Abstract
Ehrlich ascites tumours (EAT) were grown in mice by injecting 1 × 106 cells intraperitoneally. In mice which received one or more injections of 30 mg soybean trypsin inhibitor (TI) i.p. during tumour growth, the number of recoverable tumour cells was significantly reduced by up to 92%. Also, the mean size of these cells was significantly smaller.
When the rate of labelled thymidine incorporation in vitro was compared in TI-treated and control cells, no significant differences were detected. However, when the population doubling time of EAT cells in vivo was calculated, it was apparent that recoverable TI-treated cells were dividing more rapidly than controls. Consequently, the reduced number of cells recovered from TI-treated mice did not result from a reduced growth rate.
Viability, assessed by trypan blue dye exclusion and rate of labelled chromium release, was the same in TI-treated and control cell populations. Thus TI was nontoxic to EAT cells and the reduced number of cells from treated tumours was not therefore due to cytotoxicity.
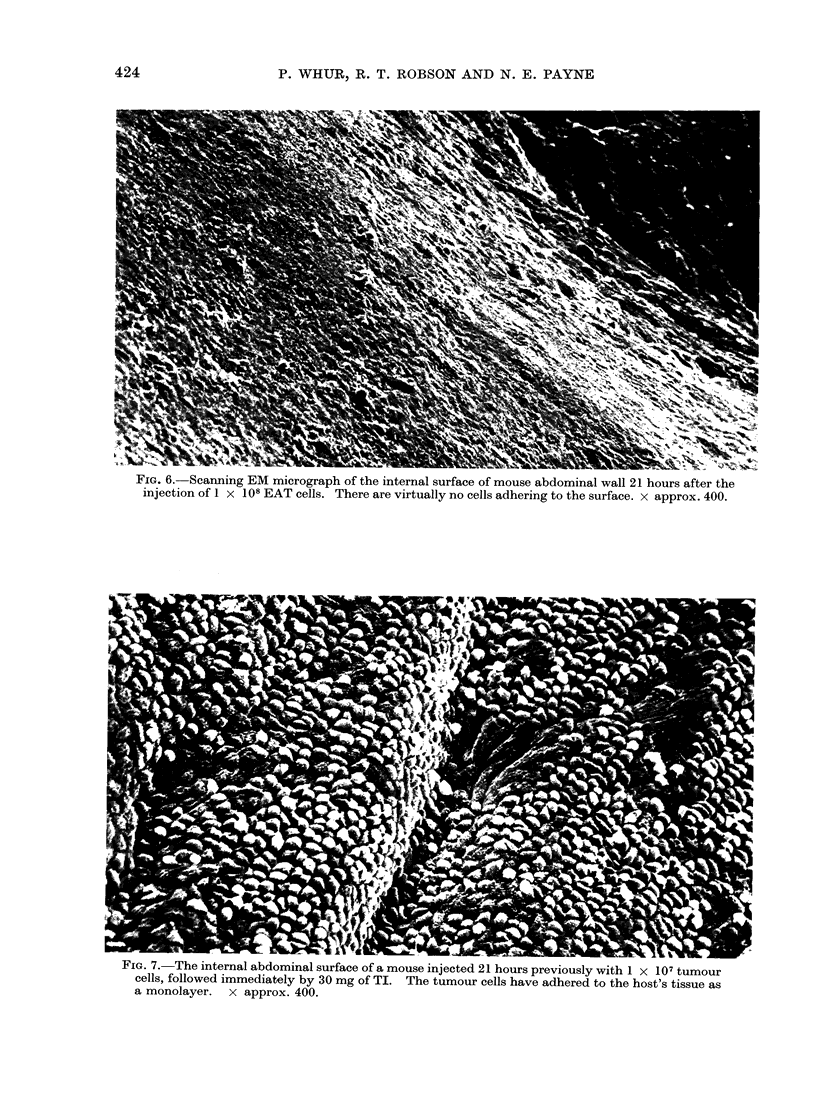
Scanning electron microscopy revealed that normal EAT cells did not adhere to internal host surfaces but that after TI treatment they adhered in large numbers to produce an appearance which resembled a confluent monolayer. This binding to host tissue accounted for the reduction in the number of cells recovered from TI-treated animals. We propose that TI acts as a protease inhibitor to prevent intrinsic proteolytic enzyme activity at the tumour cell surface. This activity would normally destroy the binding sites required for adhesion to host tissue.
Full text
PDF


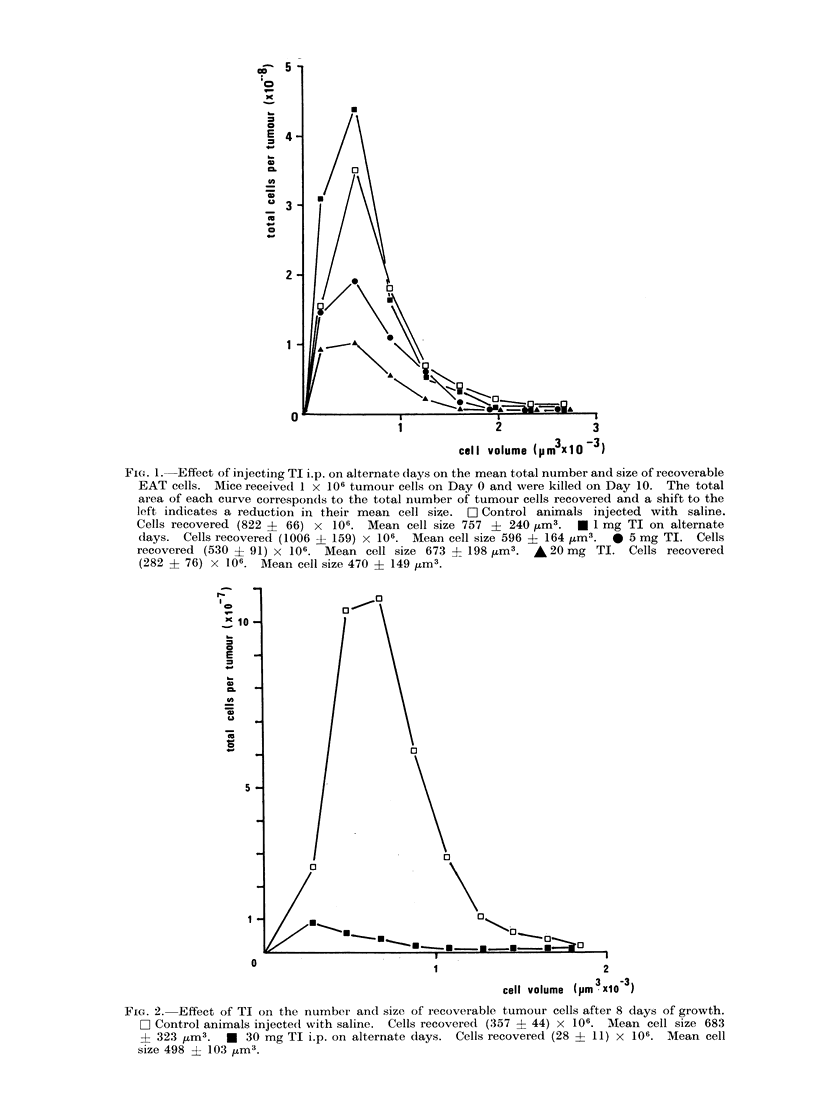
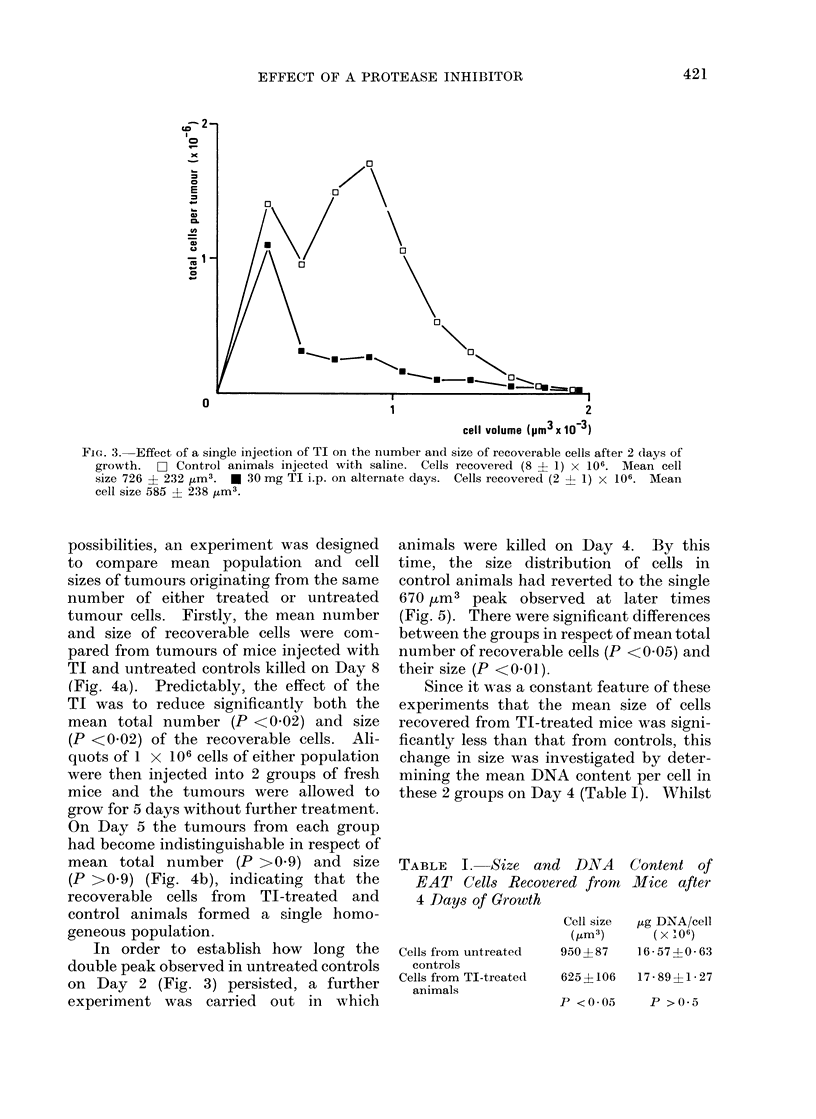
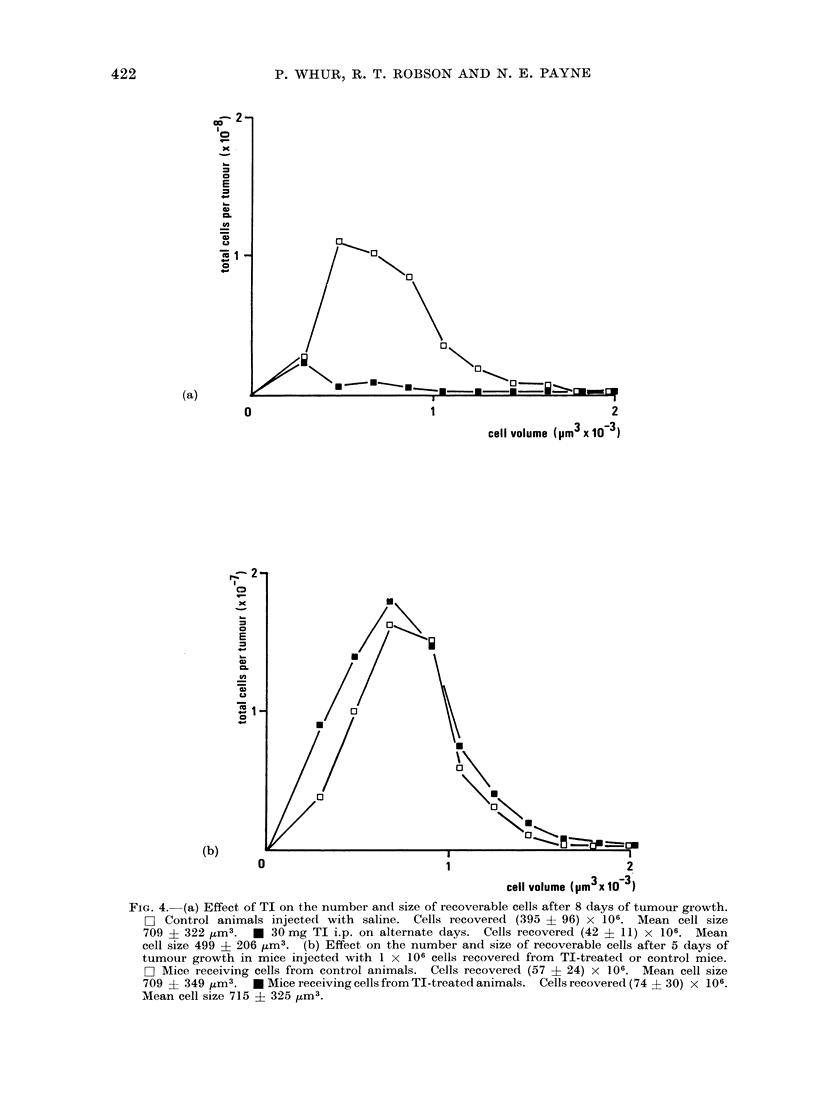
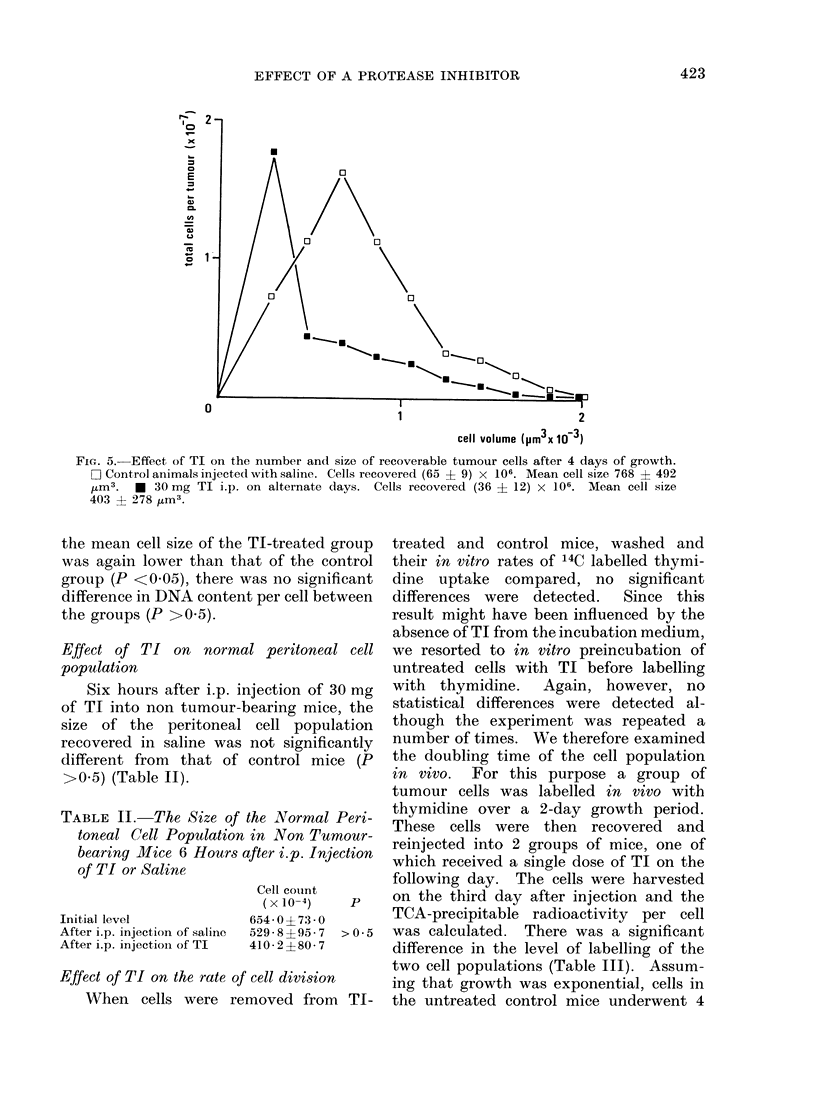




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassleer R., Desaive C. Contribution to the study of the Ehrlich-ascites tumor cells. A cytological and cytochemical analysis of the effects of sarcolysine. Eur J Cancer. 1971 Oct;7(5):441–450. doi: 10.1016/0014-2964(71)90042-9. [DOI] [PubMed] [Google Scholar]
- Burger M. M. A difference in the architecture of the surface membrane of normal and virally transformed cells. Proc Natl Acad Sci U S A. 1969 Mar;62(3):994–1001. doi: 10.1073/pnas.62.3.994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burger M. M. Proteolytic enzymes initiating cell division and escape from contact inhibition of growth. Nature. 1970 Jul 11;227(5254):170–171. doi: 10.1038/227170a0. [DOI] [PubMed] [Google Scholar]
- Dombernowsky P., Bichel P., Hartmann N. R. Cytokinetic analysis of the JB-1 ascites tumour at different stages of growth. Cell Tissue Kinet. 1973 Jul;6(4):347–357. doi: 10.1111/j.1365-2184.1973.tb01623.x. [DOI] [PubMed] [Google Scholar]
- Goetz I. E., Weinstein C., Roberts E. Effects of protease inhibitors on growth of hamster tumor cells in culture. Cancer Res. 1972 Nov;32(11):2469–2474. [PubMed] [Google Scholar]
- Inbar M., Sachs L. Interaction of the carbohydrate-binding protein concanavalin A with normal and transformed cells. Proc Natl Acad Sci U S A. 1969 Aug;63(4):1418–1425. doi: 10.1073/pnas.63.4.1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolson G. L. Topography of membrane concanavalin A sites modified by proteolysis. Nat New Biol. 1972 Oct 18;239(94):193–197. doi: 10.1038/newbio239193a0. [DOI] [PubMed] [Google Scholar]
- Ozanne B., Sambrook J. Binding of radioactively labelled concanavalin A and wheat germ agglutinin to normal and virus-transformed cells. Nat New Biol. 1971 Aug 4;232(31):156–160. doi: 10.1038/newbio232156a0. [DOI] [PubMed] [Google Scholar]
- Payne N. E., Whur P., Robson R. T. Agglutination of normal and malignant cells by concanavalin A in relation to cell surface structure. Br J Cancer. 1973 Jul;28(1):86–86. doi: 10.1038/bjc.1973.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickaver A. H., Ratcliffe N. A., Williams A. E., Smith H. Cytotoxic effects of peritoneal neutrophils on a syngeneic rat tumour. Nat New Biol. 1972 Feb 9;235(58):186–187. doi: 10.1038/newbio235186a0. [DOI] [PubMed] [Google Scholar]
- Schnebli H. P., Burger M. M. Selective inhibition of growth of transformed cells by protease inhibitors. Proc Natl Acad Sci U S A. 1972 Dec;69(12):3825–3827. doi: 10.1073/pnas.69.12.3825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whur P., Weatherhead B. Rates of incorporation of ( 3 H)leucine into protein of the pars intermedia of the pituitary in the amphibian Xenopus laevis after change of background colour. J Endocrinol. 1971 Nov;51(3):521–532. doi: 10.1677/joe.0.0510521. [DOI] [PubMed] [Google Scholar]
- Winzler R. J. Carbohydrates in cell surfaces. Int Rev Cytol. 1970;29:77–125. doi: 10.1016/s0074-7696(08)60033-9. [DOI] [PubMed] [Google Scholar]




