Abstract
Inactivation of p53-dependent apoptosis promotes oncogenic transformation, tumor development, and resistance to many cytotoxic anticancer agents. p53 can transcriptionally activate bax, a bcl-2 family member that promotes apoptosis. To determine whether bax is required for p53-dependent apoptosis, the effects of bax deficiency were examined in primary fibroblasts expressing the E1A oncogene, a setting where apoptosis is dependent on endogenous p53. We demonstrate that bax can function as an effector of p53 in chemotherapy-induced apoptosis and contributes to a p53 pathway to suppress oncogenic transformation. Furthermore, we show that additional p53 effectors participate in these processes. These p53-controlled factors act synergistically with Bax to promote a full apoptotic response, and their action is suppressed by the Bcl-2 and E1B 19K oncoproteins. These studies demonstrate that Bax is a determinant of p53-dependent chemosensitivity and illustrate how p53 can promote apoptosis by coordinating the activities of multiple effectors.
p53 mutations are associated with a poor prognosis and drug resistance in human cancer (1–5). One biological property of p53 that can explain these findings is its ability to promote apoptosis in response to anticancer drugs (6). For example, oncogenically transformed murine fibroblasts expressing p53 are extremely susceptible to the induction of apoptosis by many cytotoxic anticancer agents; however, identically derived cells from p53-deficient mice remain resistant to drug-induced apoptosis (7, 8). Similarly, p53 mutations are associated with resistance to drug-induced apoptosis in certain human tumor lines (9), and reintroduction of wild-type p53 into p53 mutant tumors can restore apoptosis and chemosensitivity (10).
Despite its clinical potential, the mechanism whereby p53 promotes apoptosis is poorly understood. p53 is a sequence-specific binding protein that can regulate transcription; however, the extent to which this activity contributes to apoptosis remains controversial (11). One transcriptional target of p53 that may be important for apoptosis is bax. Bax is a cell death agonist with homology to the antiapoptotic Bcl-2 protein (12). Forced overexpression of p53 increases bax expression in several cell types, and this increase correlates with the induction of apoptosis (13–15). Sequence-specific binding sites for p53 have been identified in the bax promoter, and these elements confer p53 responsiveness to heterologous promoters (16). In addition, the Bcl-2 and E1B 19K proteins, which can inhibit apoptosis induced by enforced p53 expression, can physically associate with Bax (12, 15), raising the possibility that these oncoproteins interfere with p53-dependent apoptosis by antagonizing Bax function.
All of the studies mentioned above have relied on forced overexpression of p53 or Bax to induce apoptosis—circumstances that may not faithfully reproduce their normal activities. Primary mouse embryo fibroblasts (MEFs) undergo p53-dependent cell cycle arrest, but remain viable after treatment with cytotoxic agents. In MEFs expressing the E1A oncogene, however, these agents induce apoptosis in a manner dependent on endogenous p53 (7). In this study, E1A was stably introduced into whole populations of primary MEFs by retroviral-mediated gene transfer, permitting their analysis without significant expansion in culture. This approach allows strict control of genetic background, thereby avoiding complications arising from unknown mutations that accumulate in immortal or tumor-derived lines. Conclusions as to the importance of bax in the p53 apoptotic pathway can be made by comparing both normal and E1A-expressing MEFs derived from wild-type, bax null (bax−/−), p53 null (p53−/−) and double mutant (bax−/−p53−/−) mice.
MATERIALS AND METHODS
Cells and Cell Culture.
Embryonic fibroblasts derived from p53 and bax knockout mice (17, 18) were prepared from day 13.5 embryos. The following crosses were used to produce the appropriate genotypes: bax+/− × bax+/−, p53+/− × p53+/−, and p53−/−bax+/− × p53−/−bax−/−. Gene transfer was performed at passage three.
Retroviral Vectors and Infections.
Retroviral vectors were as follows: LPC, control vector expressing puromycin phosphotransferase (puro); LPC-12S, a 12S E1A cDNA in LPC; LPC-HAbax, an epitope tagged bax cDNA in LPC, LPC-bcl-2, a murine bcl-2 cDNA in LPC, LPC-19K, an adenovirus E1B 19K cDNA in LPC, WZLHygro, control vector expressing hygromycin phosphotransferase (hygro); WZL-12S, a 12S E1A cDNA in WZLHygro. A detailed description of the infection protocols will be published elsewhere (A. Lin, M.E.M., and S.W.L., unpublished results). Retroviruses were produced using the BOSC23 packaging line (19). We typically infected between 60–90% of cells as judged using a control virus expressing β-galactosidase (not shown). Cell populations were selected in either 2 μg/ml puromycin for 2–3 days (LPC vectors) or 100 μg/ml hygromycin B for 4–5 days (WZLHygro vectors) to eliminate uninfected cells. Drug cytotoxicity studies were performed immediately thereafter. When two genes were introduced into MEFs, cells were infected sequentially, selecting for each resistance marker after its introduction.
Transformation Assay.
MEFs were plated at 3.5 × 105 cells per 6-cm plate and transfected the next day by calcium phosphate coprecipitation. The amounts of DNA used in experiments 1 and 2 was 1.0 μg of p1AHygro (E1A), 2.0 μg of pT24neo (T24 H-ras) and 8.0 μg of pBluescript, or 10 μg of pBluescript (vector only). In Exp. 3 and 4 1× corresponded to 0.5 μg of p1AHygro and 1.0 μg of pT24neo.
Cell Viability and Apoptosis.
Cells were plated in 12 well plates (105 cells per well) 24 h before drug addition. Adherent and nonadherent cells were pooled 24 h after drug addition and analyzed for viability by trypan blue exclusion; at least 200 cells were scored for each point. In all experiments, the wild-type and bax−/− MEFs were from littermate embryos. Apoptotic cell death was confirmed by staining with acridine orange or fluorescein isothiocyanate (FITC)-annexin V. Acridine orange was added to cells at 2.5 μg/ml for 15 min and viewed using the FITC filter on an inverted fluorescent microscope (Zeiss). For annexin staining, cells were treated with 0.1 μg/ml adriamycin for 24 h, after which adherent and nonadherent cells were pooled. Staining with FITC-annexin V and propidium iodide were performed according to the manufacturer’s instructions (BioWhitaker).
Gene and Protein Expression.
Northern and Western blots were performed according to standard procedures. RNA was prepared using RNAzol B (Cinna/Biotecx Laboratories, Friendswood, TX), and 10 μg was loaded per lane. Proteins were extracted in RIPA buffer (50 mM NaCl/50 mM Tris/1% Nonidet P-40/0.5% sodium deoxycholate/0.1% SDS) and loaded at 10 μg total protein per lane. Western blots were probed using a rabbit antipeptide antibody to the N terminus of murine bax (N20, Santa Cruz Biotechnology), and proteins were visualized using ECL (Amersham). For immunofluorescence studies, N20 was used as a primary antibody, followed by a FITC-conjugated goat anti-rabbit secondary antibody (Calbiochem). The expression of all retrovirally transduced genes was assessed by Western blot and confirmed to be equal between compared populations (not shown).
RESULTS
bax Acts in a p53 Apoptotic Pathway.
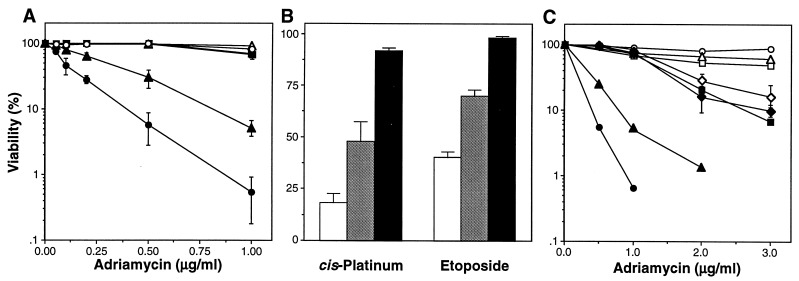
Wild-type, bax−/−, and p53−/− MEFs of various genotypes were infected with a high-titer ectopic retrovirus coexpressing adenovirus-5 E1A cDNA and a selectable marker to eliminate uninfected cells. After selection, these E1A-expressing MEFs (E1A-MEFs) were treated with adriamycin, etoposide, and cis-platinum at doses that induce p53-dependent apoptosis (7). Concordant with previous results, wild-type E1A-MEFs were extremely sensitive to apoptosis, whereas the p53−/− E1A-MEFs—and the control MEFs of each genotype—remained viable (Fig. 1A and B). With all drugs tested, disruption of bax compromised apoptosis in E1A-MEFs (Fig. 1 A and B; Fig. 2). Similar results were observed after treatment with γ-radiation and reduced serum (data not shown). Hence, bax plays a role in promoting cell death under circumstances where apoptosis proceeds through the p53 pathway. Other p53-regulated factors also must participate in this pathway, because bax−/− E1A-MEFs were not as resistant to apoptosis as those lacking p53 (Fig. 1 A and B; Fig. 2).
Figure 1.
bax acts in a p53 pathway for drug-induced apoptosis. (A) Retroviruses expressing an E1A 12S cDNA and puro (LPC-12S; closed symbols) or a puro alone (LPC; open symbols) were used to infect MEFs derived from wild-type (circles), bax−/− (triangles), and p53−/− (squares) mice. Pure populations of E1A-expressing cells or controls were plated in multiwell plates and treated with the chemotherapeutic drug adriamycin. Cell viability was assessed 24 h later by trypan blue exclusion. The wild-type and bax−/− MEFs were from littermate embryos. Each experiment used two separate MEF preparations for each genotype, and the data were pooled. Each point represents the mean ± SD from at least three separate experiments (n ≥ 6 for each genotype). (B) Wild type (white), bax−/− (gray), and p53−/− (black) MEFs expressing E1A were treated with etoposide (2 μg/ml) or pulsed for 1 h with cis-platinum (100 μM), and viability was assessed after 24 h. (C) The normal and E1A-expressing cell populations described in A, together with bax+/−p53−/− (closed diamond) and bax−/−p53−/− (open diamond) E1A-MEFs, were treated with high doses of adriamycin to induce p53-independent apoptosis. Viability was assessed 24 h later. Each experiment used two separate MEF preparations for each genotype; the p53+/−bax−/− and p53−/−bax−/− E1A-MEFs were derived from littermate embryos.
Figure 2.
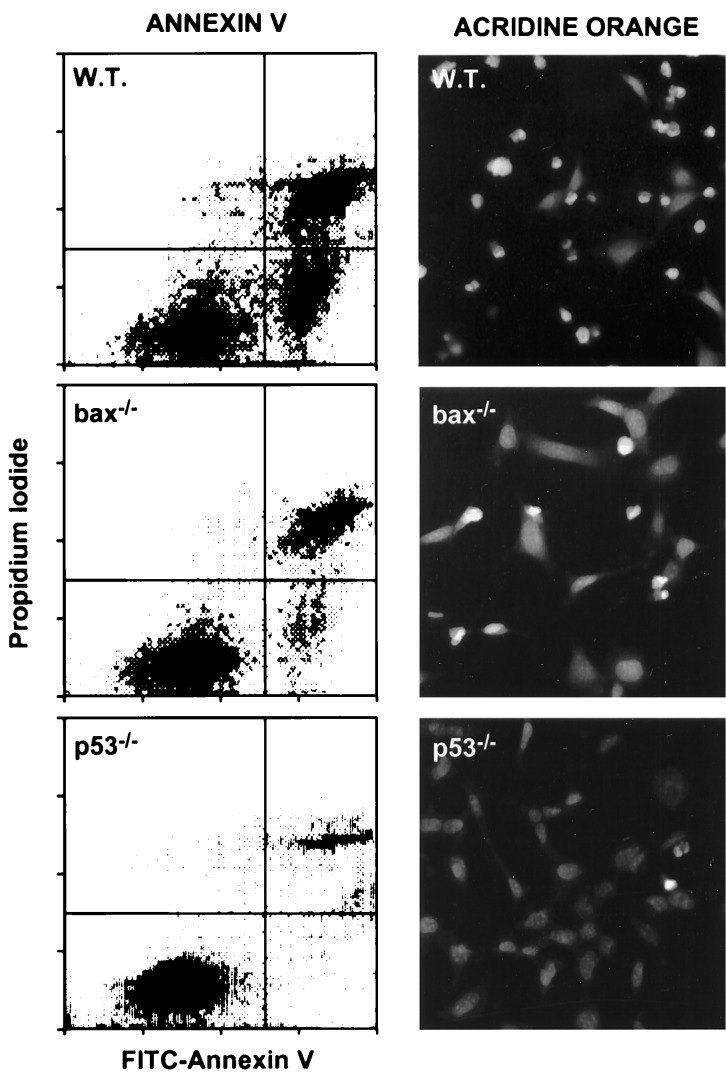
Apoptosis in bax-deficient E1A-MEFs. E1A-MEFs of the indicated genotype were treated with 0.1 μg/ml adriamycin for 24 h and analyzed for apoptosis by costaining with FITC-annexin V and propidium iodide, or by staining with acridine orange. Annexin V binds phosphotidylserine. Apoptotic changes in membrane biochemistry lead to increased concentration of phosphotidylserine on the outer plasma membrane, where it becomes accessible to annexin V (20). Propidium iodide fluorescently stains late apoptotic cells that have lost membrane integrity. Shown are representative dot plots from two-color flow cytometry: lower left quadrant, viable; lower right quadrant, early apoptotic; upper right, late apoptotic. Acridine orange staining allows visualization of the chromatin condensation characteristic of apoptotic cells.
p53-independent forms of apoptosis can be induced in this system by increasing the doses of chemotherapeutic drugs (7). To test whether bax action is limited to the p53 pathway, we compared the response of p53−/− single mutants to bax−/−p53−/− double mutants after treatment with high concentrations of adriamycin. Our data show only a slight increase in resistance of p53−/−bax−/− E1A-MEFs compared with either p53−/−bax+/− or p53−/− E1A-MEFs (Fig. 1C). Furthermore, the role of bax in apoptosis was specific to cells expressing E1A; thus, bax-deficiency did not enhance viability in the control MEFs undergoing adriamycin-induced apoptosis (Fig. 1C; data not shown). Thus in this assay bax functions primarily in the p53 dependent pathway.
bax Expression Is Controlled by p53.
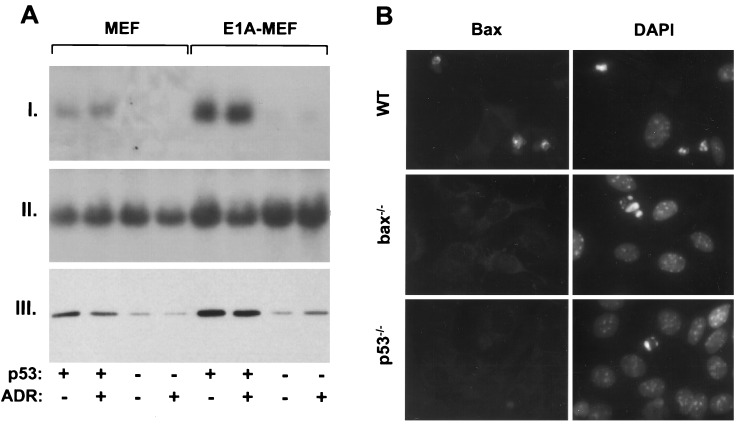
Overexpression of p53 can transcriptionally activate bax in selected settings, and two p53 response elements exist in the bax promoter (16). In MEFs, endogenous p53 also enhanced bax expression: wild-type MEFs averaged a 7-fold increase in bax mRNA and 6-fold increase in Bax protein compared with p53−/− MEFs (Fig. 3A). Previous studies demonstrate that p53 protein accumulates in MEFs following introduction of E1A, but does not further increase after an apoptotic stimulus (21, 22). Similarly, bax mRNA and protein increased 2.5-fold in a p53-dependent manner upon introduction of E1A, but no further increase was detected after adriamycin treatment (Fig. 3A). However, strong Bax immunofluorescent staining was observed in apoptotic cells derived from wild-type E1A-MEFs treated with adriamycin, but not in bax−/− and p53−/− E1A-MEFs (Fig. 3B). Perhaps Bax is further increased in the small percentage of p53-expressing cells actively undergoing apoptosis; alternatively, this increased intensity may reflect concentration of Bax in dying cells. Nevertheless, the failure to detect Bax expression in apoptotic cells lacking p53 provides supportive evidence that the Bax response resides in the p53 pathway. Furthermore, the fact that endogenous p53 regulates Bax expression argues that Bax is an effector of p53.
Figure 3.
Endogenous p53 regulates bax expression in MEFs. (A) bax mRNA and protein expression in wild-type and p53−/− MEFs infected with either the E1A-expressing or control virus. Cell populations were treated with 0.1 μg/ml adriamycin for 9 h (ADR) or left untreated. (I) Northern blot using a murine bax cDNA probe (12), or (II) a probe corresponding to the 18S ribosomal RNA. (III) Western blot using an antipeptide rabbit polyclonal primary antibody directed against Bax. (B) Wild-type (WT), bax−/−, and p53−/− E1A-MEFs treated with 0.2 mg/ml adriamycin for 24 h. (Left) Bax expression was measured by immunofluorescence using the Bax antibody described in A. (Right) 4′, 6-diamidono-2-phenylindole (DAPI) staining of the same field reveals the condensed chromatin characteristic of apoptotic cells. The strong immunofluorescent signal observed in wild-type cells was abolished when the Bax antibody was preincubated with the synthetic peptide to which it was generated (not shown).
Bax Cooperates with Other p53-Controlled Factors.
To address whether Bax is sufficient to induce apoptosis in the absence of p53, we ectopically expressed Bax in wild-type, bax−/−, and p53−/− MEFs and their E1A-MEF derivatives. Bax efficiently induced apoptosis in wild-type and bax−/− E1A-MEFs, but was ineffective in the parental MEFs and p53−/− E1A-MEFs (Fig. 4A). Exogenous Bax levels were equivalent to, or greater than, those of endogenous Bax in wild-type E1A-MEFs (not shown). Bax was not completely ineffectual in p53−/− E1A-MEFs, as these cells displayed a partially restored apoptotic response to chemotherapeutic agents (not shown). These results demonstrate that an oncogene (in this case, E1A) can dramatically enhance apoptosis in response to Bax. Furthermore, the fact that Bax did not substitute for p53 in apoptosis implies that Bax synergizes with other activities controlled by p53 to elicit a full apoptotic response.
Figure 4.
Ectopic expression of Bax, Bcl-2, and E1B 19K. (A) Primary cell populations expressing E1A and/or Bax were generated by sequential infection with recombinant retroviral vectors expressing E1A (WZL-12S) then either bax (LPC-HAbax; gray bars) or a empty vector (LPC; black bars). Infected populations were plated in multiwell plates and analyzed for viability 24 h later without drug treatment. The MEF genotypes and E1A status are indicated below the graph. Shown is a representative graph of three separate experiments that all produced the same results. (B) Wild-type (circles), bax−/− (triangles), or p53−/− (squares) MEFs were first infected with retroviruses expressing E1A (WZL-12S) and then either Bcl-2 (LPC-bcl2; open symbols) or a control vector (LPC; closed symbols). After selection, cells were treated with adriamycin, and viability was determined after 24 h. (C) E1A-expressing populations were superinfected with a retrovirus expressing E1B 19K (LPC-19K) and analyzed as in B. The symbols are as described in B, except that open symbols represent cells coexpressing E1B 19K with E1A. Each point in B and C represents the average ± SD of data obtained from at least three separate experiments.
Bcl-2 and E1B 19K Suppress p53-Dependent Apoptosis in bax-Deficient Cells.
The Bcl-2 and E1B 19K oncoproteins suppress apoptosis downstream of p53 (23, 24) and can physically associate with Bax (12, 15). To determine whether the action of Bcl-2 and E1B 19K are compromised in the absence of bax, wild-type, bax−/−, and p53−/− MEFs were sequentially infected with recombinant retroviruses expressing E1A and then either Bcl-2 (Fig. 4B) or E1B 19K (Fig. 4C). Both oncoproteins completely suppressed drug-induced apoptosis in bax−/− E1A-MEFs, at doses where cell death was p53-dependent. Consequently, Bcl-2 and E1B 19K can interfere with p53 effector functions independently of their ability to antagonize Bax.
bax-Deficiency Promotes Oncogenic Transformation.
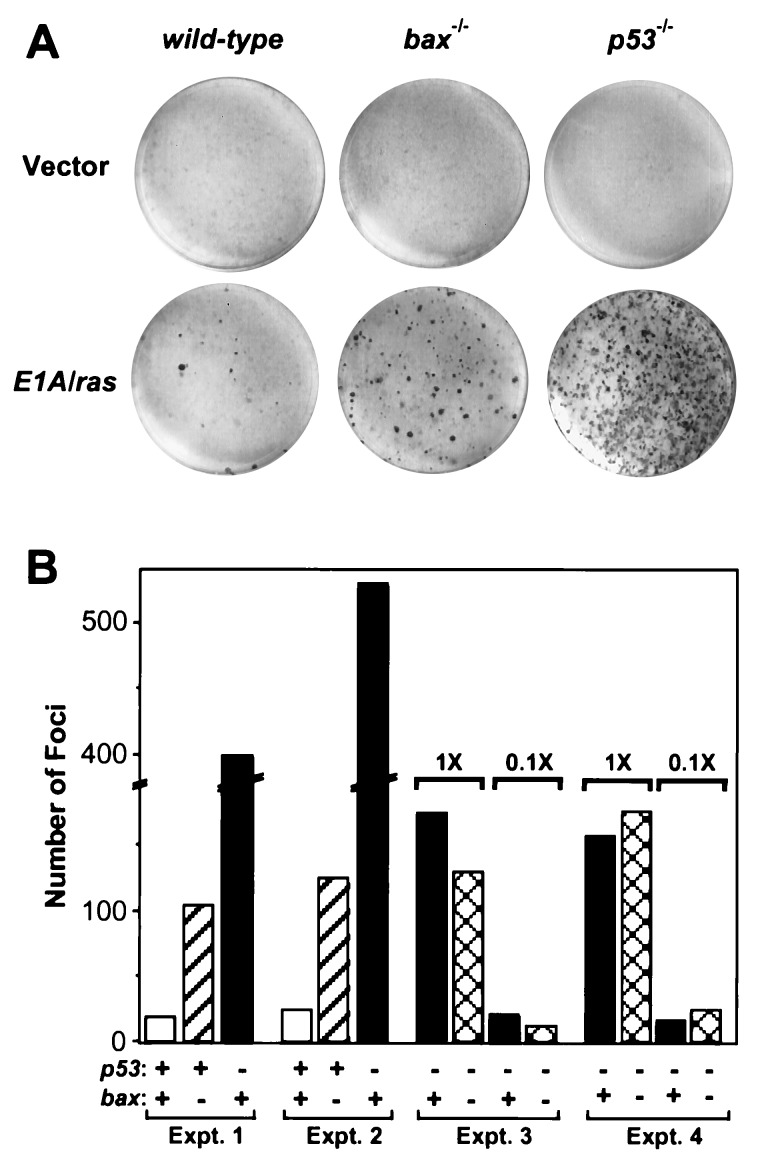
Inactivation of p53-dependent apoptosis can promote oncogenic transformation as well as resistance to cytotoxic agents (7, 22). To determine whether bax also contributes to p53’s ability to suppress transformation, we performed a focus assay using primary fibroblasts of different genotypes (wild-type, bax−/−, p53−/−, and bax−/−p53−/−). As predicted, p53 suppressed focus formation induced by E1A and oncogenic ras. Thus, p53−/− MEFs produced 20-fold more foci than wild-type MEFs (Fig. 5A and B). Bax also suppressed focus formation, because bax−/− MEFs produced a 5-fold increase in foci compared with wild-type (Fig. 5 A and B). bax−/−p53−/− double mutant cells produced the same number of foci as cells lacking only p53, consistent with p53 and bax acting in the same genetic pathway (Fig. 5B). Thus, bax not only appears to act downstream of p53 to promote chemotherapy-induced apoptosis, but also functions in a p53 pathway to suppress oncogenic transformation.
Figure 5.
bax acts in a p53 pathway to suppress oncogenic transformation. Wild-type (p53+/+bax+/+), bax null (p53+/+bax−/−), p53 null (p53−/−bax+/+) and double mutant (p53−/−bax−/−) MEFs were transfected with plasmids expressing adenovirus-5 E1A and an activated ras oncogene. Two weeks after transfection, plates were fixed and stained with Giemsa to visualize foci. (A) Photographs of representative plates. (B) Graph quantifying the number of foci from several experiments. The genotypes are indicated below the graph and are as follows: for p53, (+) indicates p53+/+ and (−) indicates p53−/−; for bax, (+) indicates bax+/+ and (−) indicates bax−/−. In Exp. 1 and 2, the number of foci on p53−/−bax+/+ were such that the numbers must be considered estimates. Exp. 3 and 4, two different amounts of input E1A/ras DNA were used to generate numbers of foci that could be accurately counted. The amount of E1A/ras DNA in the 0.1× lanes was 1/10 those marked 1×.
DISCUSSION
This study has taken a genetic approach to investigate how p53 promotes apoptosis. Earlier studies have examined p53’s apoptotic activity by overexpressing p53 in immortal or tumor-derived lines. While informative, these studies suffer from two inherent limitations: (i) Overexpressed p53 may not induce apoptosis through the same mechanism as endogenous p53; (ii) Immortal cell lines may have acquired unknown mutations that affect p53’s apoptotic activity. In contrast, the system used here exploits the observation that E1A reveals the p53-dependent apoptotic program in primary fibroblasts and combines both dominant (high-titer retroviruses) and recessive (null mutant MEFs) activities to study p53 and its genetic interactions. By emphasizing endogenous activities and maintaining strict control of genetic background, our results should provide a picture of how p53 normally operates. Using this approach, we examined the requirement for bax in two clinically relevant endpoints of p53 action: chemosensitivity and suppression of oncogenic transformation.
These studies argue that bax functions as an effector of p53. bax also proved to be a determinant of chemosensitivity, indicating that mutations that reduce Bax activity, whether in p53 or bax itself, should contribute to drug resistance in human tumors. Indeed, the fact that bax-deficiency promotes both drug resistance and oncogenic transformation underscores the relationship between factors that influence tumor progression and chemosensitivity. Although the action of Bax in p53-dependent apoptosis may be controlled by tissue-specific factors (14, 17) the fact that an oncogene revealed a crucial role for bax in this pathway gives it relevance for both tumorigenesis and cancer therapy. Consistent with this view, inactivation of bax promotes the progression of brain tumors in oncogene-expressing transgenic mice (T. Van Dyke, personal communication). Furthermore, attenuated Bax expression is associated with a poor response to chemotherapy in human breast cancer (25).
This study illustrates the complexity of the p53 pathway, because bax deficiency did not confer the same resistance to apoptosis, nor susceptibility to oncogenic transformation, as loss of p53. Furthermore, enforced Bax expression did not substitute for p53 in apoptosis, but rather acted synergistically with additional factors controlled by p53. The identity of these p53-regulated factors remains to be determined, but the Bcl-2 and E1B 19K oncoproteins can suppress their action. Just as p53’s apoptotic activity does not proceed through a simple linear pathway, MEFs lacking the p21Cip1/Waf1 cyclin-dependent kinase inhibitor are only partially defective in p53-mediated cell cycle arrest (26, 27). Thus, it appears that p53 produces its complex biology by coordinating the activities of multiple effectors. This complexity will need to be recognized when designing novel therapies to exploit this pathway.
Acknowledgments
We thank P. Burfeind for flow cytometry, G. Brown for animals, G. Hannon for advice, and J. Morgenstern for the generous gift of retroviral vectors. We also thank Y. Lazebnik, M. Hengartner, and B. Stillman for critically reading the manuscript. T.M.F.C. is supported by a fellowship from the President’s Council at Cold Spring Harbor Laboratory. This work was supported by National Institutes of Health Grants CA49712 (S.J.K.) and CA13106 (S.W.L.).
ABBREVIATIONS
- MEFs
primary mouse embryo fibroblasts
- bax−/−
bax null
- p53−/−
p53 null
- bax−/−p53−/−
bax and p53 double mutant
- E1A-MEFs
E1A-expressing MEFs
- FITC
fluorescein isothiocyanate
References
- 1.Elrouby S, Thomas A, Costin D, Rosenberg C R, Potmesil M, Silber R, Newcomb E W. Blood. 1993;82:3452–3459. [PubMed] [Google Scholar]
- 2.Wattel E, Preudhomme C, Hecquet B, Vanrumbeke M, Quesnel B, Dervite I, Morel P, Fenaux P. Blood. 1994;84:3148–3157. [PubMed] [Google Scholar]
- 3.Bergh J, Norberg T, Sjogren S, Lindgren A, Holmberg L. Nat Med. 1995;1:1029–1034. doi: 10.1038/nm1095-1029. [DOI] [PubMed] [Google Scholar]
- 4.Aas T, Borresen A, Geisler S, Smith-Sorrensen B, Johnsen H, Varhaug J E, Akslen L A, Lonning P E. Nat Med. 1996;2:811–814. doi: 10.1038/nm0796-811. [DOI] [PubMed] [Google Scholar]
- 5.Righetti S C, Della Torre G, Pilotti S, Menard S, Ottone F, Colnaghi M I, Pierotti M A, Lavarino C, Cornarotti M, Oriana S, Bohm S, Bresciani G L, Spatti G, Zunino F. Cancer Res. 1996;56:689–693. [PubMed] [Google Scholar]
- 6.Lowe S W. Curr Opin Oncol. 1995;7:547–553. doi: 10.1097/00001622-199511000-00013. [DOI] [PubMed] [Google Scholar]
- 7.Lowe S W, Ruley H E, Jacks T, Housman D E. Cell. 1993;74:954–967. doi: 10.1016/0092-8674(93)90719-7. [DOI] [PubMed] [Google Scholar]
- 8.Lowe S W, Bodis S, McClatchey A, Remington L, Ruley H E, Fisher D, Housman D E, Jacks T. Science. 1994;266:807–810. doi: 10.1126/science.7973635. [DOI] [PubMed] [Google Scholar]
- 9.Fan S J, Eldeiry W S, Bae I, Freeman J, Jondle D, Bhatia K, Fornace A J, Magrath I, Kohn K W, O’Connor P M. Cancer Res. 1994;54:5824–5830. [PubMed] [Google Scholar]
- 10.Fujiwara T, Grimm E A, Mukhopadhyay T, Zhang W W, Owenschaub L B, Roth J A. Cancer Res. 1994;54:2287–2291. [PubMed] [Google Scholar]
- 11.Ko L J, Prives C. Genes Dev. 1996;10:1054–1072. doi: 10.1101/gad.10.9.1054. [DOI] [PubMed] [Google Scholar]
- 12.Oltvai Z N, Milliman C L, Korsmeyer S J. Cell. 1993;74:609–619. doi: 10.1016/0092-8674(93)90509-o. [DOI] [PubMed] [Google Scholar]
- 13.Selvakumaran M, Lin H K, Miyashita T, Wang H G, Krajewski S, Reed J C, Hoffman B, Liebermann D. Oncogene. 1994;9:1791–1798. [PubMed] [Google Scholar]
- 14.Miyashita T, Krajewski S, Krajewska M, Wang H G, Lin H K, Liebermann D A, Hoffman B, Reed J C. Oncogene. 1994;9:1799–1805. [PubMed] [Google Scholar]
- 15.Han J, Sabbatini P, Perez D, Rao L, Modha D, White E. Genes Dev. 1996;10:461–477. doi: 10.1101/gad.10.4.461. [DOI] [PubMed] [Google Scholar]
- 16.Miyashita T, Reed J C. Cell. 1995;80:293–299. doi: 10.1016/0092-8674(95)90412-3. [DOI] [PubMed] [Google Scholar]
- 17.Knudson C M, Tung K S, Tourtellotte W G, Brown G A, Korsmeyer S J. Science. 1995;270:96–99. doi: 10.1126/science.270.5233.96. [DOI] [PubMed] [Google Scholar]
- 18.Jacks T, Remington L, Williams B O, Schmitt E M, Halachmi S, Bronson R T, Weinberg R A. Curr Biol. 1994;4:1–7. doi: 10.1016/s0960-9822(00)00002-6. [DOI] [PubMed] [Google Scholar]
- 19.Pear W S, Nolan G P, Scott M L, Baltimore D. Proc Natl Acad Sci USA. 1993;90:8392–8396. doi: 10.1073/pnas.90.18.8392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Andree H A, Reutelingsperger C P, Hauptmann R, Hemker H C, Hermens W T, Willems G M. J Biol Chem. 1990;265:4923–4928. [PubMed] [Google Scholar]
- 21.Lowe S W, Ruley H E. Genes Dev. 1993;7:535–545. doi: 10.1101/gad.7.4.535. [DOI] [PubMed] [Google Scholar]
- 22.Lowe S W, Jacks T, Housman D E, Ruley H E. Proc Natl Acad Sci USA. 1994;91:2026–2030. doi: 10.1073/pnas.91.6.2026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chiou S K, Rao L, White E. Mol Cell Biol. 1994;14:2556–2563. doi: 10.1128/mcb.14.4.2556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Debbas M, White E. Genes Dev. 1993;7:546–554. doi: 10.1101/gad.7.4.546. [DOI] [PubMed] [Google Scholar]
- 25.Krajewski S, Blomqvist C, Franssila K, Krajewska M, Wasenius V M, Niskanen E, Nordling S, Reed J C. Cancer Res. 1995;55:4471–4478. [PubMed] [Google Scholar]
- 26.Deng C, Zhang P, Harper J W, Elledge S J, Leder P. Cell. 1995;82:675–684. doi: 10.1016/0092-8674(95)90039-x. [DOI] [PubMed] [Google Scholar]
- 27.Brugarolas J, Chandrasekaran C, Gordon J I, Beach D, Jacks T, Hannon G J. Nature (London) 1995;377:552–557. doi: 10.1038/377552a0. [DOI] [PubMed] [Google Scholar]