The presence of patent fallopian tubes, normal ovulation, and normal sperm parameters may still be associated with subfertility because of distortion of the uterine cavity or the presence of intraperitoneal endometriosis. Frustratingly, in some cases, no abnormality is found on routine investigation and the infertility is labelled “unexplained.”
Unexplained subfertility
A couple is usually referred for investigation of subfertility after trying unsuccessfully to conceive for a year. Although many may despair of ever conceiving, the chance of successful spontaneous conception during the subsequent year is about 50%. However, the chance is reduced if the woman has never been pregnant (primary subfertility) or is aged over 30, or the duration of subfertility is longer than three years.
Figure 1.

Unexplained subfertility can be a frustrating diagnosis for any couple trying to conceive
Diagnosis of unexplained subfertility
Unexplained subfertility is a diagnosis of exclusion. Up to 25% of patients who present for investigation in a reproductive medicine clinic are diagnosed with unexplained fertility. The diagnosis is usually made after investigations show normal semen parameters, ovulatory concentrations of serum progesterone in the mid-luteal phase, tubal patency, and a normal uterine cavity.
A frustrating diagnosis for patients
It is important to emphasise to couples with a diagnosis of unexplained subfertility that they have only had essential, simple fertility tests that do not always assess function. For example, despite showing tubal patency, normal transport of eggs and sperm in tubes has not been evaluated as no test for this is available. Although a woman may have an ovulatory concentration of serum progesterone and this indicates formation of a corpus luteum, it does not necessarily mean that an egg has been released nor that an egg has been picked up in the fallopian tubes. Even for women who ovulate, there is no information about oocyte quality and consequent embryo quality after fertilisation. Despite normal semen parameters, the sperm may fail one of the steps needed to fertilise the oocyte. Some or all of these potential causes of infertility may be avoided by using intrauterine insemination with superovulation, in vitro fertilisation, or intracytoplasmic sperm injection.
Figure 2.
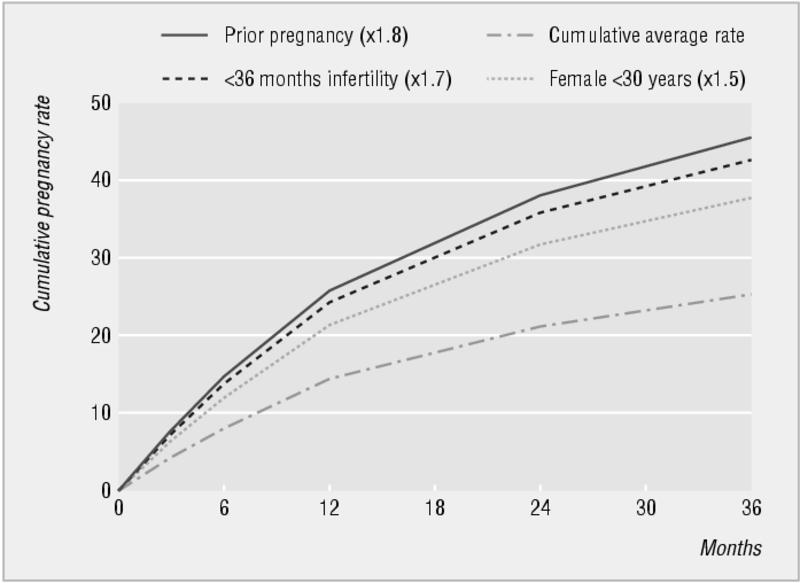
Cumulative live birth rate and prognostic influence of history and findings in couples not conceiving in the first year of trying
Should further tests be done?
Further tests can be done but they seldom alter management. The “postcoital” test should no longer be done routinely as it is unreliable and seldom alters management.1 What couples want is not so much to find out “what is wrong,” but “what can be done for us.” Hence, a pragmatic approach to their treatment should be taken.
Treatment options
“Expectant” management
The decision as to when it is appropriate to treat a couple for unexplained subfertility or to wait for spontaneous pregnancy is dictated largely by duration of subfertility, the woman's age, and the couple's wishes. A woman over 35 should be advised to start treatment earlier than a younger woman.
A diagnosis of unexplained fertility can be highly frustrating for patients, who may interpret this as meaning that there is apparently “no cause” for their subfertility and hence no effective treatment
Couples want a baby, not exhaustive and prolonged investigation
Superovulation and intrauterine insemination
Intrauterine insemination with superovulation is favoured as the treatment of choice for unexplained subfertility, although if the woman is over 37 years she may be advised to proceed directly to in vitro fertilisation. Intrauterine insemination is less invasive and cheaper than in vitro fertilisation and can achieve pregnancy rates of about 10-17% each cycle, with 85% of conceptions occurring within the first four cycles.2 It may be appropriate for couples to have up to six cycles of intrauterine insemination before stopping this treatment and moving on to in vitro fertilisation. The point at which the treatment is stopped will depend on the woman's age, the duration of subfertility, and local funding policies.
Table 1.
Superovulation and intrauterine insemination
| • This treatment cycle starts with the onset of the menses, with daily, or alternate day ovarian stimulation using injections of follicular stimulating hormone |
| • Follicular development is monitored using ultrasonography. When one or two follicles are at least 18 mm in size, ovulation is triggered by human chorionic gonadotrophin hormone injection |
| • The sperm, washed free of seminal fluid in the laboratory, are placed in the uterine cavity via a catheter either immediately or within 24 hours of the human chorionic gonadotrophin hormone injection |
| • No additional hormonal support is required to sustain the endometrium |
Endometriosis
Endometriosis is present in 20-40% of women who complain of subfertility, although it can be found in 5% of fertile women. Mild endometriosis that is not associated with adhesions and tubal defects may be associated with protracted infertility in some women but not others, and it is unclear why. Postulated explanations include intraperitoneal inflammation, immunological factors, unruptured luteinised follicles, and an increase in the rate of miscarriage.
Endometriosis should be suspected when there is dyspareunia, severe dysmenorrhoea, or unexplained abdominal pain, although the symptoms experienced are a poor indicator of the severity of disease. Pelvic examination may show tenderness, nodules of endometriosis on the uterosacral ligaments, or an enlarged ovary, which may be secondary to an ovarian endometrioma. The diagnosis of endometriosis is generally confirmed by laparoscopy. Preoperative ultrasonography is helpful to diagnose the likely cause of a tender and enlarged ovary.
Figure 3.
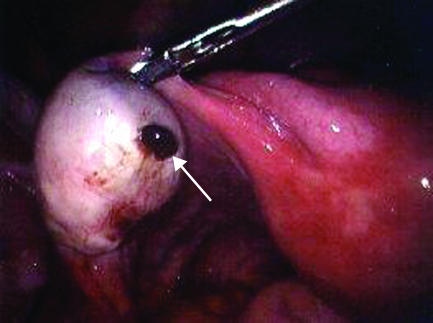
Laparoscopy of an enlarged ovary containing an endometriotic cyst leaking “chocolate” fluid (arrow). These endometriomas develop as endometriotic implants that may bleed slowly into the ovary over months. This patient complained of worsening left sided pain. A small rupture was found and there was a small amount of blood in her abdomen. The cyst was removed and the normal ovarian tissue was saved
Treatment options
Drug treatment to control the symptoms of endometriosis is usually counterproductive to the immediate fertility prospects for a couple. Although some drugs are effective in suppressing deposits of the disease during the course of treatment, most prevent the woman conceiving (for example, gonadotrophin releasing hormone analogues, sequential oral contraception). The woman may even be advised not to attempt to conceive while taking certain drugs (for example, danazol), thereby prolonging the period of subfertility, and with little or no chance of improving fecundity after treatment. Thus, after a period of infertility where endometriosis is present, the choice of approach is surgery or assisted conception.
Endometriosis is characterised by the presence and growth of endometrial tissue outside the uterus and is often associated with symptoms of dysmenorrhoea, dyspareunia, and subfertility
Where endometriosis is minimal without tubal damage, intrauterine insemination with superovulation may be a reasonable option. For minimal or mild endometriosis, surgical ablation using laparoscopic laser treatment, bipolar coagulation of endometriotic deposits, or excision of the deposits has been shown to be more effective than expectant management.3 For severe disease the most cost effective management is in vitro fertilisation.
Figure 4.
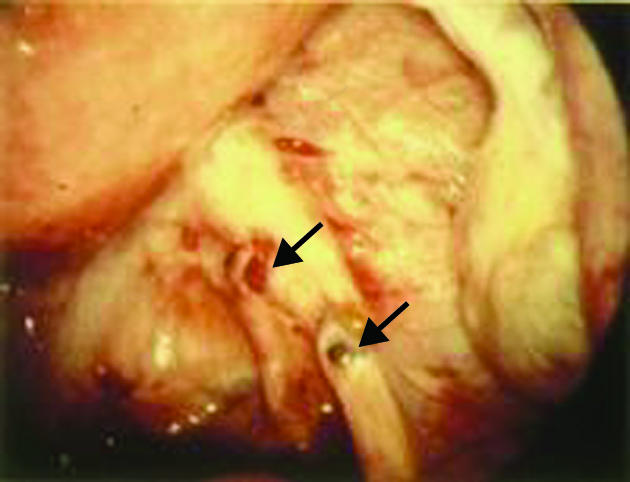
Mild pelvic endometriosis seen at the time of diagnostic laparoscopy. Arrows show typical endometrioic deposits
Fibroids
Fibroids (leiomyomata) are benign tumours of the myometrium which occur in up to 30% of women. They are more common in African and Afro-Caribbean women, non-smokers, and women who postpone childbearing voluntarily or involuntarily. Most women with fibroids do not know that they are present and have no symptoms from them.
Figure 5.
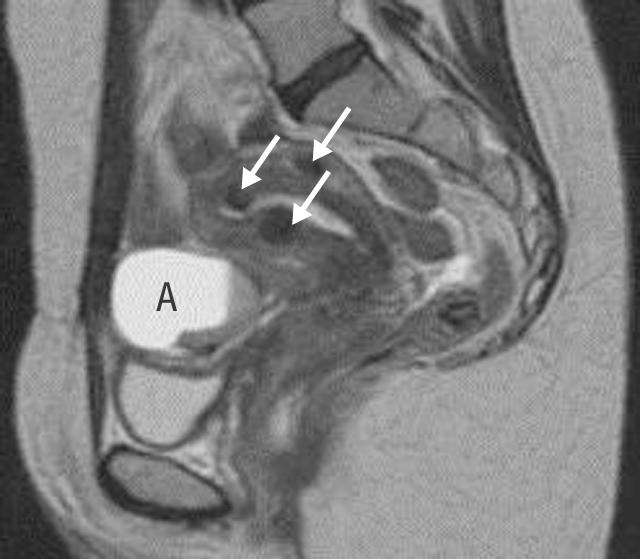
Magnetic resonance scan showing a bright endometrioma (A) with a dependent clot. The arrows show small intramural fibroids
Rarely, women will present because they can feel a lump or a “pelvic fullness” caused by the size of the fibroids. More often they present with menorrhagia or subfertility. Fibroids are more likely to reduce the chance of an embryo implanting if the fibroid is intracavity.
Figure 6.
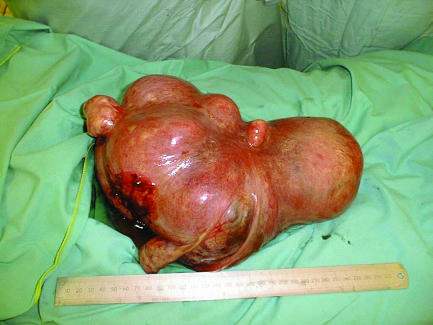
Uterus containing multiple fibroids, which may interfere with fertility even after surgical myomectomy because of distortion of the uterus
Fibroids are estimated to have a detrimental effect on fertility in up to 10% of cases. They are also associated with an increased risk of miscarriage in women who conceive and half the live birth rate in in vitro fertilisation cycles.4 Apart from the mass effect, the precise mechanism by which fibroids may cause subfertility is unknown.
Figure 7.
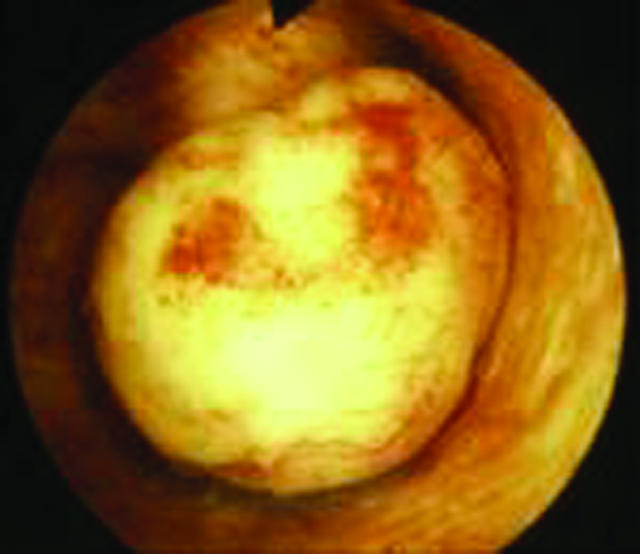
Intracavity fibroid seen by hysteroscopy
Medical management
The size of fibroids can be reduced, albeit temporarily, by administration of superactive gonadotrophin releasing hormone analogues (for example, goserelin, buserelin, nafarelin). The expected reduction in size can be around 30% after four months' use. However, when the fibroids are again exposed to the restored oestrogen-rich environment, they will continue to grow. Consequently a surgical approach is a more realistic alternative. Laparoscopic myomectomy may be suitable for smaller subserosal or intramural fibroids. However, there is still a risk of rupture during a subsequent labour. 5
Surgical management
If the fibroids are mainly intracavity (submucosal), they can be resected easily hysteroscopically with good long term results for fertility and the treatment of menorrhagia.
Figure 8.
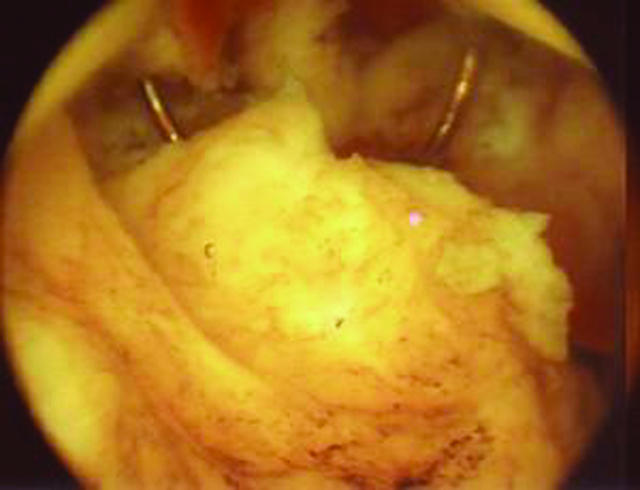
Hysteroscopic resection of an intracavity fibroid, with part of the diathermy cutting loop visible
However, if the fibroids are intramural, an abdominal procedure (laparotomy or laparoscopic myomectomy) is needed. Gonadotrophin releasing hormone analogues should be used before surgery to shrink the fibroid, to make the surgery less vascular, and often to allow improvement in haemoglobin concentration.
Myolysis—the thermal destruction of fibroids using a laser fibre—is not recommended for women who want to remain fertile. Myolysis carries the risk of adhesion formation and rupture. The success rate of a subsequent spontaneous conception after a hysteroscopic, abdominal, or a laparoscopic myomectomy is about 60% if infertility was the sole reason for the surgery.6
Endometriomas should be excised or drained before treatment as (a) they may limit the potential of the ovary to generate follicles during the treatment or (b) they may inadvertently be inoculated with vaginal bacteria during egg retrieval, leading to a pelvic or ovarian abscess
Fibroid embolisation
Bilateral uterine artery embolisation (fibroid embolisation) is a new technique that has gained some favour in the treatment of fibroids. However, relatively few successful pregnancies have been reported, and there is a risk of hysterectomy because of sepsis of necrotic fibroids. The joint report of the Royal College of Obstetricians and Gynaecologists and the Royal College of Radiologists does not recommend fibroid embolisation for infertile women until more is known about outcome.7
Table 2.
Practice points
| • Couples should be referred for assessment of their subfertility if they have failed to conceive after one year of frequent unprotected intercourse |
| • Unexplained infertility should be treated initially with superovulation and intrauterine insemination except if a couple has more than three years of subfertility or if the woman is aged ⩾38 years (in which case early recourse to in vitro fertilisation is recommended) |
| • Women with endometriosis who fail to conceive should have surgical ablation of their deposits except in severe disease, when in vitro fertilisation is recommended after treatment of endometriomas |
| • Fibroids are a cause of subfertility. Surgery should always be considered if no other explanation for subfertility is found and is essential if the fibroid is intracavity |
Fibroids and in vitro fertilisation
In women about to begin a course of in vitro fertilisation treatment, there is evidence that intramural fibroids reduce the chance of treatment success because they decrease the implantation potential of an embryo. Evidence also exists that the incidence of miscarriage may be increased in women with an intramural fibroid having in vitro fertilisation treatment. However, no randomised trials show whether myomectomy done on these women will increase their chances of conception.
Figure 9.
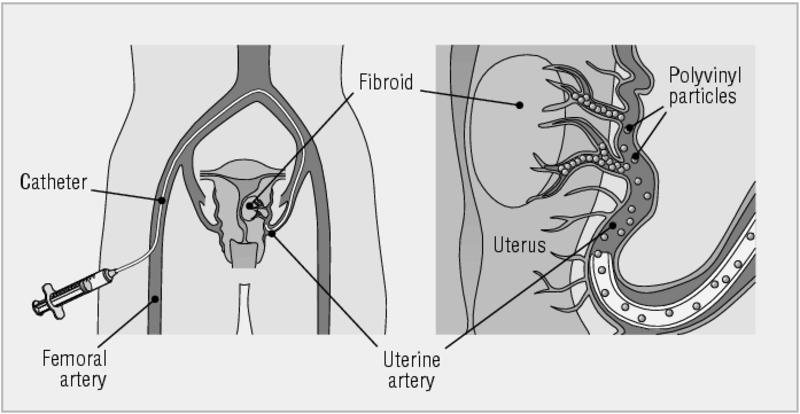
Fibroid embolisation—both uterine arteries are occluded using a transfemoral approach. Small polyvinyl alcohol beads obstruct the blood supply to the fibroids, causing necrosis and shrinkage
Table 3.
Further reading
| • Buttram VC, Reiter RC. Uterine leiomyomata: etiology, symptomology and management. Fertil Steril 1981;30:644-7 |
| • Philips Z, Barraza-Llorens M, Posnett J. Evaluation of the relative cost-effectiveness of treatments for infertility in the UK. Hum Reprod 2000;15:95-106 |
| • Templeton A, Cooke I, O'Brien PMS, eds. Evidence based fertility treatment. London: RCOG, 1998 |
The ABC of subfertility is edited by Peter Braude, professor and head of department of women's health, Guy's, King's, and St Thomas's School of Medicine, London, and Alison Taylor, consultant in reproductive medicine and director of the Guy's and St Thomas's assisted conception unit. The series will be published as a book in the winter.
Competing interests: None declared.
The photograph of a couple in bed is from Elinor Carucci/Photonica. The figure showing the cumulative birth rate and prognostic influence of history uses data from Collins JA et al. Fertil Steril 1995;64: 22-8. The photograph of an endometrioic cyst taken at laparoscopy is reproduced with permission of Dr D A Hill, Florida hospital family practice residency, Orlando, Florida. The magnetic resonance scan showing the bright endometrioma is reproduced with permission of B Cooper, St Paul's Hospital, Vancouver, British Columbia. The figures showing fibroid embolisation are adapted courtesy of Dr J Spies, Georgetown University Medical Center, Maryland.
References
- 1.Royal College of Obstetricians and Gynaecologists. Evidence based guidelines. The management of the infertile couple. London: RCOG Press, 1998.
- 2.Nuojua-Huttunen S, Tomas C, Bloigu R, Tuomivaara L, Martikainen H. Intrauterine insemination treatment in subfertility: an analysis of factors affecting outcome. Hum Reprod 1999;14: 698-703. [DOI] [PubMed] [Google Scholar]
- 3.Marcoux S, Maheux R, Berube S. Canadian Collaborative Group on Endometriosis. Laparoscopic surgery in infertile women with minimal or mild endometriosis. N Engl J Med 1997;337: 217-22. [DOI] [PubMed] [Google Scholar]
- 4.Hart R, Khalaf Y, Yeong CT, Seed P, Taylor A, Braude P. A prospective controlled study of the effect of intramural uterine fibroids on the outcome of assisted conception. Hum Reprod 2001;16: 2411-7. [DOI] [PubMed] [Google Scholar]
- 5.Hart R, Molnar BG, Magos A. Long term follow-up of hysteroscopic myomectomy assessed by survival analysis. Br J Obstet Gynaecol 1999;106: 700-5. [DOI] [PubMed] [Google Scholar]
- 6.Vercellini P, Maddalena S, De Giotgi O, Aimi G, Crosignani PG. Abdominal myomectomy for infertility: a comprehensive review. Hum Reprod 1998;13: 873. [DOI] [PubMed] [Google Scholar]
- 7.Clinical recommendations on the use of uterine artery embolisation in the management of fibroids. Report of a joint working party. London: RCOG Press, 2000.


