23 For the search strategies see appendix 1 on bmj.com.
A manual follow up of references comprised the most frequently encountered journal (Preventive Medicine), contacting seven most frequently encountered authors (B K Rimer, C Lerman, M D Schwartz, V Champion, M W Kreuter, C S Skinner, and R Bastani) and citation index searches for these authors. We also checked other prominent reviews and a trials register in the field.24,25
Methods of the review
Two reviewers (AE and SU) independently selected publications from search outputs (titles and abstracts). Disagreements were resolved by discussion. In cases of doubt papers were retrieved in full for final assessment, including circulation of rejected papers to other members of the group to review the decision.
Data extraction
AE and SU extracted the data on to a template covering country of origin, group of health professionals involved, screening programme, group of patients involved, setting, sample size, and key outcomes. We extracted data on differences in baseline risk to assess effect modifiers. We extracted data on the nature and design of the intervention, including the type of individualised risk communication in the main intervention (see types of interventions). Outcome data comprised the absolute changes in numbers between groups (for dichotomous variables) and the mean change and standard deviation of the mean change (for continuous variables). We also recorded statistical significance of results. We extracted data to assess the quality of the study against methodological checklists.4,26 The checklist by Edwards et al assesses the quality of the trial and its report in the literature, but does not, for example, score on blinding of participants as it is often not feasible to do so in trials of risk communication.4 Differences in assessment of publications were resolved by discussion between the assessors.
Data combination
We first examined studies for qualitative synthesis. We then applied standard statistical methods for the consumers and communication review group to combine data by using MetaView where feasible, subject to assessment of homogeneity. We calculated odd ratios for dichotomous variables, and weighted mean differences for continuous outcomes. Sensitivity analyses entailed analysing different sections of the data—for example, according to different screening programme or studies based only on participants at high risk (as defined in the studies themselves, due to particular risk factors) rather than people at average risk. We also used the categorisation of individualised risk communication into three levels of detail given to consumers, to examine for evidence of different effect from heterogeneous groups of interventions.
Insufficient data were provided in many studies to enable (raw) data entry into MetaView. In view of the heterogeneity of the studies in terms of the screening programmes addressed, participants, and design of intervention we used a random effects model (giving a more conservative confidence interval on estimates of effect sizes).
Results
Description of studies
Thirteen studies met the inclusion criteria (please note that Lerman et al 199532 and Schwartz et al 199936 arise from the same study). Ten of these described interventions for mammography screening programmes or in relation to breast cancer risk and gene testing. Two addressed screening for (high) cholesterol, and one each addressed cervical, prostate, and colorectal screening (some studies covered more than one topic). Four studies were based on samples of people thought or known to be at higher risk than average for the population. Eleven studies were from the United States. The interventions were delivered by a range of healthcare professionals, ranging from doctors and nurses to staff specifically recruited and trained for the study.
With regard to outcomes reported, 12 studies measured uptake of screening, but only 10 had reliable data for meta-analysis. Four measured changes in the perception of risk and susceptibility. Beyond this there were only occasional outcomes with reliable data for extraction. One study reported changes in knowledge with data that could be extracted, two measured intentions to undergo screening, and two measured movement across stages of change. We found no extractable data on costs, “appropriate” uptake, or health status (other than anxiety).
Some studies were considered closely for inclusion but were eventually excluded. Broadly these fell into two groups. Some examined individualised risk communication but had either no control data or inadequate control data for comparison. Others did not specifically examine individualised risk communication as a separable intervention effect.
Methodological quality of included studies
The studies were of variable quality, but almost all method scores were above average for risk communication studies in health care.4 Although all were randomised trials, description of allocation concealment was adequate in two studies, inadequate in one, and unclear in the remainder.
Review findings and meta-analysis
The table shows principal data on the types of intervention, outcomes, and method scores of the 13 included studies. Appendix 2 on bmj.com and the Cochrane review contain further descriptions of the studies and outcomes.14 Overall we found evidence that individualised risk communication (whether written, spoken, or visually presented) was associated with an increased uptake of screening tests (odds ratio 1.5, 95% confidence interval 1.11 to 2.03) (figure for data from random effects models). The study outcomes showed statistical evidence of heterogeneity. Insufficient data were available (for example, on knowledge, perceived risk levels, attitudes to tests, and intentions to take or actual taking of tests) to assess whether this increase in test uptake was related to informed decision making.
Effect modifiers
Within this overall increase in uptake we found limited evidence that more detailed communication of individualised risk may lead to smaller increases in the uptake of tests (see figure). For individualised risk communication that used and presented numerical calculations of risk, the odds ratio for test uptake was 1.22 (0.56 to 2.68). For risk estimates or calculations that were categorised—for example, into high, medium, or low strata of risk—the odds ratio was 1.42 (1.07 to 1.88). For risk communication that simply listed personal risk factors the odds ratio was 1.7 (1.17 to 2.48). Differences between the categories did not reach significance.
Figure 1.
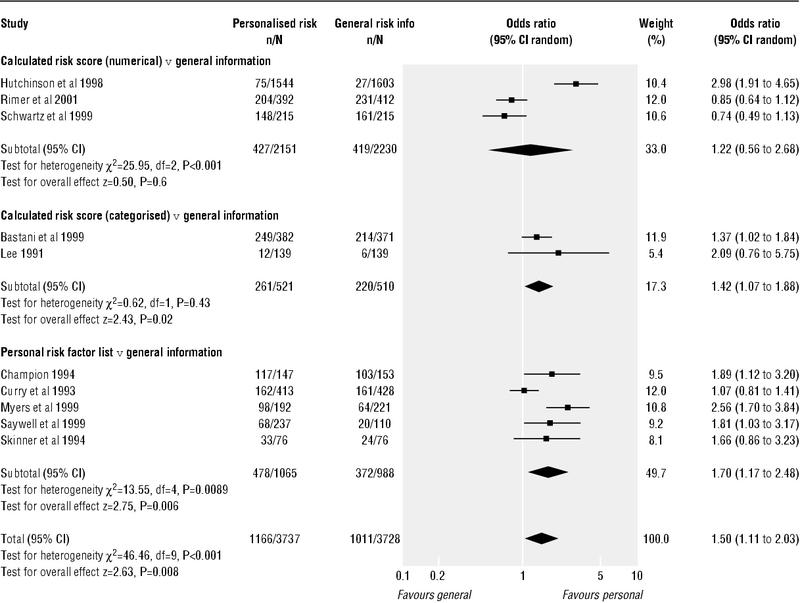
Uptake of test outcomes from 10 studies addressing decisions by patients on whether or not to undergo screening that were used in the review: random effects meta-analysis
The results for the studies addressing mammography showed slightly smaller effects than among the whole group of studies (odds ratio for mammography studies 1.13, 0.98 to 1.29). The four studies examining risk communication in individuals at “higher risk”19,27,28,32,36 showed larger odds ratios (1.99, 1.52 to 2.6) for uptake of tests than the other studies.
Discussion
Principal findings
Few studies in the field of screening have examined the effects of communicating individualised risk. Individualised risk communication is associated with higher uptake of tests, but the evidence that this is necessarily informed decision making by consumers is insufficient. We found insufficient studies to assess effect modifiers reliably, but some evidence showed that effects (on uptake) were greater among individuals at higher risk of disease.
Strengths and weaknesses of the review and its findings
The weaknesses of these data lie in the small number of studies. Findings or hypotheses arising from these data require further evaluation when more primary studies are available. The results are dominated by findings from the topic area of mammography. Caution is required in generalising from these results, and particularly for clinical topics other than mammography. Against this, the strength of these data lies in the fact that the studies have been gleaned from systematic searches of several key databases and contact with key authors in the field, and represent a synthesis of the existing literature base.
Table 1.
Characteristics of studies included in this review
| Study | Design | Participants | Interventions | Outcomes | Allocation concealment | Method score | Notes |
|---|---|---|---|---|---|---|---|
| Bastani et al 199927 | Randomised controlled trial | Women aged over 30; breast cancer in first degree relative; resident in United States or Canada | Mailed notification of individualised risk assessment and other theoretically driven (adherence model) materials tailored for women at high risk | Uptake of mammography one year after baseline survey | Unclear | 15/22 | |
| Champion 199428 | Randomised controlled trial | Women aged ≥35 who had never had breast cancer; United States | In-home interviews conducted by graduate nursing research assistants. Discussion about individual risk factors—susceptibility intervention—as part of a belief modifying intervention | Change in beliefs and knowledge (including susceptibility scores) after the intervention; mammography compliance one year after the intervention | Unclear | 16/22 | |
| Champion et al 199529 | Randomised controlled trial | Women aged ≥35; not diagnosed with breast cancer; United States (analysis of intervention effect only on women aged ≥40 | Interviews conducted at home by graduate nursing students. Discussion about individual risk factors—susceptibility intervention—as part of a belief modifying intervention | Change in beliefs and knowledge (including susceptibility (scores); mammography compliance; movement across stages of change | Unclear | 12/22 | Raw data for compliance outcomes not available for inclusion in meta-analysis; other results included in appendix 2 on bmj.com and Cochrane review14 |
| Curry et al 199319 | Randomised controlled trial | Women aged ≥50; newly enrolled in a health maintenance organisation without prior history of breast cancer or of mammography use in the previous 12 months; United States | Mailed risk factor questionnaire plus personal risk invitation detailing personal risk factors | Use of mammography within one year of invitation | Unclear | 18/22 | |
| Hutchison et al 199830 | Randomised controlled trial | Patients aged 20-69 years, from two primary care group practices; Canada | Risk appraisal questionnaire (yielding risk score). People with scores above 2 were advised to go for screening | Rate of cholesterol testing during the three months of follow up | Unclear | 14/22 | |
| Kreuter et al 199631 | Randomised controlled trial | Patients aged 18-75 from eight family medical practices; North Carolina, United States | Mailed health risk appraisal—risk information tailored to information given at baseline questionnaire | Rate of uptake of Pap smear, mammography, and cholesterol screeing after six months in participants contemplating these behaviours at baseline | Inadequate | 15/22 | Multiple outcomes from overlapping groups of patients, so single figure not included in meta-analysis; separate figures reported in appendix 2 on bmj.com and Cochrane meta-analysis14 |
| Lee 199118 | Randomised controlled trial, stratified for previous screening history and risk status | Federal employees aged ≥40; United States | Appraisal of risk of colorectal—categorised as high, medium, or low personal risk | Knowledge, intention to take test, and uptake | Unclear | 16/22 | |
| Lerman et al 199532 | Randomised controlled trial | Women aged 35 years and older with a family history of breast cancer in a first degree relative; United States | Counselling for risk of breast cancer, including discussion of factors contributing to heightened risk and presentation of individualised risk data | Changes or improvement in risk comprehension | Adequate | 13/22 | Additional paper33 addresses effects on general and breast cancer specific distress |
| Lerman et al 199734 | Randomised controlled trial | Women aged 18-75 who had at least one first degree relative with breast or ovarian cancer; United States | Educational session including a review of individual risk factors for breast and ovarian cancers | Changes in risk perception; testing intentions | Unclear | 16/22 | No data on taking test in control group |
| Myers et al 199920 | Randomised controlled trial | African American men, aged 40-70; patients at the University of Chicago, United States | A personalised “pro-record,” which included a form with tailored risk factors and symptoms | “Adherence”—men who made an office visit for prostate cancer education and early detection within a year | Unclear | 15/22 | |
| Rimer et al 200117 | Randomised controlled trial | Women in their 40s and 50s, and members of Blue Cross Blue Shield (a health maintenance organisation); North Carolina, United States | Tailored print and counselling detailing a woman's personal risk (numerical and graphical) of breast cancer | Accuracy of risk perceptions; mammography uptake | Unclear | 18/22 | |
| Saywell et al 199935 | Randomised controlled trial | Women aged 50-85, non-compliant with mammography guidelines, no history of breast cancer; United States | Telephone and in person counselling including discussion of personal risk factors | Mammography compliance four to six weeks after counselling | Adequate | 12/22 | |
| Schwartz et al 199936 | Randomised controlled trial | Women with family history of breast cancer (first degree relative of sufferer) aged ≥40; United States | Risk counselling including individualised risk figures | Self reported mammography use one year after (compared with baseline) | Unclear | Follow up to the Lerman et al 1995 trial | |
| Skinner et al 199437 | Randomised controlled trial, stratified between clinics | Female family practice attenders aged 40-65; United States | Tailored text re: beliefs, mammography stages, risk factors, and barriers | Mammography stage and uptake | Unclear | 15/22 |
The relative paucity of data in the screening area reflects the difficulties in communicating individualised risk to consumers. The range of clinical topics covered here is narrow. Calculation or estimation of risk is dependent on adequate epidemiological data, and feasible means of converting this into information for individual consumers on the basis of their own risk factors. Examples include the Gail model for risk of breast cancer5 and methods for calculating cardiovascular risk,38 but only the latter have permeated into routine clinical practice. Equivalent epidemiological research into a broader range of clinical topics is needed to enable calculation of individualised risk estimates for individuals with these conditions. Then further evaluation of the effects of providing such information on individualised risk to consumers would be possible.
High risk status as an effect modifier
The intervention effects seemed greater among consumers or patients deemed to be at higher risk than average.19,27,28,32,36 Thus a “high risk status” of consumers may be an important effect modifier for individualised risk communication, indicating substantial potential to modify choices made by such people. This may be a positive opportunity for clinicians to influence decisions among people with risk factors for a condition, but equally it could be potentially harmful if interventions are not introduced carefully.
Informed decision making
Two studies provided the most detailed risk estimates to patients, but were the only ones to show a reduction in uptake of tests.17,36 These effects of communicating risk are consistent with the effects of framing and other manipulations of data representation in health care7—that more information, especially information that is most pertinent to the individual and the decision on treatment in question, often makes consumers more wary of the treatments or tests on offer. Provision of a service may lead patients to perceive that professionals desire use of the service (in this case uptake of tests) rather than informed choices by patients. When the direction of effects is for lower uptake of tests, as in these two studies,17,36 it implies that information can have an important effect. This shows that the overall effect (of increasing uptake) in this review should be regarded with caution.
Implications for screening programmes
As above, the effects of interventions in this review may provide positive opportunities for clinicians to influence decisions among people with risk factors for a condition, but interventions must be introduced carefully to avoid harm. The opportunities to mislead are endless.39 The fact that screening decisions by consumers can be influenced substantially by the type of information generates tension between individual health policy (which seeks to enhance informed decision making) and objectives of population health. The data in this review indicate that the effects of informing patients about individualised risk are variable. In some instances the objectives of population health might be compromised (by lower uptake). The cost effectiveness of screening programmes in reducing the burden of disease depends on reaching a threshold level of coverage of the tests in the target population. Interventions using individualised risk communication may sometimes limit the ability of a programme to reach this threshold and sometimes enhance it.
These tensions between the goals of population health and individual choices must be recognised.40 An informed debate should be conducted among the relevant interest groups—government, public health, clinicians, and particularly patients and their representative groups—and signs show that this is starting.41 Policy developments could explore whether individually appropriate choices, which may include choosing not to take tests, can be accommodated in standards and assessments of achieving best practice at population level.
Further research
If informed decision making by consumers is important, one consequence is that valid instruments will be needed to show that it has occurred. Some measures for specific conditions exist, such as the multidimensional measure of informed choice in screening for Down's syndrome.42 It examines the consistency between knowledge, attitudes to tests, intentions, and choices of uptake of tests. Further research should develop measures for other screening choices, or whether a generic measure of informed choice for screening decisions is feasible.
Research should also evaluate individual cognitive and affective outcomes of interventions using risk communication. These include decisional conflict, satisfaction with decision making, and anxiety measures.43-45 Further intervention studies in this field need to incorporate such measures into their evaluations as they address some of the core constructs that build towards informed decision making by consumers.
These developments may make the effects of individualised risk communication interventions clearer, both in terms of informed decision making and uptake of tests. Until such data are available it seems premature to advocate the use of individualised risk communication simply on the basis that increased uptake of screening programmes has been shown.
Conclusions
Communication of individualised risk generally leads to increased uptake of screening programmes. This may meet professionally or policy driven agendas in health care, but it is not yet clear that the increased uptake is associated with informed decision making by consumers. Further evaluation of strategies to promote informed decision making is still required, and this must include detailed assessment of the cognitive and affective outcomes that may influence subsequent behavioural outcomes (namely, uptake of tests).
What is already known on this topic
Screening programmes are increasingly looking towards ways of promoting informed choices by people invited to have tests
Individualised risk communication—using specific risk estimates based, for example, on the individual's own risk factors for a condition (such as age or family history)—is associated with larger effects on patients' outcomes in general health care, but its effects in screening programmes are uncertain
What this study adds
Individualised risk communication leads to increased participation in screening programmes
It may be useful for purposes of population health, which depend on maximising participation
Few data indicated that the increased uptake could be attributed to informed decision making by people invited to screening
More developed strategies to promote informed decision making are still required
Supplementary Material
 Search strategies and descriptions of the included studies and their outcomes appear on bmj.com
Search strategies and descriptions of the included studies and their outcomes appear on bmj.com
We thank everyone who has commented on the project protocol and the parallel Cochrane protocol and review at various stages, helping to make it feasible. In particular this includes the Consumer Advisory Group within the Cochrane Consumers and Communication Review Group: Hilda Bastian and Genny Nolan (Australia); Teenah Handiside (New Zealand); Christine Brunswick (United States); the contact editor, Dominique Broclain; the editorial team; the review group coordinator, Megan Prictor; the coordinating editor, Sophie Hill; and external peer reviewers.
Contributors: AE designed the protocol, assessed studies for inclusion and data extraction, and led the drafting of this report. SU conducted the literature searches, assessed studies for inclusion and data extraction, and contributed to the drafting of this report. GE contributed to protocol design, was a third reviewer for inclusion and data extraction where required, and contributed to the drafting of this report. KH contributed to protocol design, the statistical data extraction and analysis, and contributed to the drafting of this report. AE is the guarantor.
Funding: Cochrane Health Promotion and Public Health Field.
Competing interests: None declared.
Note about process: AE is the guest editor for this theme issue of the BMJ but he submitted this research to the BMJ in the normal way and it was handled through the BMJ 's normal decision making mechanisms. He played no part in the decision making over this paper.
A fuller version of this review and its findings is available through the Cochrane Library. Issue 1. Update Software, 2003. (Consumers and Communication Review Group.)
References
- 1.Raffle A, Alden B, Quinn M, Babb P, Brett M. Outcomes of screening to prevent cancer: analysis of cumulative incidence of cervical abnormality and modelling of cases and deaths prevented. BMJ 2003;326: 901-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.General Medical Council. Seeking patients consent: the ethical considerations. London: GMC, 1999.
- 3.Slaytor E, Ward JE. How risks of breast cancer and benefits of screening are communicated to women: analysis of 58 pamphlets. BMJ 1998;317: 263-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Edwards A, Hood K, Matthews EJ, Russell D, Russell IT, Barker J, et al. The effectiveness of one-to-one risk communication interventions in health care: a systematic review. Med Dec Making 2000;20: 290-7. [DOI] [PubMed] [Google Scholar]
- 5.Gail MH, Brinton LA, Byar DP, Corle DK, Green SB, Schairer C, et al. Projecting individualized probabilities of developing breast cancer for white females who are being examined annually. J Natl Cancer Inst 1989;81: 1879-86. [DOI] [PubMed] [Google Scholar]
- 6.Sarfati D, Howden-Chapman P, Woodward A, Salmond C. Does the frame affect the picture? A study into how attitudes to screening for cancer are affected by the way benefits are expressed. J Med Screening 1998;5: 137-40. [DOI] [PubMed] [Google Scholar]
- 7.Edwards A, Elwyn G, Covey J, Mathews E, Pill R. Presenting risk information—a review of the effects of “framing” and other manipulations on patient outcomes. J Health Commun 2001;6: 61-82. [DOI] [PubMed] [Google Scholar]
- 8.Raffle A. Information about screening—is it to achieve high uptake or to ensure informed choice? Health Expect 2001;4: 92-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stewart-Brown S, Farmer A. Screening could seriously damage your health. BMJ 1997;314: 533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Covello VT, von Winterfeldt D, Slovic P. Risk communication: a review of the literature. Risk Abstracts 1986;3: 171-81. [Google Scholar]
- 11.Fischoff B. Why (cancer) risk communication can be hard. J Nat Cancer Inst Monographs 1999;25: 7-14. [DOI] [PubMed] [Google Scholar]
- 12.Keeney RL, von Winterfeldt D. Improving risk communication. Risk Anal 1986;6: 417-24. [DOI] [PubMed] [Google Scholar]
- 13.Bottoroff J, Ratner P, Johnson J, Lovato CY, Joab SA. Communicating cancer risk information: the challenges of uncertainty. Pat Educ Counseling 1998;33: 67-81. [DOI] [PubMed] [Google Scholar]
- 14.Edwards A, Unigwe S, Elwyn G, Hood K. Personalised risk communication in health screening programs. Cochrane Library. Issue 1. Update Software, 2003. [DOI] [PubMed]
- 15.National Centre for Reviews and Dissemination. Undertaking systematic reviews of research on effectiveness. CRD guidelines for those carrying out or commissioning reviews. York: University of York, 1996. (Report No 4.)
- 16.Edwards A, Elwyn G, Hood K, Rollnick S. Judging the “weight of evidence” in systematic reviews: introducing rigour into the qualitative overview stage by assessing Signal and Noise. J Eval Clin Pract 2000;6: 177-84. [DOI] [PubMed] [Google Scholar]
- 17.Rimer B, Halabi S, Skinner C, Kaplan E, Crawford Y, Samsa G, et al. The short-term impact of tailored mammography decision-making interventions. Pat Educ Counseling 2001;43: 269-85. [DOI] [PubMed] [Google Scholar]
- 18.Lee CY. A randomized controlled trial to motivate worksite fecal occult blood testing. Yonsei Med J 1991;32: 131-8. [DOI] [PubMed] [Google Scholar]
- 19.Curry SJ, Taplin SH, Anderman C, Barlow WE, McBride C. A randomized trial of the impact of risk assessment and feedback on participation in mammography screening. Prev Med 1993;22: 350-60. [DOI] [PubMed] [Google Scholar]
- 20.Myers R, Chodak G, Wolf T, Burgh D, McGrory G, Marcus S, et al. Adherence by African American men to prostate cancer education and early detection. Cancer 1999;86: 88-104. [DOI] [PubMed] [Google Scholar]
- 21.Llewellyn-Thomas HA. Patients' health care decision-making: a framework for descriptive and experimental investigations. Med Dec Making 1995;15: 101-6. [DOI] [PubMed] [Google Scholar]
- 22.Edwards A, Elwyn G. How should `effectiveness' of risk communication to aid patients' decisions be judged? A review of the literature. Med Dec Making 1999;19: 428-34. [DOI] [PubMed] [Google Scholar]
- 23.Matthews EJ, Edwards A, Barker J, Bloor M, Covey J, Hood K, et al. Efficient literature searching in diffuse topics: lessons from a systematic review of research on communicating risk to patients in primary care. Health Libr Rev 1999;16: 112-20. [DOI] [PubMed] [Google Scholar]
- 24.O'Connor A, Rostom A, Fiset V, Tetroe J, Entwistle V, Llewellyn-Thomas HA, et al. Decision aids for patients facing health treatment or screening decisions: systematic review. BMJ 1999;319: 731-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jepson R, Clegg A, Forbes C, Lewis R, Sowden A, Kleijnen J. The determinants of screening uptake and interventions for increasing uptake: a systematic review. London: Stationery Office, 2000. (Health Technology Assessment Programme.) [PubMed]
- 26.Jadad A, Moore M, Carrol D, Jenkinson C, Reynolds DJM, Gavaghan DJ, et al. Assessing the quality of reports of randomised clinical trials: is blinding necessary? Control Clin Trials 1996;17: 1-12. [DOI] [PubMed] [Google Scholar]
- 27.Bastani R, Maxwell A, Bradford C, Das I, Yan K. Tailored risk notification for women with a family history of breast cancer. Prev Medicine 1999;29: 355-64. [DOI] [PubMed] [Google Scholar]
- 28.Champion VL. Strategies to increase mammography utilization. Med Care 1994;32: 118-29. [DOI] [PubMed] [Google Scholar]
- 29.Champion VL, Huster G. Effect of interventions on stage of mammography adoption. J Behav Medicine 1995;18: 169-87. [DOI] [PubMed] [Google Scholar]
- 30.Hutchison B, Birch S, Evans C, Goldsmith L, Markham B, Frank J. Screening for hypercholesterolaemia in primary care: randomised controlled trial of postal questionnaire appraising risk of coronary heart disease. BMJ 1998;316: 1208-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kreuter MW, Strecher VJ. Do tailored behavior change messages enhance the effectiveness of health risk appraisal? Results from a randomized trial. Health Ed Res 1996;11: 97-105. [DOI] [PubMed] [Google Scholar]
- 32.Lerman C, Lustbader E, Rimer B, Daly M, Miller S, Sands C, et al. Effects of individualized breast cancer risk counseling: a randomized trial. J Nat Cancer Inst 1995;87: 286-92. [DOI] [PubMed] [Google Scholar]
- 33.Lerman C, Narod S, Schulman K, Hughes C, Gomez-Caminero A, Bonney G, et al. BRCA1 testing in families with hereditary breast-ovarian cancer. A prospective study of patient decision making and outcomes. JAMA 1996;275: 1885-92. [PubMed] [Google Scholar]
- 34.Lerman C, Biesecker B, Benkendorf JL, Kerner J, Gomez-Caminero A, Hughes C, et al. Controlled trial of pretest education approaches to enhance informed decision-making for BRCA1 gene testing. J Nat Cancer Inst 1997;89: 148-57. [DOI] [PubMed] [Google Scholar]
- 35.Saywell R, Champion V, Skinner C, McQuillen D, Martin D, Maraj M. Cost-effectiveness comparison of five interventions to increase mammography screening. Prev Med 1999;29: 374-82. [DOI] [PubMed] [Google Scholar]
- 36.Schwartz M, Rimer B, Daly M, Sands C, Lerman C. A randomized trial of breast cancer risk counseling: the impact on self-reported mammography use. Am J Public Health 1999;89: 924-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Skinner CS, Strecher VJ, Hospers H. Physicians' recommendations for mammography: do tailored messages make a difference? Am J Public Health 1994;84: 43-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.British Cardiac Society, British Hyperlipidaemia Association, British Hypertension Society, endorsed by the British Diabetic Association. Joint British recommendations on prevention of coronary heart disease in clinical practice. Heart 1998;80(suppl 2): S1-29. [PMC free article] [PubMed] [Google Scholar]
- 39.Gigerenzer G. How innumeracy can be exploited. In: Reckoning with risk—learning to live with uncertainty. 1st ed. London: Penguin, 2002: 201-10.
- 40.Rogers W. Are guidelines ethical? Some considerations for general practice. Br J Gen Pract 2002;52: 663-9. [PMC free article] [PubMed] [Google Scholar]
- 41.Marteau TM, Kinmonth A-L. Screening for cardiovascular risk: public health imperative or matter for individual informed choice? BMJ 2002;325: 78-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Marteau TM, Dormandy E, Michie S. A multi-dimensional measure of informed choice for use in Down's Syndrome screening. Health Expect 2001;4: 99-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.O'Connor AM. Validation of a decisional conflict scale. Med Dec Making 1995;15: 25-30. [DOI] [PubMed] [Google Scholar]
- 44.Holmes-Rovner M, Kroll J, Schmitt N, Rovner D, Breer L, Rothert ML, et al. Patient satisfaction with health care decisions: the satisfaction with decision scale. Med Dec Making 1996;16: 58-64. [DOI] [PubMed] [Google Scholar]
- 45.Marteau TM, Bekker H. The development of a six-item short form of the state scale of the Spielberger state-trait anxiety inventory. Br J Clin Psychol 1992;31: 301-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


