Abstract
A malignant invasive fibrosarcoma in hamsters and a malignant mammary carcinoma in mice were each challenged with the broad spectrum proteinase inhibitor aprotinin (Trasylol). In both tumour systems, significant anti-tumour effects of aprotinin were observed. Variations in the site and dosage of aprotinin application were made in an attempt to improve the chemotherapeutic response.
Full text
PDF


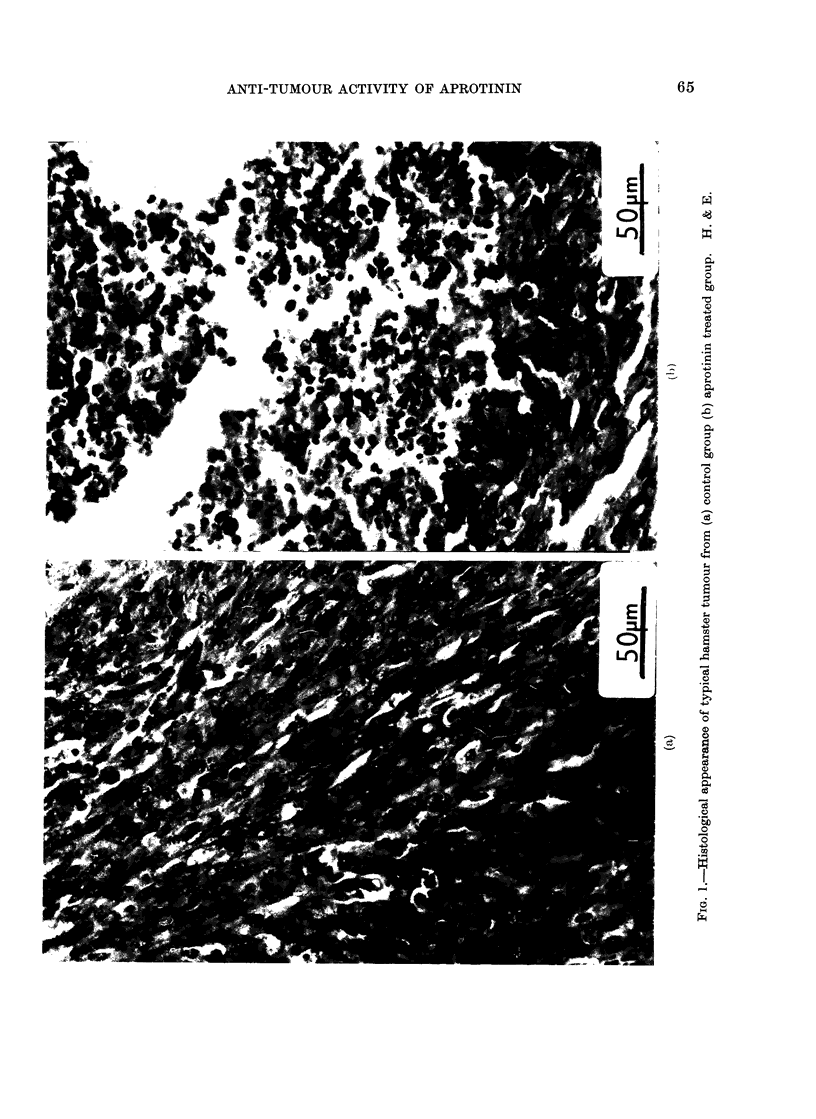
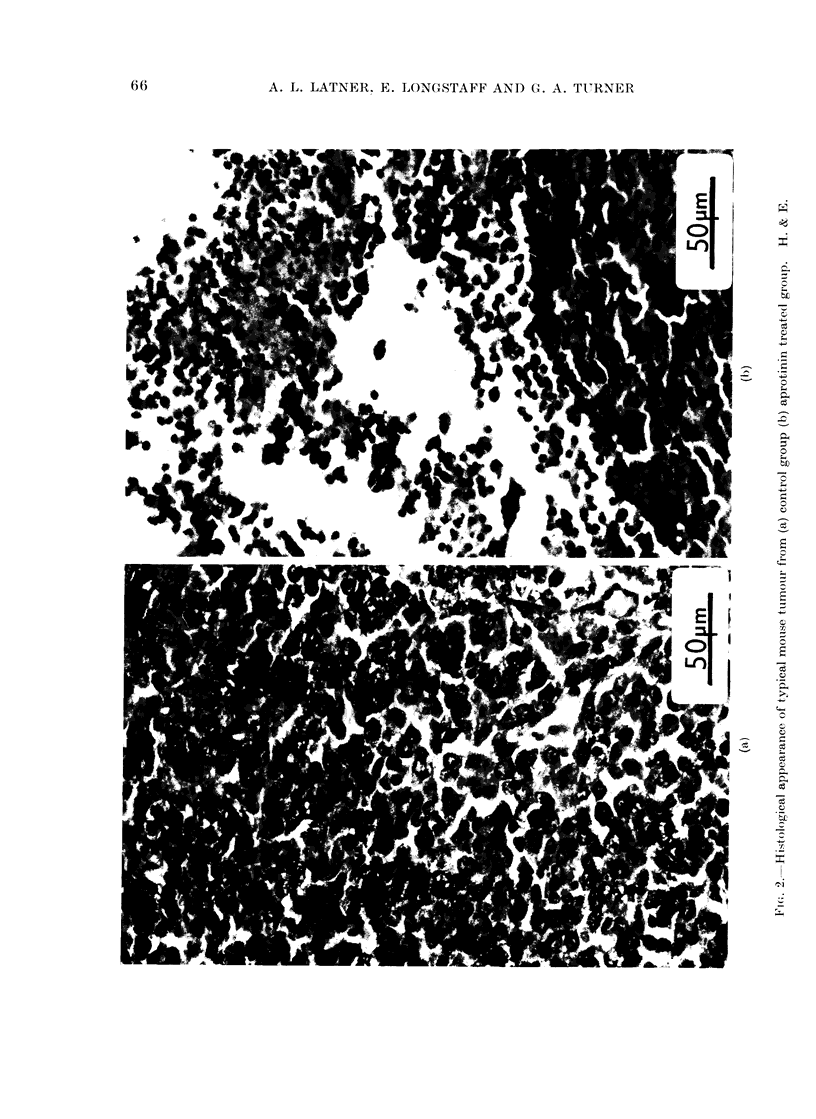

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burger M. M. Proteolytic enzymes initiating cell division and escape from contact inhibition of growth. Nature. 1970 Jul 11;227(5254):170–171. doi: 10.1038/227170a0. [DOI] [PubMed] [Google Scholar]
- Dresden M. H., Heilman S. A., Schmidt J. D. Collagenolytic enzymes in human neoplasms. Cancer Res. 1972 May;32(5):993–996. [PubMed] [Google Scholar]
- Goetz I. E., Weinstein C., Roberts E. Effects of protease inhibitors on growth of hamster tumor cells in culture. Cancer Res. 1972 Nov;32(11):2469–2474. [PubMed] [Google Scholar]
- HOLMBERG B. On the in vitro release of cytoplasmic enzymes from ascites tumor cells as compared with strain L cells. Cancer Res. 1961 Nov;21:1386–1393. [PubMed] [Google Scholar]
- Hozumi M., Ogawa M., Sugimura T., Takeuchi T., Umezawa H. Inhibition of tumorigenesis in mouse skin by leupeptin, a protease inhibitor from Actinomycetes. Cancer Res. 1972 Aug;32(8):1725–1728. [PubMed] [Google Scholar]
- Latner A. L., Longstaff E., Pradhan K. Inhibition of malignant cell invasion in vitro by a proteinase inhibitor. Br J Cancer. 1973 Jun;27(6):460–464. doi: 10.1038/bjc.1973.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latner A. L., Longstaff E., Turner G. A. Enhanced malignant behaviour of cells treated with crude rat liver histone. Br J Cancer. 1973 Mar;27(3):218–229. doi: 10.1038/bjc.1973.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sefton B. M., Rubin H. Release from density dependent growth inhibition by proteolytic enzymes. Nature. 1970 Aug 22;227(5260):843–845. doi: 10.1038/227843a0. [DOI] [PubMed] [Google Scholar]
- Shamberger R. J., Rudolph G. Increase of lysosomal enzymes in skin cancers. Nature. 1967 Feb 11;213(5076):617–618. doi: 10.1038/213617a0. [DOI] [PubMed] [Google Scholar]
- Taylor A. C., Levy B. M., Simpson J. W. Collagenolytic activity of sarcoma tissues in culture. Nature. 1970 Oct 24;228(5269):366–367. doi: 10.1038/228366a0. [DOI] [PubMed] [Google Scholar]
- Troll W., Klassen A., Janoff A. Tumorigenesis in mouse skin: inhibition by synthetic inhibitors of proteases. Science. 1970 Sep 18;169(3951):1211–1213. doi: 10.1126/science.169.3951.1211. [DOI] [PubMed] [Google Scholar]
- Whur P., Robson R. T., Payne N. E. Effect of a protease inhibitor on the adhesion of Ehrlich ascites cells to host cells in vivo. Br J Cancer. 1973 Nov;28(5):417–428. doi: 10.1038/bjc.1973.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamanishi Y., Dabbous M. K., Hashimoto K. Effect of collagenolytic activity in basal cell epithelioma of the skin on reconstituted collagen and physical properties and kinetics of the crude enzyme. Cancer Res. 1972 Nov;32(11):2551–2560. [PubMed] [Google Scholar]