Abstract
Groups of immature and mature mice were treated once with DMBA by oral or intraperitoneal route, and the subsequent bilateral sequence of ovarian changes leading to the development of unilateral granulosa cell tumour was studied.
Early post-treatment changes included disappearance of oocytes and follicles as well as increase of the stroma mass. The neoplastic development was closely correlated to the rate of oocyte disappearance. The faster oocytes were eliminated, the earlier tumours appeared. The early post-treatment changes led to a stage of potential preneoplasia, characterized by diffuse luteinization of the ovarian parenchyma. In some preneoplastic ovaries the luteinized tissue underwent neoplastic transformation and developed into invasive luteoma. In other preneoplastic ovaries foci of granulosa-like tumour cells appeared in the luteinized tissue, representing the stage of microscopic granulosa cell tumour. However, such microtumours could also develop within pre-existing luteomata. Autoradiography after injection of thymidine-3H suggested that the granulosa-like tumour cells developed as the result of undifferentiated proliferation of luteinized cells.
So far the pathological ovarian evolution occurred bilaterally as well as unilaterally. However, when a microscopic granulosa cell tumour by further growth became a macroscopic granulosa cell tumour the contralateral ovary invariably atrophied. This ultimate unilateral development coincided with a continuous production of oestrogen by the granulosa cell tumour. The reason for the contralateral atrophy is discussed in relation to the influence of the hormonal balance on ovarian tumorigenesis.
Full text
PDF







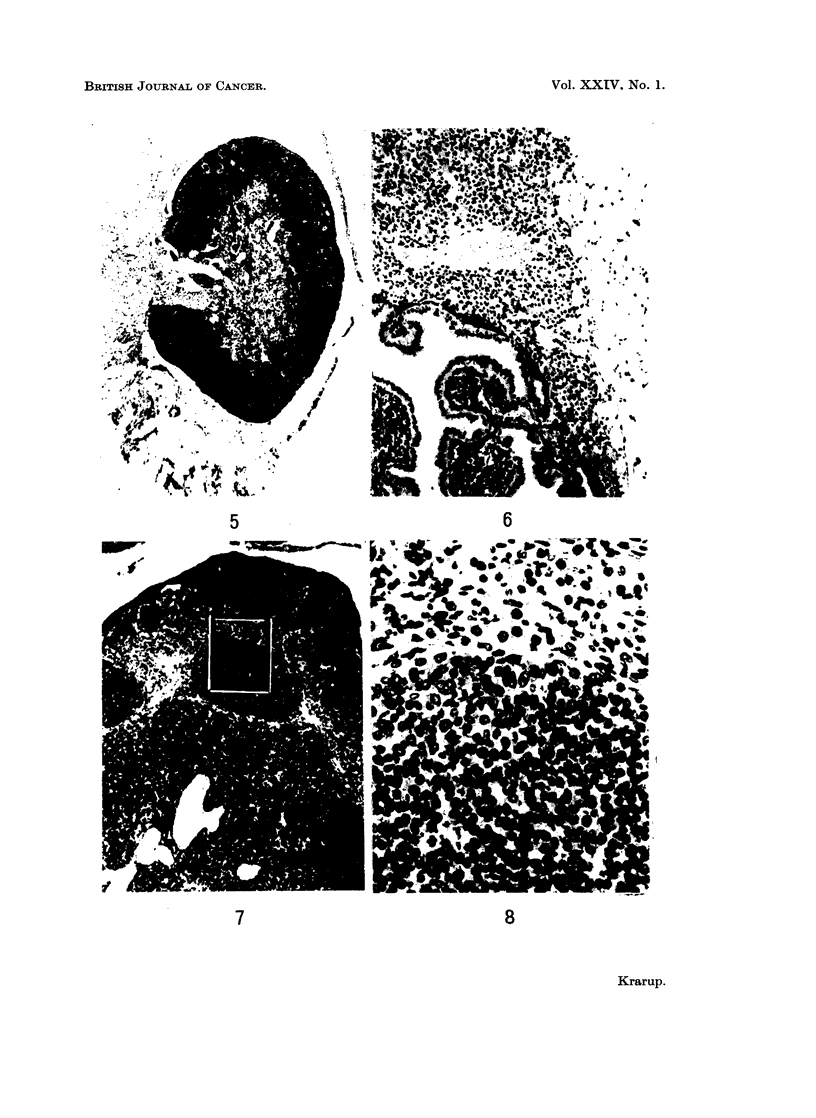
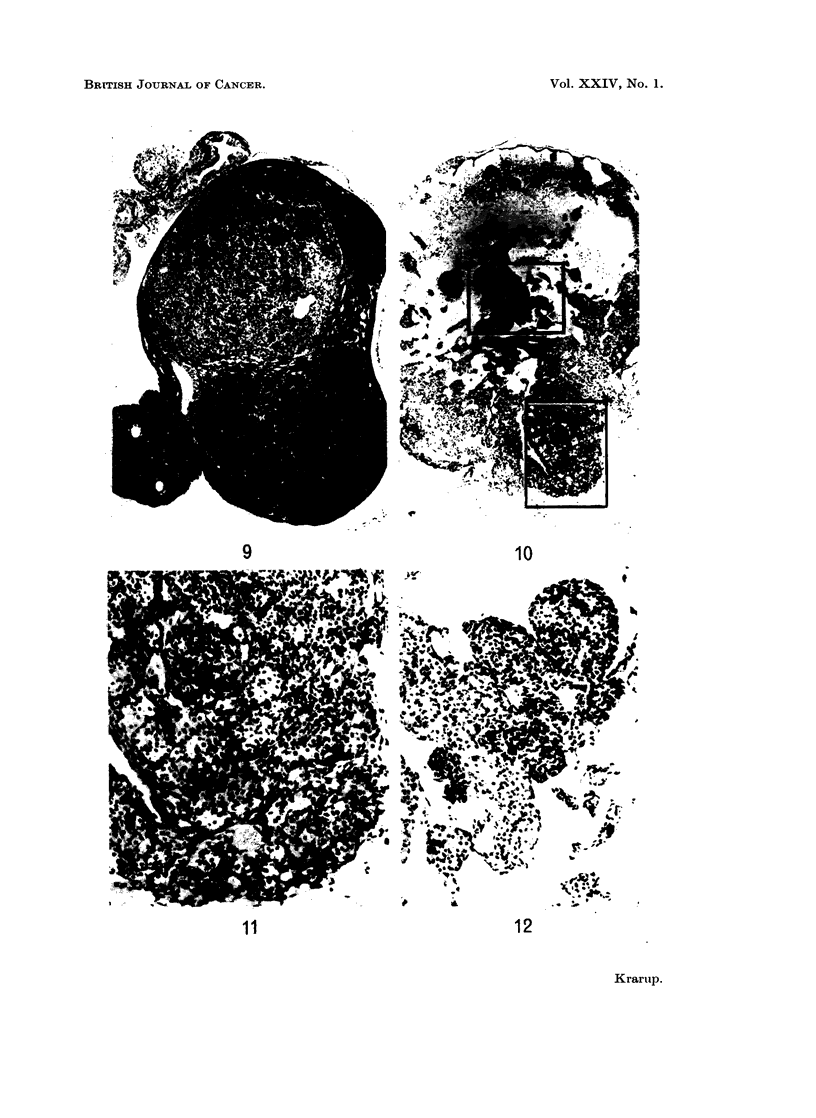














Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Biskind G. R., Biskind M. S. Atrophy of Ovaries Transplanted to the Spleen in Unilaterally Castrated Rats; Proliferative Changes Following Subsequent Removal of Intact Ovary. Science. 1948 Aug 6;108(2797):137–138. doi: 10.1126/science.108.2797.137. [DOI] [PubMed] [Google Scholar]
- DEANE H. W., FAWCETT D. W. Pigmented interstitial cells showing brown degeneration in the ovaries of old mice. Anat Rec. 1952 Jun;113(2):239–245. doi: 10.1002/ar.1091130208. [DOI] [PubMed] [Google Scholar]
- HOWELL J. S., MARCHANT J., ORR J. W. The induction of ovarian tumours in mice with 9:10-dimethyl-1:2-benzanthracene. Br J Cancer. 1954 Dec;8(4):635–646. doi: 10.1038/bjc.1954.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jull J. W., Streeter D. J., Sutherland L. The mechanism of induction of ovarian tumors in the mouse by 7,12-dimethylbenz-[alpha]anthracene. I. Effect of steroid hormones and carcinogen concentration in vivo. J Natl Cancer Inst. 1966 Oct;37(4):409–420. [PubMed] [Google Scholar]
- Krarup T. 9:10-dimethyl-1:2-benzantracene induced ovarian tumours in mice. Acta Pathol Microbiol Scand. 1967;70(2):241–248. doi: 10.1111/j.1699-0463.1967.tb01287.x. [DOI] [PubMed] [Google Scholar]
- Krarup T., Pedersen T., Faber M. Regulation of oocyte growth in the mouse ovary. Nature. 1969 Oct 11;224(5215):187–188. doi: 10.1038/224187a0. [DOI] [PubMed] [Google Scholar]
- LIPSCHUTZ A., CERISOLA H., PANASEVICH V. I. THE ROLE OF PITUITARY HORMONES IN OVARIAN TUMOURIGENESIS. Acta Unio Int Contra Cancrum. 1964;20:1412–1416. [PubMed] [Google Scholar]
- MARCHANT J. The development of ovarian tumours in ovaries grafted from mice pretreated with dimethylbenzanthracene. Inhibition by the presence of normal ovarian tissue. Br J Cancer. 1960 Sep;14:514–518. doi: 10.1038/bjc.1960.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MODY J. K. The actio of four carcinogenic hydrocarbons on the ovaries of IF mice and the histogenesis of induced tumours. Br J Cancer. 1960 Jun;14:256–266. doi: 10.1038/bjc.1960.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MURPHY E. D., RUSSELL E. S. OVARIAN TUMORIGENESIS FOLLOWING GENIC DELETION OF GERM CELLS IN HYBRID MICE. Acta Unio Int Contra Cancrum. 1963;19:779–782. [PubMed] [Google Scholar]
- Pedersen T., Krarup T. Cell population kinetics in the mouse ovary after treatment with a chemical carcinogen (DMBA). Int J Cancer. 1969 Jul 15;4(4):495–506. doi: 10.1002/ijc.2910040415. [DOI] [PubMed] [Google Scholar]
- Peters H., Pedersen T. Origin of follicle cells in the infant mouse ovary. Fertil Steril. 1967 May-Jun;18(3):309–313. doi: 10.1016/s0015-0282(16)36303-8. [DOI] [PubMed] [Google Scholar]
- RUSSELL E. S., FEKETE E. Analysis of W-series pleiotropism in the mouse: effect of WvWv substitution on definitive germ cells and on ovarian tumorigenesis. J Natl Cancer Inst. 1958 Aug;21(2):365–381. [PubMed] [Google Scholar]
- Zylicz E., Sips D., Levy E., Peters H. The vaginal smear in mice. A correlation of smear type and oocyte number. Acta Cytol. 1967 Nov-Dec;11(6):483–485. [PubMed] [Google Scholar]







