Abstract
Crocidolite asbestos fibres, suspended in physiological saline, were injected subcutaneously into one or both flanks of 95 CBA/Lac female mice; 75 control mice received injections of saline only. Most animals were killed at chosen intervals of between 2 and 42 days after injection but some were left for longer periods of up to 623 days. At autopsy, many lymphoid and non-lymphoid structures were removed and examined for the presence of asbestos by the following techniques: haematoxylin and eosin staining followed by conventional and polarized light microscopy; Perl's stain; microincineration followed by phase-contrast microscopy; maceration with KOH followed by phase-contrast microscopy; and electron microscopy.
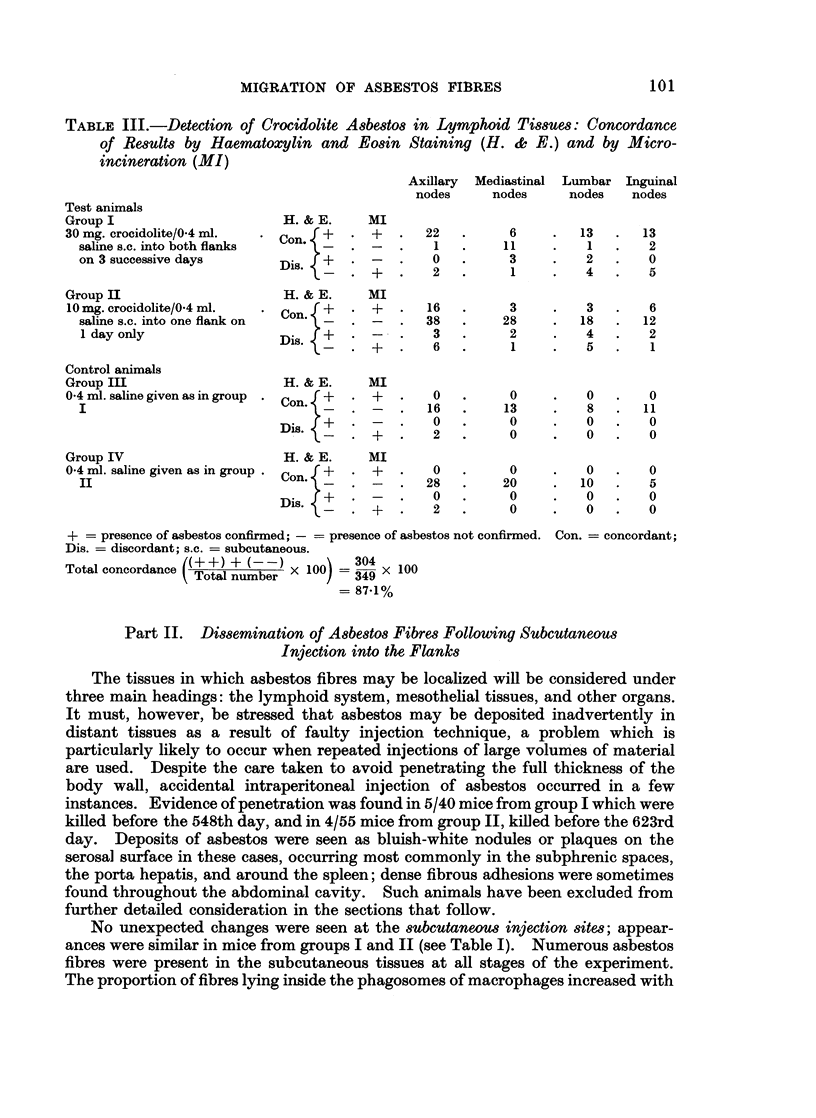
A combination of haematoxylin and eosin staining and microincineration was found to be the most convenient and reliable method for demonstrating asbestos fibres in the tissues. Electron microscopy was essential for detecting very small fibres and for locating them to specific intracellular structures.
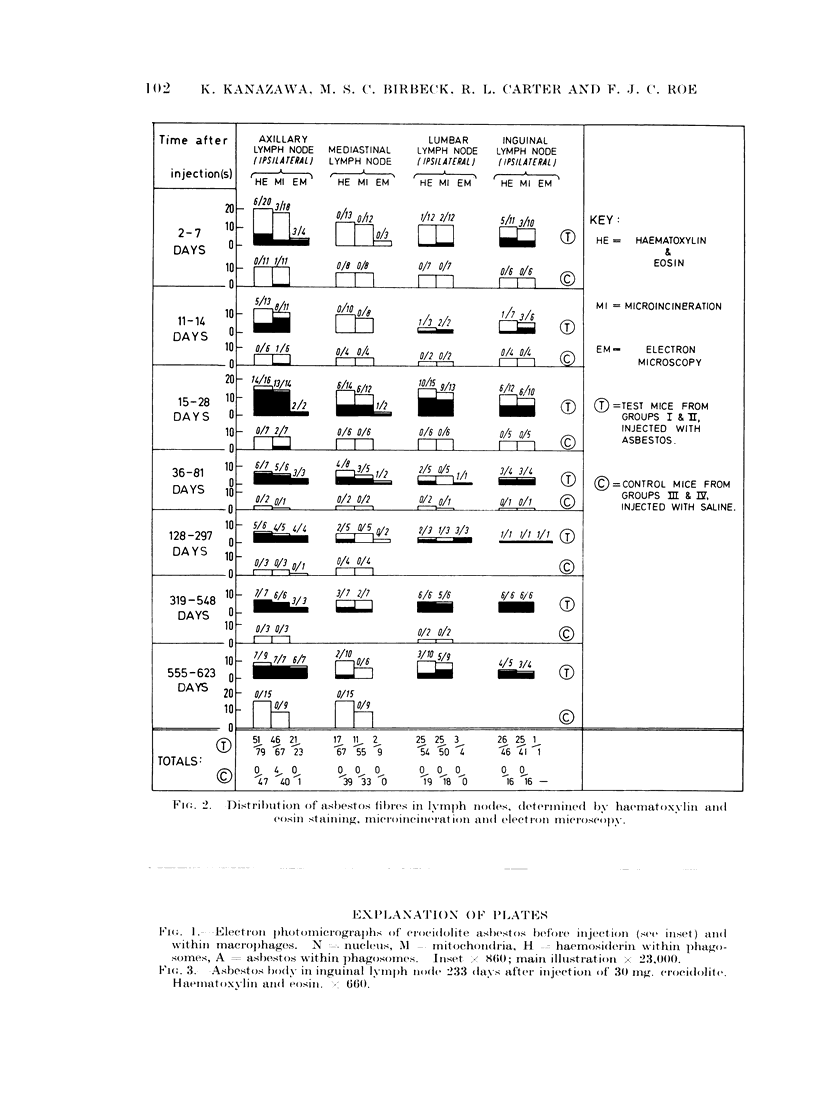
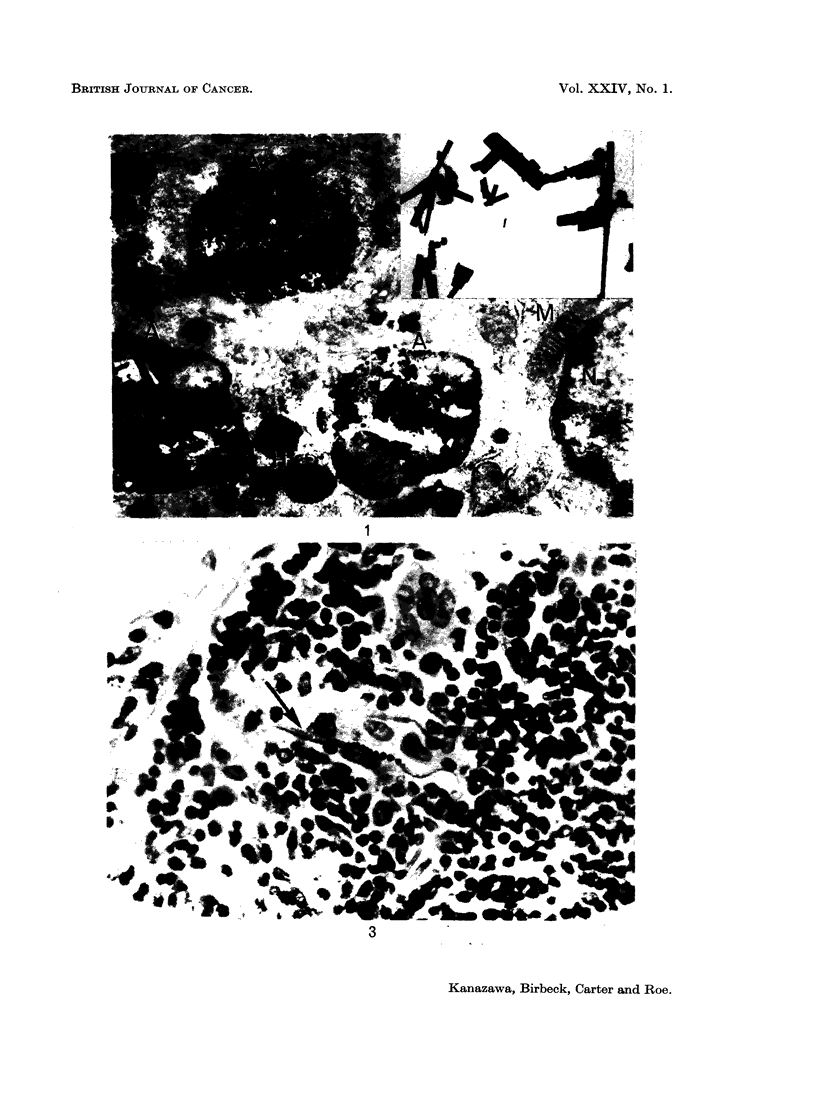
The morphological findings indicate that some migration of asbestos fibres away from the initial site of injection takes place. Dissemination is usually along lymphatic pathways and fibres tend to accumulate in the lymphoid tissues, particularly in the regional (axillary) lymph nodes; smaller amounts were found in inguinal, mediastinal and lumbar nodes. The fibres were usually intracellular, lying inside the phagosomes of macrophages, but larger fibres weresometimes encountered lying free. Small numbers of fibres were seen in the spleen and also in non-lymphoid organs such as the liver, kidneys and brain—suggesting that some asbestos may enter the blood stream. There was no evidence of massive or selective spread to subserosal tissues in the thorax or abdomen, though trapping of asbestos fibres was observed in pleural “milky spots” in long-term survivors. The possible role of milky spots in the development of pleural plaques and mesotheliomata is discussed.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Botham S. K., Holt P. F. The mechanism of formation of asbestos bodies. J Pathol Bacteriol. 1968 Oct;96(2):443–453. doi: 10.1002/path.1700960223. [DOI] [PubMed] [Google Scholar]
- Davis J. M. Electron-microscope studies of asbestosis in man and animals. Ann N Y Acad Sci. 1965 Dec 31;132(1):98–111. doi: 10.1111/j.1749-6632.1965.tb41093.x. [DOI] [PubMed] [Google Scholar]
- Gaensler E. A., Addington W. W. Asbestos or ferruginous bodies. N Engl J Med. 1969 Feb 27;280(9):488–492. doi: 10.1056/NEJM196902272800907. [DOI] [PubMed] [Google Scholar]
- Gough J. Differential diagnosis in the pathology of asbestosis. Ann N Y Acad Sci. 1965 Dec 31;132(1):368–372. doi: 10.1111/j.1749-6632.1965.tb41117.x. [DOI] [PubMed] [Google Scholar]
- Gross P., de Treville R. T., Cralley L. J., Davis J. M. Pulmonary ferruginous bodies. Development in response to filamentous dusts and a method of isolation and concentration. Arch Pathol. 1968 May;85(5):539–546. [PubMed] [Google Scholar]
- Holt P. F., Young D. K. The mechanism of production of asbestos bodies from anthophyllite fibres. J Pathol Bacteriol. 1967 Apr;93(2):696–699. doi: 10.1002/path.1700930235. [DOI] [PubMed] [Google Scholar]
- Hourihane D. O. A biopsy series of mesotheliomata, and attempts to identify asbestos within some of the tumors. Ann N Y Acad Sci. 1965 Dec 31;132(1):647–673. doi: 10.1111/j.1749-6632.1965.tb41143.x. [DOI] [PubMed] [Google Scholar]
- LANG J. [On peculiar capillary convolutions of the parietal pleura. I]. Z Zellforsch Mikrosk Anat. 1962;58:487–523. [PubMed] [Google Scholar]
- Miotti R. Die Lymphknoten und Lymphgefässe der Weissen Ratte (Rattus Norvegicus Berkenhout, Epimys Norvegicus) Acta Anat (Basel) 1965;62(4):489–527. [PubMed] [Google Scholar]
- Newhouse M. L., Thompson H. Epidemiology of mesothelial tumors in the London area. Ann N Y Acad Sci. 1965 Dec 31;132(1):579–588. doi: 10.1111/j.1749-6632.1965.tb41138.x. [DOI] [PubMed] [Google Scholar]
- PRESSMAN J. J., SIMON M. B. Experimental evidence of direct communications between lymph nodes and veins. Surg Gynecol Obstet. 1961 Nov;113:537–541. [PubMed] [Google Scholar]
- Roe F. J., Carter R. L., Walters M. A., Harington J. S. The pathological effects of subcutaneous injections of asbestos fibres in mice: migration of fibres to submesothelial tissues and induction of mesotheliomata. Int J Cancer. 1967 Nov 15;2(6):628–638. doi: 10.1002/ijc.2910020624. [DOI] [PubMed] [Google Scholar]
- SELIKOFF I. J., CHURG J., HAMMOND E. C. RELATION BETWEEN EXPOSURE TO ASBESTOS AND MESOTHELIOMA. N Engl J Med. 1965 Mar 18;272:560–565. doi: 10.1056/NEJM196503182721104. [DOI] [PubMed] [Google Scholar]
- Smith W. E., Miller L., Elsasser R. E., Hubert D. D. Tests for carcinogenicity of asbestos. Ann N Y Acad Sci. 1965 Dec 31;132(1):456–488. doi: 10.1111/j.1749-6632.1965.tb41127.x. [DOI] [PubMed] [Google Scholar]
- Stumphius J., Meyer P. B. Asbestos bodies and mesothelioma. Ann Occup Hyg. 1968 Oct;11(4):283–293. doi: 10.1093/annhyg/11.4.283. [DOI] [PubMed] [Google Scholar]
- Tang J., Mills J., Chiang L., de Chiang L. Gastric pepsin, mucus and clinical secretory studies. I. Gastric pepsin and pepsin inhibitors. Comparative studies on the structure and specificity of human gastricsin, pepsin and zymogen. Ann N Y Acad Sci. 1967 Jan 26;140(2):688–696. doi: 10.1111/j.1749-6632.1967.tb50994.x. [DOI] [PubMed] [Google Scholar]
- WAGNER J. C. Experimental production of mesothelial tumours of the pleura by implantation of dusts in laboratory animals. Nature. 1962 Oct 13;196:180–181. doi: 10.1038/196180a0. [DOI] [PubMed] [Google Scholar]
- WAGNER J. C., SLEGGS C. A., MARCHAND P. Diffuse pleural mesothelioma and asbestos exposure in the North Western Cape Province. Br J Ind Med. 1960 Oct;17:260–271. doi: 10.1136/oem.17.4.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEBSTER I. Asbestosis in non-experimental animals in South Africa. Nature. 1963 Feb 2;197:506–506. doi: 10.1038/197506a0. [DOI] [PubMed] [Google Scholar]



