Abstract
Macrophage-derived chemokine (MDC) is a potent chemoattractant for antigen-specific T lymphocytes. We hypothesized that Adenovirus- (Ad-) transduced dendritic cells (DCs) overexpressing MDC would enhance the T cell–mediated humoral immune response specific for antigens presented by the DC. We challenged two strains of mice with lethal Pseudomonas aeruginosa infection 3 weeks after immunization with AdMDC-modified DCs pulsed with heat-killed P. aeruginosa. MDC-expressing DCs specifically attracted T lymphocytes and preserved typical DC surface phenotypes without growth factors in vitro. Mice immunized with AdMDC/Pseudomonas/DCs developed high levels of serum anti-Pseudomonas Ab’s and were protected from a lethal respiratory challenge with Pseudomonas. The in vivo protective immunity required CD4+ T cells, B cells, and IL-4, but not CD8+ T cells and IL-12. AdMDC/DCs pulsed with Pseudomonas yielded significant but not absolute cross-protection against different strains of P. aeruginosa. Pseudomonas-pulsed AdMDC/DCs protected mice from Pseudomonas but not Escherichia coli and vice versa; this microbe-specific protection correlated with microbe-specific induction of CD4+ T cell proliferation and IL-4 secretion. Based on these observations, AdMDC-modified DCs pulsed with a killed bacteria may be a useful approach to vaccination against infectious disorders.
Introduction
Dendritic cells (DCs) are antigen-presenting cells specialized for interaction with T lymphocytes to initiate and modulate immune responses (1, 2). DCs capture antigens in peripheral tissues and migrate to lymphoid organs, where they present peptide antigens in the context of the surface MHC to antigen-specific T cell antigen receptors (TCR) (1, 2). Extracellular antigens (e.g., bacteria) entering through the endocytic pathway of DCs are processed and usually displayed in the context of MHC class II molecules to CD4+ Th cells. This TCR-mediated antigenic signal, together with cytokines secreted from the DCs and costimulatory molecules on the DC surface, cooperatively activates CD4+ T lymphocytes. The clonal CD4+ T cells then expand and regulate antigen-specific immune responses via two paths, Th1 or Th2 subsets (1, 2). Th1 cells produce IFN-γ, TNF-β, and IL-2, whereas Th2 cells produce IL-4, IL-5, IL-6, IL-10, and IL-13 (3). Together with the help of various cytokines, these different classes of CD4+ Th cells primed by DCs directly interact with, and stimulate, antigen-specific immune effectors such as B cells (1–3).
Macrophage-derived chemokine (MDC; also referred to as stimulated T cell chemotactic protein, STCP-1, or the recently proposed nomenclature, CCL22) is a 8-kDa CC chemokine constitutively produced by DCs, B cells, macrophages, and thymic medullary epithelial cells (4–6). MDC exerts a potent chemoattraction for activated T lymphocytes, monocytes, monocyte-derived DCs, and natural killer (NK) cells (4, 5, 7). The functional receptor for MDC is the CC chemokine receptor 4 (CCR4), which is preferentially expressed on blood T cells, particularly CD4+ T cells of the Th2 phenotype (8–10). Thymus- and activation-regulated chemokine (TARC), a CC chemokine closely related to MDC, is also a specific ligand for CCR4. MDC and TARC are thought to play a dual role in the selective migration of antigen-stimulated T cells, especially Th2 cells, toward antigen-presenting cells during T cell–mediated host responses (11–16). Recently, a murine orthologue, which shows a high amino acid sequence homology with human MDC (64% identity and 83% similarity), has been identified (17, 18). Human and murine MDC share the chemotactic properties across species barriers, and both mouse and human MDC have been described as a functional ligand for murine CCR4 (19).
Based on these considerations, we hypothesized that enhancement of the ability of DCs to attract Th2 phenotype T lymphocyte would augment antigen-specific humoral immunity by increasing the frequency of interactions between DCs and Th2 T cells recognizing specific antigens presented by DCs. To evaluate this concept, we genetically engineered murine bone marrow–derived DCs with an E1– recombinant adenovirus (Ad) vector expressing MDC (AdMDC) and assessed the ability of these MDC-modified DCs to induce immunity against a lethal respiratory challenge with Pseudomonas aeruginosa. The data demonstrate that immunization with AdMDC-modified DCs pulsed with heat-killed P. aeruginosa renders mice resistant to the lethal infection of Pseudomonas with a Th2-dominant humoral immune response in a microbe-specific manner.
Methods
Mice.
Six- to eight-week-old female wild-type, CD4+ T cell–deficient (C57BL/6-CD4tm1Mak), CD8+ T cell–deficient (C57BL/6-CD8atm1Mak), B cell–deficient (C57BL/6-Igh-6tm1Cgn), MHC class I–deficient (C57BL/6-B2mtm1Unc), IL-4–deficient (C57BL/6-Il4tm1Cgn), IL-12–deficient (C57BL/6-Il12btm1Jm) C57BL/6 mice and wild-type BALB/c mice (The Jackson Laboratory, Bar Harbor, Maine, USA) and MHC class II–deficient C57BL/6 mice (C57BL/6-Abbtm1; Taconic Farms, Germantown, New York, USA) were housed under specific pathogen-free conditions until infection.
Ad vectors.
The E1–, E3– Ad vectors based on human Ad5 genome used in this study included AdMDC, expressing the human MDC cDNA under the control of the cytomegalovirus (CMV) early-immediate promoter/enhancer, and the AdNull, an identical vector, but with no transgene (5, 20). Both Ad vectors were prepared as described previously and were free of replication competent Ad (21–23).
DCs.
Bone marrow-derived DCs from C57BL/6 or BALB/c mice were grown in complete RPMI-1640 media (10% FBS and antibiotics) supplemented with recombinant murine GM-CSF (10 ng/ml; R&D Systems , Minneapolis, Minnesota, USA) and recombinant murine IL-4 (2 ng/ml; R&D Systems Inc.) (24, 25). DCs were genetically modified with AdMDC, using AdNull and PBS, pH 7, as controls, at an moi of 100 for 4 hours, washed, and then cultured for 48 hours at 37°C.
DC expression of MDC.
To demonstrate that AdMDC expressed MDC at the mRNA level, total cellular RNA was extracted from the AdMDC-modified (or control-modified) DCs using TRIzol (Life Technologies Inc., Grand Island, New York, USA), and RNA (1 μg) was subjected to reverse transcription using oligo(dT)18 primer (CLONTECH Laboratories Inc., Palo Alto, California, USA) at 42°C in a total volume of 20 μl. The PCR reaction was performed with the following specific primers and the serial dilution (2 μl) of RT reaction mixture using AdvanTaq Plus DNA polymerase (CLONTECH Laboratories Inc.): for exogenous MDC, 5′-TGCCAAGAGTGACGTGTCCA-3′ and 5′-AGAGTAGGATCCTCATTGGCTCAGCTTATT-3′; for endogenous MDC, 5′-GTGGCTCTCGTCCTTCTTGC-3′ and 5′-GGACAGTTTATGGAGTAGCTT-3′; for control GAPDH, 5′-ATGGTGAAGGTCGGTGTGAACGGA-3′ and 5′-TTACTCCTTGGAGGCCATGTAGGC-3′. The amplification profile was 95°C for 1 minute, 30 cycles of 95°C for 30 seconds, and 68°C for 90 seconds, followed by 68°C for 3 minutes.
To demonstrate the function of the MDC protein expressed by the AdMDC-modified DCs, human T lymphocyte CEM cells or murine T lymphocyte EL4 cells (no. CCL-119 and TIB-39, respectively; American Type Culture Collection, Manassas, Virginia, USA) were suspended in 100 μl of RPMI-1640 media containing 1 mg/ml BSA at 107 cells/ml and loaded to the upper wells of 5-μm pore, polycarbonate, transwell chambers in 24-well plates (Corning Costar Inc., Corning, New York, USA). Serially diluted culture supernatant of DCs genetically modified as described above was placed in the lower well in a volume of 600 μl. Where indicated, neutralizing anti-human MDC mAb or isotype-matched mouse IgG control Ab (R&D Systems Inc.) was added to the condition medium in the lower chamber at 10 μg/ml, or recombinant human MDC or recombinant human TARC (R&D Systems Inc.) was added to the cell suspension in the upper chamber at 100 ng/ml. After a 4-hour incubation at 37°C, the number of migrating cells in the lower chamber was determined by FACS analysis. A migration index was calculated as the number of cells migrating in the presence of conditioned media divided by the number of cells migrating in the presence of medium only (26–28).
To assess the in vivo chemoattracting activity of AdMDC-modified DCs for cells expressing CCR4, a receptor for MDC, DCs were labeled with PKH26 (Zynaxis, Cell Science Inc., Malvern, Pennsylvania, USA) after AdMDC or AdNull modification and injected intravenously to C57BL/6 mice. Four days after the injection, spleens were dissected, and frozen sections (5 μm thick) were incubated with rabbit anti-mouse CCR4 Ab diluted at 1:500 (Alexis Corp., San Diego, California, USA). The specimens were then treated with 20 μg/ml anti-rabbit IgG FITC Ab (Vector Laboratories, Burlingame, California, USA), followed by nuclear staining with 1 μg/ml 4′, 6-diamidino-2-phenylindole (DAPI; Dojindo Laboratories, Tokyo, Japan).
Assessment of DCs by flow cytometry.
DCs were incubated with the FITC- and phycoerythrin-conjugated (PE-conjugated) mAbs against the surface molecules CD40 and I-Ab (MHC class II), CD86 (B7-2), and CD80 (B7-1), or appropriate isotype-matched control Ab (PharMingen, San Diego, California, USA). Stained cells were analyzed by a contour plot of 530 nm for FITC and 585 nm for PE, and 1% of false positive events was accepted in the control Ab staining.
Bacterial strains and immunization.
P. aeruginosa strains used in this study (all generously provided by A. Prince, Columbia University, New York, New York, USA) included the well-characterized laboratory strains (referred here as “laboratory strains”) PAO1 (serogroup O2), PAO-NP (Pil– mutant of PAO1), PAK (serogroup O1), and PA103 (serogroup O11). In addition, several other strains (referred here as “clinical strains”) cultured from the sputum of individuals with cystic fibrosis included PA514 (Fla+, Pil+), PA515 (Fla+, Pil+), PA516 (Fla–, Pil+), and PA517 (Fla–, Pil+). DCs were incubated with AdMDC, AdNull, or PBS at an moi of 100 for 4 hours at 37°C in the presence of heat-killed (56°C, 30 minutes) P. aeruginosa or (as a control) Escherichia coli (25922 strain; ATCC) at a ratio of ten bacteria per one DC. DCs were incubated for a further 30 minutes with 200 μg/ml gentamicin sulfate to kill the remaining bacteria, washed extensively with PBS, and injected intravenously at 105 cells per mouse. Where indicated, genetically modified DCs pulsed with heat-killed P. aeruginosa were incubated with 10 μg/ml anti-mouse CD40 Ab (PharMingen) for 2 hours before injection. AdMDC- and AdNull-modified DCs were shown to be capable of equally loading an antigen as naive DCs because all of the DCs equally stimulated IL-4 secretion from ovalbumin-specific (OVA-specific) Th2 cells (provided by M. Ono, Sendai, Tohoku University, Japan) in the presence of 50 μg/ml OVA (Sigma Chemical Co., St. Louis, Missouri, USA) (not shown).
Anti-pseudomonas Ab’s.
Ab’s in sera were assessed by ELISA in microtiter plates (Bio-Rad Laboratories Inc., Hercules, California, USA) coated with 107 CFU heat-killed PAO1 per well using Mouse Typer sub-isotyping kit (Bio-Rad Laboratories Inc.) (29). End-point titers were determined as the inverse of the dilution that gave a fixed absorbance value of 0.1 at OD415. Negative results were given a titer of 20, the starting dilution.
Infection of mice.
Lethal respiratory infection with P. aeruginosa was established using the method of Starke et al. (30). Previous studies from our laboratory demonstrated that PAO1, a well-characterized laboratory strain of P. aeruginosa, enmeshed in agar beads provides a reproducible example of a lethal infection in both C57BL/6 and BALB/c mice (29). To establish a parallel model with a clinical strain of Pseudomonas, a similar model was assessed using PA514, PA515, PA516, and PA517. Based on the observation that PA514-impregnated agar beads gave the most reproducible lethal model, PA514 was used in all subsequent studies as an example of a clinical strain. Briefly, a log-phase bacteria diluted in warm (52°C) tryptic soy agar was added to heavy mineral oil and stirred vigorously, followed by cooling with ice. The bacteria-containing beads were washed extensively with PBS, and the number of viable P. aeruginosa in beads was determined by plating serial dilutions of homogenized bead suspension. Anesthetized mice were placed in a supine position, and 50 μl of 2 × 105 CFU Pseudomonas-impregnated beads was inoculated via the trachea into the lung. All animals were monitored daily for 14 days after the inoculation. Mice that appeared moribund were sacrificed, and this was recorded as the date of death.
In vitro proliferation and cytokine assays.
Splenic CD4+ T cells were collected from mechanically disrupted spleens of immunized mice using anti-CD4 magnetic beads (Miltenyi Biotec, Auburn, California, USA). Proliferation and cytokine release by CD4+ T cells was assessed using 96-well plates with 5 × 104 microbe-pulsed DCs as stimulators and 5 × 105 immunized CD4+ T cells as responders in complete RPMI-1640 media. Microbe-pulsed DCs were prepared by incubation with heat-killed PAO1, PA103, or E. coli (DC/bacteria ratio, 1:10) for 4 hours at 37°C, irradiated for 30 Gy, and washed extensively before use. Cell proliferation was measured daily by an MTS colorimetric assay kit (Promega Corp., Madison, Wisconsin, USA) and calculated as 100 × ([experimental absorbance] – [background absorbance]) / ([absorbance at start of the culture] – [background absorbance]) (%). After 4 days of culture, the supernatant was collected and analyzed for mouse IL-4 content with an ELISA kit (R&D Systems Inc.).
Statistics.
All data are reported as mean plus or minus SE, unless otherwise described. Statistical comparison was made using the two-tailed student’s t test, and a P value of less than 0.05 was accepted as indicating significance. Survival evaluation was carried out using Kaplan-Meier analysis as described previously (29).
Results
Genetic modification of DCs.
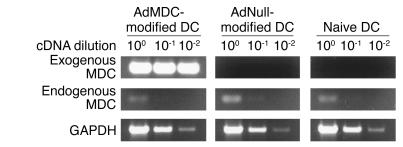
Genetic modification of DCs by AdMDC resulted in high levels of exogenous MDC mRNA expression, but in no detectable change in the low level of endogenous MDC mRNA expression (Figure 1). Serial dilutions of the cDNA prepared from AdMDC-modified, AdNull-modified, or naive DCs were subjected to PCR amplification with primer sets specific for AdMDC-derived MDC mRNA, endogenous MDC mRNA, or ubiquitously expressed GAPDH mRNA. Exogenous MDC mRNA expression was detected at high levels only in AdMDC-modified DCs; this AdMDC-derived transcript was strongly amplified even from cDNA diluted 100-fold. In contrast, similar low levels of endogenous MDC mRNA expression were found in these DCs with or without genetic modification. Comparable levels of expression of the housekeeping gene GAPDH demonstrated the intactness of the RNA samples.
Figure 1.
RT-PCR analysis for exogenous and endogenous MDC mRNA expression in genetically modified DC. Total cellular RNA (1 μg) from DCs transduced with AdMDC, AdNull, or PBS alone (naive control) were reverse transcribed using the oligo(dT)18 primer. Serial 1:10 dilution of RT-generated cDNA were used as a template to amplify exogenous MDC, endogenous MDC, and GAPDH cDNA fragments by PCR. Samples were separated by 1.5% agarose gel electrophoresis and stained with ethidium bromide.
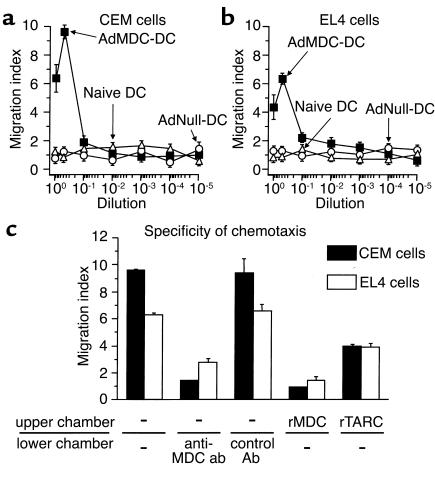
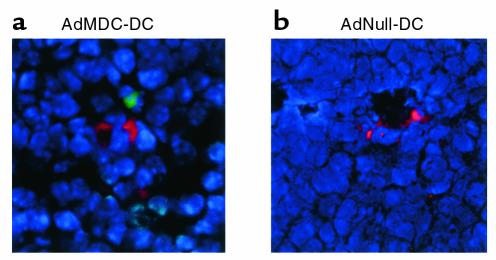
The function of the MDC protein produced by AdMDC vector–modified DCs was confirmed by chemotaxis assays using human and murine T cell lines in vitro and by immunohistochemistry in vivo (Figures 2 and 3). A dose-dependent migratory response of human T lymphocyte CEM was observed only in the culture supernatant of AdMDC-modified DCs, but not in that of AdNull-modified or naive DCs (Figure 2a). Similar results were achieved in the migration assay of murine T lymphocyte EL4, although the peak migration of EL4 cells against the supernatant of AdMDC-modified DCs was less intense (Figure 2b). The relevance of the observed chemotaxis to Ad-mediated MDC expression was demonstrated by the observation that both human and murine T lymphocyte chemotactic activities of the AdMDC-conditioned media were significantly inhibited by an addition of neutralizing anti-human MDC mAb to the media (CEM, P < 0.001; EL4, P < 0.005), but unaffected by that of isotype-matched control Ab (CEM, P > 0.5; EL4, P > 0.5; Figure 2c). Furthermore, when recombinant human MDC or TARC was added in the upper chambers to disturb the gradients, the induced chemotaxis of both human and murine T cells was also suppressed using CEM and EL4 cells (rMDC: CEM, P < 0.001; EL4, P < 0.001; rTARC: CEM, P < 0.005; EL4, P < 0.01; Figure 2c). The in vitro chemoattracting activity was relevant in vivo because cells expressing CCR4, a receptor for MDC, were observed around AdMDC-modified DCs in the spleen of the mouse 4 days after administration of AdMDC-modified and PKH26-labeled DCs (Figure 3a). In contrast, no CCR4-positive cells were observed around AdNull-modified and PKH26-labeled DCs (Figure 3b).
Figure 2.
Chemotaxis of human and murine T lymphocytes to conditioned media of genetically modified DCs. (a) CEM cells. Human T lymphocyte CEM cells placed in the upper chamber of a transwell chamber were assayed for chemotaxis in response to serial dilution of supernatant from DCs transduced with AdMDC, AdNull, or PBS alone (naive control) in the lower chamber. (b) EL4 cells. The study was similar to that in a, but the murine T lymphocyte EL4 cell line was used. (c) Suppression of AdMDC-mediated chemotaxis. CEM and EL4 cells were assayed for chemotaxis in response to 1:2 dilution of supernatant from AdMDC-modified DCs. Where indicated, anti–MDC neutralizing Ab or mouse IgG control Ab was added to the supernatant in the lower chambers at 10 μg/ml at the initiation of assay or cells were placed to the upper chamber in the presence of recombinant (r) MDC or TARC at 100 ng/ml. For all parts, the number of cells migrating to the lower chamber at 37°C for 4 hours was counted by FACS analysis. Migration index was calculated as the number of cells migrating to the conditioned media over the number of cells migrating to control medium. Results represent the mean ± SE (n = 3 per data point).
Figure 3.
Chemoattracting activity of AdMDC-modified DCs for CCR4-expressing cells in vivo. To demonstrate the in vivo function of AdMDC-modified DCs, C57BL/6 mice were intravenously injected with AdMDC- or AdNull-modified DCs. Genetically modified DCs were labeled with PKH26 (red fluorescence) before the injection for in vivo cell tracking. After 4 days, spleens were dissected, and frozen spleen sections were stained using rabbit anti-mouse CCR4 Ab followed by visualization with anti-rabbit IgG Ab labeled with FITC (green fluorescence). Nuclei were stained with DAPI (blue fluorescence).
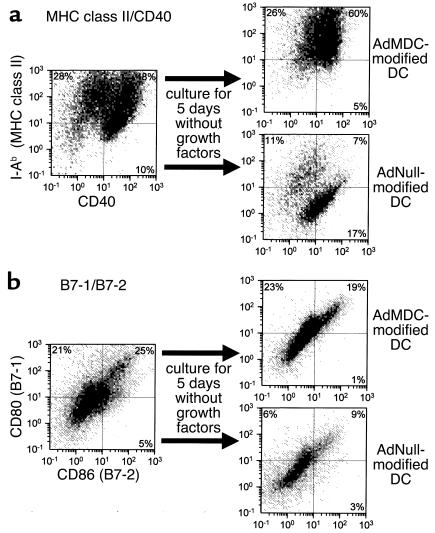
Surface phenotype of AdMDC-modified DCs.
Characteristic DC surface molecules did not change significantly following DC modification with AdMDC (Figure 4). Interestingly, the surface markers MHC class II, CD40, B7-1, and B7-2 in DCs modified with AdMDC remained stable in cultures for 5 days without growth factors, while these surface markers diminished in control DCs in the same conditions. DCs generated with GM-CSF and IL-4 were transduced with AdMDC or AdNull vectors and then cultured for 5 days without GM-CSF and IL-4 to analyze for their expression of I-Ab (MHC class II) and CD40 by two-color FACS analysis (Figure 4a). Compared with DCs before subsequent culture without growth factors, the percentages of positive cells for both I-Ab and CD40 were consistently higher in AdMDC-modified DCs (I-Ab+ 76% vs. AdMDC 86%; CD40+ 58% vs. AdMDC 65%; I-Ab+CD40+ 48% vs. AdMDC 60%). The comparable percentage expression of these surface molecules was strikingly lower in AdNull-modified DCs (I-Ab+, 76% vs. AdNull 18%; CD40+ 58% vs. AdNull 24%; I-Ab+CD40+ 48% vs. AdNull 7%). Similar results were observed with CD80 (B7-1) and CD86 (B7-2) expression (Figure 4b). There was considerable variance in the percentages of positive cells for CD80 and CD86 between AdMDC- and AdNull-modified DCs cultured for 5 days in the absence of growth factors (CD80+ AdMDC 42% vs. AdNull 15%; CD86+ AdMDC 20% vs. AdNull 12%; CD80+CD86+ AdMDC 19% vs. AdNull 9%), although these percentages of AdMDC-transduced DCs were slightly lower than that before the cytokine-depleted culture (CD80+ 46% vs. AdMDC 42%; CD86+ 30% vs. AdMDC 20%; CD80+CD86+ 25% vs. AdMDC 19%).
Figure 4.
Surface phenotype of DCs genetically modified with MDC differentiated under growth factor–withdrawal conditions. DCs generated from bone marrow of C57BL/6 mice with GM-CSF and IL-4 were transduced with AdMDC or AdNull at MOI of 100 for 4 hours. Transduced cells were then washed and cultured in complete RPMI-1640 media without GM-CSF and IL-4 for 5 days. Cells before and after a withdrawal of cytokines were analyzed by two-color flow cytometry for the expression of (a) I-Ab (MHC class II) and CD40 or (b) CD80 (B7-1) and CD86 (B7-2). The percentage of cells in each quadrant is listed.
In vivo protective effects of AdMDC-modified DCs.
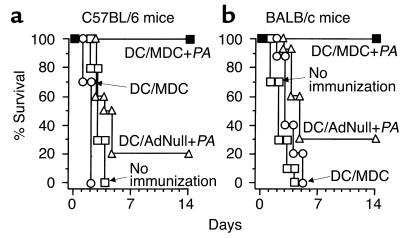
C57BL/6 and BALB/c mice immunized with AdMDC-modified DCs pulsed with P. aeruginosa were protected against lethal Pseudomonas bronchopulmonary infection (Figure 5, a and b). Immunization of C57BL/6 mice with AdMDC-modified DCs pulsed with heat-killed P. aeruginosa provided complete survival against a lethal challenge with Pseudomonas-impregnated (PAO1 strain) beads (P < 0.001, AdMDC-modified DCs pulsed with P. aeruginosa compared with all other groups; Figure 5a). In contrast, only 20% of mice immunized with AdNull-modified DCs pulsed with P. aeruginosa were protected, and immunization with AdMDC-modified DCs alone or no immunization resulted in no survival against the lethal challenge of P. aeruginosa. Similar results were achieved with immunized BALB/c mice (Figure 5b). BALB/c mice undergoing immunization of AdMDC-modified DCs pulsed with heat-killed P. aeruginosa also completely survived the lethal infection of Pseudomonas, whereas immunization with AdNull-modified DCs pulsed with P. aeruginosa, AdMDC-modified DCs alone, or no immunization led to only 30% or no survival, respectively, of the challenged mice (P < 0.005, AdMDC-modified DCs pulsed with P. aeruginosa compared with all other groups; Figure 5b). We obtained similar results using genetically modified DCs that were stimulated by triggering CD40 with the agonist Ab against CD40 before administration: AdMDC- or AdNull-modified DCs pulsed with heat-killed P. aeruginosa provided 80% or 10% protection of immunized C57BL/6 mice, respectively (not shown).
Figure 5.
Immunization with syngenic AdMDC-modified DCs pulsed with P. aeruginosa protects mice against lethal P. aeruginosa respiratory challenge. Mice were immunized intravenously with AdMDC-modified DCs pulsed with heat-killed P. aeruginosa strain PAO1 (DC/MDC + PA), AdNull-modified DCs pulsed with heat-killed P. aeruginosa strain PAO1 (DC/AdNull + PA), or AdMDC-modified DCs alone (DC/MDC) at 105 DCs per mouse. Controls included naive mice without any immunization (no immunization). After 3 weeks, 2 × 105 CFU P. aeruginosa (PAO1) enmeshed in agar beads was administered intratracheally. Survival was recorded as the percentage of surviving animals (n = 10 mice per group). (a) C57BL/6 mice. (b) BALB/c mice.
Anti-pseudomonas Ab responses.
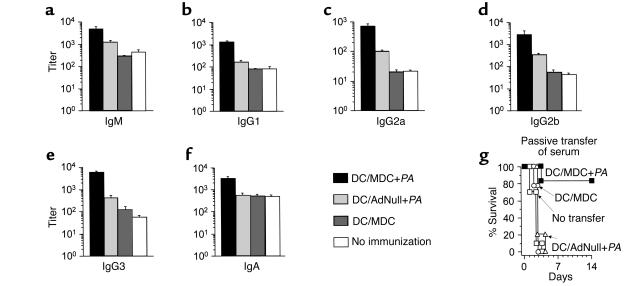
Enhanced serum levels of anti-Pseudomonas Ab’s were observed in AdMDC/DC/Pseudomonas-immunized mice, and its in vivo relevance was determined (Figure 6). In all Ab isotypes examined, immunization with AdMDC-modified DCs pulsed with heat-killed Pseudomonas significantly increased the end-point titers of anti-Pseudomonas Ab on day 14 after immunization compared with all other control groups (IgM, P < 0.0005, Figure 6a; IgG1, P < 0.005, Figure 6b; IgG2a, P < 0.0005, Figure 6c; IgG2b, P < 0.0005, Figure 6d; IgG3, P < 0.01, Figure 6e; IgA, P < 0.01, Figure 6f). Controls included immunization with AdNull-modified DCs pulsed with heat-killed Pseudomonas, AdMDC-modified DCs alone, and no immunization. This observed difference in anti-Pseudomonas Ab levels was sufficient to protect recipient mice from a lethal infection of P. aeruginosa in passive transfer of serum (Figure 6g). Passive transfer of 100 μl serum collected 3 weeks after immunization of AdMDC-modified DCs pulsed with P. aeruginosa resulted in 80% protection from a lethal challenge of P. aeruginosa (P < 0.005, AdMDC-modified DCs pulsed with P. aeruginosa compared with all other groups, Figure 6g). Control serum from mice immunized with AdNull-modified DCs pulsed with P. aeruginosa or AdMDC-modified DCs alone mediated no protection, nor did no transfer of serum.
Figure 6.
In vivo Pseudomonas-specific Ab responses of mice immunized with AdMDC-modified DCs pulsed with Pseudomonas. (a–f) Titer of anti-Pseudomonas Ab in serum. C57BL/6 mice were immunized with heat-killed P. aeruginosa-pulsed (PAO1 strain) and AdMDC-modified (or AdNull-modified) DCs. Two weeks after immunization, each isotype of anti-Pseudomonas (PAO1 strain) Ab was assessed in serum using a standard ELISA protocol. Data are presented as mean ± SE (n = 3 mice per data point) of end-point titers. (a) IgM, (b) IgG1, (c) IgG2a, (d) IgG2b, (e) IgG3, (f) IgA. (g) Passive transfer of serum. Three weeks after immunization, serum was collected and pooled from C57BL/6 mice immunized as in a–f. Each recipient C57BL/6 mouse received 100 μl of heat-inactivated (at 56°C for 30 minutes) serum intravenously. After 6 hours, recipient animals were challenged by intratracheal injection with 2 × 105 CFU P. aeruginosa strain PAO1 enmeshed in agar beads (day 0). Controls included mice without any infusion of serum. Survival was recorded as the percentage of surviving animals (n = 10 mice per group).
Transferable immunity in splenocytes.
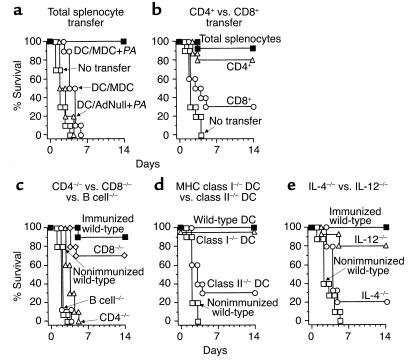
The protective effect provided by the AdMDC/Pseudomonas/DC immunization was transferred to naive mice by CD4+ T cell splenocytes (Figure 7, a and b). To determine whether the AdMDC/Pseudomonas/DC–mediated protection against P. aeruginosa resides in the spleens of immunized mice, mice were adoptively immunized with 5 × 107 splenocytes isolated 2 weeks after preimmunization of AdMDC-modified DCs pulsed with P. aeruginosa and 7 days before an intratracheal instillation of Pseudomonas to the recipient mice (Figure 7a). Spleen cells from mice preimmunized with AdMDC-modified DCs pulsed with Pseudomonas completely protected the recipient mice against a lethal infection (P < 0.0001, compared with all other groups). In contrast, control splenocytes from mice immunized with AdNull-modified DCs pulsed with Pseudomonas or AdMDC-modified DCs alone mediated no protection of recipients, nor did no transfer of splenocytes. To assess the pivotal cell components for this transferable immunity, CD4+ or CD8+ T cells were separated from the spleens of mice immunized with AdMDC-modified DCs pulsed with Pseudomonas, and each cell collection was transferred to naive recipient mice to determine its protective ability against lethal infection of P. aeruginosa (Figure 7b). Adoptive transfer of splenic total splenocytes or CD4+ T cells of AdMDC/Pseudomonas/DC–immunized mice provided 90% or 80% protection, respectively, against Pseudomonas challenge to recipient mice. In contrast, only 30% of mice adoptively receiving CD8+ T cells were protected (P < 0.05, CD4+ cells compared with CD8+ cells; P < 0.001, CD4+ cells compared with naive mice; P > 0.06, CD8+ cells compared with naive mice).
Figure 7.
Immunization with AdMDC-modified DCs pulsed with P. aeruginosa protects mice against lethal P. aeruginosa infection by Th2-dominant immunity. The experiments were carried out in a fashion identical to that in Figures 5 and 6 unless otherwise noted. All respiratory challenges were with P. aeruginosa PAO1 3 weeks after the immunization (c–e) or 1 week after the transfer of splenocytes (a and b). Survival was recorded as the percentage of surviving animals (n = 10 mice per group). (a) Adoptive transfer of spleen cells. Splenocytes from C57BL/6 mice immunized as described in Figure 5 were collected 2 weeks after immunization and transferred intravenously to nonimmunized recipients. (b) CD4+ or CD8+ splenocyte transfer. Two weeks after the immunization, splenocytes were collected from C57BL/6 mice immunized with AdMDC-modified DCs pulsed with P. aeruginosa. After separation into CD4+ or CD8+ cells using magnetic beads, each cell fraction (5 × 106 CD4+ or CD8+ cells) or 5 × 107 total splenocytes were transferred intravenously to nonimmunized recipients. (c) Role of CD4+ T cells, CD8+ T cells, and B cells. CD4+ T cell–deficient, CD8+ T cell–deficient, B cell–deficient, or wild-type C57BL/6 mice were immunized with AdMDC-modified DCs pulsed with Pseudomonas. (d) MHC class II presentation of DCs. Wild-type C57BL/6 mice were immunized with AdMDC-modified DCs pulsed with Pseudomonas using DCs prepared from MHC class I–deficient, class II–deficient, or wild-type C57BL/6 mice. (e) Th2-dominant response of immunization. IL-4-deficient, IL-12-deficient, or wild-type C57BL/6 mice were immunized with AdMDC-modified DCs pulsed with Pseudomonas.
Th2-dominant humoral immunity.
Using relevant knockout mice, we observed that the AdMDC/Pseudo-monas/DC immunization required CD4+ T lymphocytes, B lymphocytes, MHC class II antigen presentation of administrated DCs, and the ability to produce IL-4 (i.e., Th2 T cells), but not IL-12 (i.e., Th1 T cells; Figure 7, c, d, and e). In this context, wild-type, CD8+ T cell–deficient (CD8–/–), CD4+ T cell–deficient (CD4–/–), and B cell–deficient (B cell–/–) C57BL/6 mice were immunized with AdMDC-modified DCs pulsed with P. aeruginosa, and after 3 weeks, challenged with intratracheal instillation of Pseudomonas-impregnated beads (Figure 7c). Survival of CD4–/– and B cell–/– mice was not improved by the AdMDC/Pseudomonas/DC immunization compared with that of nonimmunized wild-type mice, whereas immunized CD8–/– mice were significantly protected from Pseudomonas challenge, comparable to that of immunized wild-type mice (P > 0.9 or P > 0.3, CD4–/– or B–/– mice compared with nonimmunized wild-type mice, respectively; P < 0.0005, CD8–/– mice compared with CD4–/–, B–/–, and nonimmunized wild-type mice).
To assess the role of MHC antigen presentation by DCs in the AdMDC/Pseudomonas/DC immunization strategy, DCs were prepared from wild-type, MHC class I–deficient (class I–/–), or MHC class II–deficient (class II–/–) C57BL/6 mice for AdMDC modification and Pseudomonas pulse and used to immunize wild-type C57BL/6 mice with these MHC-deficient DCs 3 weeks before Pseudomonas challenge (Figure 7d). When modified with AdMDC and pulsed with Pseudomonas, class II–/– DCs provided only partial protection against Pseudomonas challenge (30%), which was not significantly different from the result with nonimmunized wild-type mice, but immunization with class I–/– DCs was completely protective as wild-type DCs (P > 0.7, class II–/– DCs compared with nonimmunized wild-type mice; P < 0.005, class I–/– DCs compared with class II–/– DCs and nonimmunized wild-type mice). The specificity of Th1 and Th2 responses involved in the protective immunity of the AdMDC/Pseudomonas/DC was assessed using IL-12 and IL-4 knockout mice, respectively. Since IL-12 or IL-4 is a critical cytokine for the differentiation of naive CD4+ T cells to the Th1 or Th2 subset, respectively, the survival of immunized wild-type, IL-12–deficient (IL-12–/–) or IL-4–deficient (IL-4–/–) C57BL/6 mice was assessed after the challenge with Pseudomonas (Figure 7e). In IL-4–/– mice, the AdMDC/Pseudomonas/DC immunization was not significantly different than nonimmunization in the protection of mice from challenge of Pseudomonas (P > 0.1). In contrast, 100% or 80% of immunized wild-type or IL-12–/– mice, respectively, survived until the end of the experiment on day 14 (P < 0.001 or P < 0.05, immunized wild-type or IL-12–/– mice, respectively, compared with both IL-4–/– and nonimmunized wild-type mice).
Microbe-specific immunity.
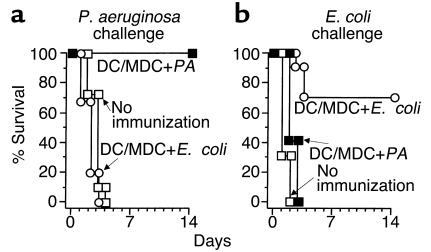
The microbe specificity of protective immunity developed by AdMDC-modified DCs was ascertained using E. coli as a control (Figure 8). Naive C57BL/6 mice and mice immunized with AdMDC-modified DCs pulsed with either P. aeruginosa or E. coli were challenged 3 weeks later with Pseudomonas-impregnated beads. Only Pseudomonas-pulsed DCs conferred complete protection; no protective effect was observed with E. coli–pulsed DCs (P < 0.001, AdMDC/DCs pulsed with P. aeruginosa compared with all other groups; P > 0.7, AdMDC/DCs pulsed with E. coli compared with naive mice; Figure 8a). In marked contrast, when these immunized mice were challenged with a lethal intratracheal infection of 108 CFU E. coli, only E. coli–pulsed DCs were significantly protective with 70% mice surviving. All of naive mice and mice immunized with P. aeruginosa-pulsed DCs died within 2 days after the E. coli instillation (P < 0.005, AdMDC/DCs pulsed with E. coli compared with all other groups; P > 0.6, AdMDC/DCs pulsed with P. aeruginosa compared with naive mice; Figure 8b).
Figure 8.
Immunization with AdMDC/DCs induces protective immunity specific to the pathogen used for pulsing DCs. (a) P. aeruginosa challenge. C57BL/6 mice were immunized with AdMDC-modified DCs pulsed with heat-killed P. aeruginosa or E. coli (25922 strain). After 3 weeks, immunized animals were infected intratracheally with 2 × 105 CFU P. aeruginosa PAO1 enmeshed in agar beads. (b) E. coli challenge. Immunization of mice was identical to that in a, but 108 CFU E. coli (25922 strain) were used for the intratracheal infection. For both parts, controls included mice without any immunization. Survival was recorded as the percentage of surviving animals (n = 10 mice per group).
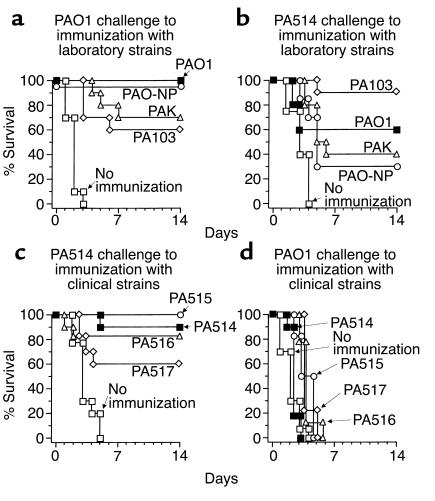
Protection against different strains of Pseudomonas.
Given that the AdMDC/Pseudomonas/DC immunity has been shown to be specific to P. aeruginosa and to provide no protection against another gram-negative bacteria (E. coli), we next determined whether the AdMDC/Pseudomonas/DC immunity was cross-protective for different strains of Pseudomonas (Figure 9). In these studies, C57BL/6 mice were immunized with AdMDC/DCs pulsed with well-characterized laboratory strains of Pseudomonas (PAO1, PAO-NP, PAK, and PA103; Figure 9, a and b) or clinical strains of P. aeruginosa (PA514, PA515, PA516, and PA517; Figure 9, c and d) 3 weeks before challenge with P. aeruginosa laboratory strain PAO1 (Figure 9, a and d) or clinical strain PA514 (Figure 9, b and c). All AdMDC/DC immunization with laboratory-characterized Pseudomonas strains led to significant improvement in survival of the PAO1- and PA514-challenged mice compared with naive mice, although there was some variation in the efficacy of protection among the Pseudomonas strains used in immunization (P < 0.0001 or P < 0.005, naive mice compared with all other groups; Figure 9, a or b, respectively). In comparison, at least 60% of animals following AdMDC/DC immunization pulsed with the clinical strains of P. aeruginosa were significantly protected against PA514 challenge, whereas none of immunization with clinical isolated Pseudomonas strains influenced the survival of mice challenged with the PAO1 strain (P < 0.05 or P > 0.05, naive mice compared with all other groups; Figure 9, c or d, respectively). Thus, while there is significant cross-strain protection, it is not absolute. Interestingly, the use of clinical strains as the immunogens appeared to afford less overall protection against challenge with the laboratory strains than did immunization with the laboratory strains.
Figure 9.
Cross-protective immunity of AdMDC-modified DCs against laboratory or clinical strains of P. aeruginosa. (a and b) Immunization resulting from AdMDC-modified DCs pulsed with laboratory strains of Pseudomonas. C57BL/6 mice were immunized with AdMDC-modified DCs pulsed with heat-killed P. aeruginosa laboratory strains PAO1, PAO-NP, PAK, or PA103 at 105 DCs per mouse. Three weeks after immunization, mice were challenged intratracheally with 2 × 105 CFU P. aeruginosa laboratory PAO1 strain (a) or clinical PA514 strain (b) enmeshed in agar beads. (c and d) Immunization resulting from AdMDC-modified DCs pulsed with clinical strains of Pseudomonas. C57BL/6 mice were immunized with AdMDC-modified DCs pulsed with heat-killed P. aeruginosa clinical strains PA514, PA515, PA516, or PA517 at 105 DCs per mouse. Three weeks after immunization, mice were challenged intratracheally with 2 × 105 CFU P. aeruginosa laboratory PAO1 strain (d) or clinical PA514 strain (c) enmeshed in agar beads. In all parts, controls included naive mice without any immunization. Survival was recorded as the percentage of surviving animals (n = 10 mice per group).
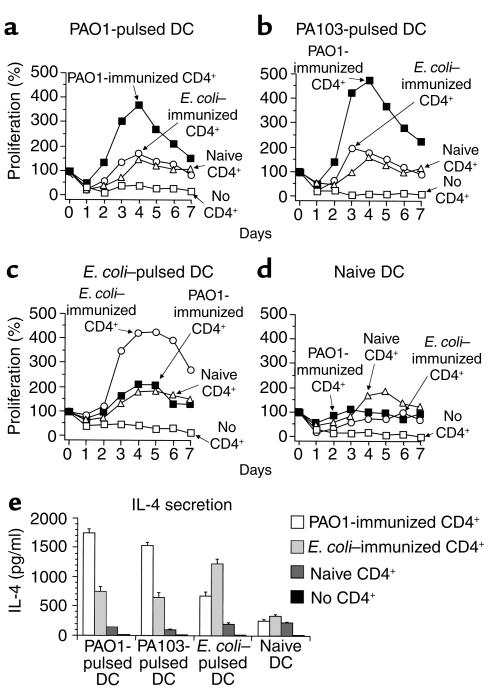
CD4+ T cell responses in vitro.
When cocultured in vitro with DCs pulsed with the specific microbe used in immunization, CD4+ T cells from AdMDC/DC–immunized mice proliferated and secreted the Th2-associated cytokine IL-4 (Figure 10). Splenic CD4+ T cells isolated from naive C57BL/6 mice or mice immunized with AdMDC-modified DCs pulsed with P. aeruginosa strain PAO1 or E. coli were stimulated in vitro with naive, PAO1-pulsed, PA103-pulsed, or E. coli–pulsed DCs. Only CD4+ T cells from AdMDC/DC/PAO1–immunized mice responded to PAO1-pulsed DC. CD4+ T cells from both naive and AdMDC/DC/E. coli–immunized mice did not proliferate in the presence of PAO1-pulsed DCs (Figure 10a). A similar proliferative response of each CD4+ T cell group was achieved using DCs pulsed with another P. aeruginosa strain, PA103 (Figure 10b). In contrast, coculture of CD4+ T cells from the AdMDC/DC/E. coli–immunized mice with E. coli–pulsed DCs resulted in a strong proliferative response, whereas E. coli–pulsed DCs did not stimulate CD4+ T cell proliferation from the naive and AdMDC/DC/PAO1–immunized mice (Figure 10c). Additional control experiments demonstrated that splenic CD4+ T cells from PAO1-immunized mice, naive mice, or E. coli–immunized mice did not proliferate after incubation with naive DCs (Figure 10d). The microbe-specific CD4+ T cell proliferation from immunized mice was reflected in the IL-4 level secreted from CD4+ T cells 4 days after the initiation of the cocultures (Figure 10e). A microbe-specific response was observed, with a significant enhancement of IL-4 production in the cocultures of AdMDC/DC/PAO1–immunized CD4+ T cells with PAO1- and PA103-pulsed DCs, and AdMDC/DC/E. coli–immunized CD4+ T cells with E. coli–pulsed DCs (P < 0.005, P < 0.005, or P < 0.05; PAO1-CD4+ and PAO1-DC, PAO1-CD4+ and PA103-DC, or E. coli-CD4+ and E. coli-DCs compared with all other CD4+ T cell groups in each DC type, respectively). Both Pseudomonas- and E. coli–pulsed DCs moderately induced nonspecific IL-4 release from CD4+ T cells from mice immunized with a different microbe than that used in pulsing the DCs (P < 0.0005, P < 0.001, or P < 0.001, E. coli-CD4+ and PAO1-DC, E. coli-CD4+ and PA103-DC, or PAO1-CD4+ and E. coli-DCs compared with naive CD4+ T cells in each DC type, respectively). Naive DCs did not induce IL-4 release from any CD4+ T cell groups (P > 0.1). This microbe-specific secretion of IL-4 was not observed for the Th1-associated cytokine IFN-γ from CD4+ T cells of immunized mice (not shown). In all proliferation and cytokine assays, no cell proliferation and no detectable cytokine release was observed with naive and microbe-pulsed DCs alone without CD4+ T cells, confirming that only CD4+ T cells were responsible for the observed responses (Figure 10, a–e).
Figure 10.
Responses of CD4+ T cells from immunized mice in a microbe-specific manner. (a–d) Proliferation. C57BL/6 mice were immunized with AdMDC-modified DCs pulsed with P. aeruginosa PAO1 strain or E. coli. Three weeks after immunization, splenic CD4+ T cells were isolated from immunized or nonimmunized mice using the magnetic beads. In 96-well culture plates, 5 × 105 CD4+ T cells were stimulated with 5 × 104 DCs that had been pulsed with heat-killed P. aeruginosa strain PAO1 (a), heat-killed P. aeruginosa strain PA103 (b), heat-killed E. coli (c), or without any microbes (d), and irradiated. In (a–d), controls included DC culture without CD4+ T cells. The number of viable cells was determined using the MTS assay, as described in Methods. The data are presented as the mean percentage increase over baseline of duplicate wells. (e) IL-4 secretion. The culture medium was collected 4 days after the initiation of the coculture described above, and the levels of murine IL-4 were assayed by ELISA. The data are presented as means ± SE (n = 3 per data point).
Discussion
In this study, we hypothesized that DCs modified with a recombinant Ad vector expressing MDC would easily encounter rare T cells bearing specific TCR for a bacterial antigen displayed on the DC surface, resulting in stimulation of specific humoral immune responses that would protect the immunized host from the relevant microbe infection. Several pieces of evidence substantiate that this hypothesis is valid. Immunization with AdMDC-modified DCs pulsed with heat-killed P. aeruginosa led to complete protection against a subsequent lethal infection with P. aeruginosa in two different strains of mice. This beneficial effect was mediated by Pseudomonas-specific humoral immunity, and an involvement of Th2-dominant immune responses in its generation was suggested by observation with in vivo immunization-infection models using knockout mice and in vitro CD4+ T cell proliferation and cytokine analyses.
Ab-mediated humoral immunity is the principal specific immune response to defend a host against pathogenic extracellular bacteria (31). Whereas non-protein antigens such as polysaccharides and glycolipids induce low-affinity Ab responses without a requirement for CD4+ T cell help, competent Ab responses against conventional protein antigens require that B cells contact activated (i.e., antigen-specific) CD4+ T cells to be stimulated by multiple ligand-receptor pairs such as CD40 on B cells and CD40 ligand on activated CD4+ T cells (31). In this context, extracellular microbial protein antigens that are endocytosed, processed, and presented in association with MHC class II molecules by antigen-presenting cells, especially DCs, enable antigen-specific CD4+ T cells to proliferate clonally, develop into memory cells, and differentiate to subsets of Th1 and Th2 effector T cells (1–3). Therefore, the direct interaction of DCs and T cells is of central importance in initiating specific immune responses (1, 2). This is achieved not only by the DCs migrating to the T cell–rich area of lymphoid organs, but also by the DCs themselves secreting T cell–attracting chemokines such as MDC (1, 2, 11, 16). Recently, MDC has been shown in vitro and in vivo to attract subsets of activated T cells, in particular Th2 CD4+ Th cells, but not naive T cells, suggesting that MDC may enhance the encounter of antigen-specific T cells with antigen-bearing DCs and thus retain the interactions for a longer time to complete the T cell–stimulation process. Consistent with this knowledge, the present study has demonstrated that genetic engineering of bacteria-pulsed DCs to express MDC efficiently induced bacterial antigen-relevant humoral immune responses with MHC class II–restricted and type 2–dominant CD4+ T cell help, resulting in protection of immunized mice against a relevant lethal infection. When administrated in vivo for the immunization, DCs that have been modified with AdMDC and pulsed with heat-killed bacteria most likely migrate to lymphoid organs and present processed protein antigens of bacterial origin on MHC class II molecules. In the context of the present study, Ad vector–mediated MDC probably enhanced the rate of encounter of antigen-presenting DCs and antigen-specific CD4+ T cells, thus more efficiently amplifying the relevant antigen-specific immune responses. In addition, the novel observation that AdMDC-transduced DCs helped maintain the surface phenotypes characteristic of DCs during in vitro culture, including MHC class II and CD40 molecule expression, may help explain one of the mechanisms by which this strategy of AdMDC/DCs pulsed with pathogenic microorganisms provides in vivo protective immunity.
Although the MDC receptor CCR4 (preferentially expressed by T lymphocytes, especially the Th2 subset of CD4+ Th cells), is the only receptor identified thus far for MDC, the following evidence from the literature and the present study suggests there are yet unidentified additional receptors for MDC. First, MDC can fully desensitize and abrogate a calcium flux in CCR4-transfected cells by a subsequent stimulation of TARC, another CCR4 ligand (9). In contrast, TARC only partly inhibits calcium mobilization of a subsequent response to MDC. This partial blocking effect of TARC was also demonstrated in the T cell chemotaxis assays of the present study. Second, monocytes and DCs, both of which express little or no CCR4, respond to MDC by chemotaxis (5, 12). In the present study, we also observed that MDC helped maintain the differentiated state of the DCs in culture. Third, NH2 terminally truncated MDC loses chemotactic activity for the T cell line HUT-78 (CCR4+), but retains its capacity to attract monocytes (CCR4–) and anti–HIV-1 activity (32, 33). Finally, murine MDC also binds to a second, unknown receptor on a separate T cell population, which is not recognized by murine TARC (34). The possible diversity of receptors for MDC appears to suggest a novel activity of MDC. In this regard, MDC has been shown to enhance the phagocytic and killing activities of macrophages in vitro and to protect mice in experimental E. coli sepsis by the innate immune responses in vivo (35). In regard to the present study, the mechanism whereby AdMDC-modified DCs exerted a microbe-specific protection in vivo can not be fully explained by nonspecific activation of macrophages, but could depend, in part, on MDC-induced increased bactericidal activity of phagocytic leukocytes.
In regard to clinical relevance of the AdMDC/DC strategy described in the present study, DCs genetically modified to overexpress MDC would require close monitoring, since excessive Th2 development could theoretically be associated with pathogenesis of atopic diseases, such as atopic dermatitis, asthma, and hay fever (36). In this context, high levels of MDC have been detected in the sera of the majority of individuals with atopic dermatitis (37), and overproduction of MDC and TARC has been reported in the atopic dermatitis-like lesions of NC/Nga mice (38). In addition, Gonzalo et al. (19) have shown that, in a murine model of lung allergic inflammation, MDC expression is upregulated during the course of inflammation and that blockage of MDC with neutralizing Ab’s results in prevention of airway hyperreactivity and significant reduction of eosinophils in the lung. In this context, the use of Ad vectors to genetically modify DCs with MDC is likely the optimal vector to use, since Ad vector–mediated expression of transgenes are only of limited duration (39–41).
It is conceivable that in vivo MHC class II- and CD4+ T cell–dependent protective immunity of bacteria-pulsed AdMDC/DCs and in vitro CD4+ T cell proliferation cocultured with bacteria-pulsed DCs were mediated by bacterial superantigens rather than by conventional protein antigens (42). Superantigens directly bind to class II MHC molecules outside the peptide-binding clefts without any intracellular processing and can interact with less restricted structural determinants of the TCR Vβ domain, leading to a massive polyclonal expansion of T cells bearing multiple distinct variable regions of TCRs (42). However, this scenario is highly unlikely for several reasons. First, although in the murine MHC class II-TCR system, class II MHC molecule I-E is required for the presentation of viral and bacterial superantigens, AdMDC/Pseudomonas/DC–mediated protection against the subsequent P. aeruginosa infection did not depend on whether immunized mice were in an I-E negative strain (C57BL/6) or an I-E positive strain (BALB/c) (42, 43). Second, although exotoxin A, the only superantigen identified from P. aeruginosa, is structurally different between the PAO1 and PA103 strains, immunization with PA103-pulsed AdMDC/DCs brought about cross-reactive protection against a lethal challenge of PAO1. Third, Matsumoto et al. (44) have shown that intraperitoneal injection of heat-killed P. aeruginosa does not increase the serum anti–exotoxin A Ab level despite the improved survival rate in a murine gut–derived sepsis model, suggesting that superantigens of heat-killed Pseudomonas have no antigenic activity to induce a marked production of specific Ab’s such as that observed in mice immunized with AdMDC/DCs pulsed with heat-killed Pseudomonas. Finally, Kersten et al. (45) have identified conventional protein antigens that are specific either for P. aeruginosa or E. coli and can be recognized by TCR. Taken together, such microbe-specific antigens derived from MHC-restricted conventional proteins may be responsible for the results observed in the in vivo immunization-challenge experiments in the present study, as well as in vitro CD4+ T cell analyses. However, it is unclear why AdMDC/DCs pulsed with clinical strains of Pseudomonas could not protect against subsequent challenge with PAO1, a well-characterized laboratory strain of P. aeruginosa, despite other well cross-reactivity among laboratory and clinical P. aeruginosa strains. The elucidation of these mechanisms awaits further studies characterizing clinical Pseudomonas strains used in this study more definitely and identifying responsible antigens for immunization with AdMDC/DCs.
Acknowledgments
We thank P. Leopold and M. Ono for help with these studies and N. Mohamed for help in preparing this manuscript. These studies were supported, in part, by P01 HL51746-06A1 and R01 HL 59861-01; the Will Rogers Memorial Fund; the Cystic Fibrosis Foundation; and GenVec Inc. (Gaithersburg, Maryland, USA).
References
- 1.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 2.Banchereau J, et al. Immunobiology of dendritic cells. Annu Rev Immunol. 2000;18:767–811. doi: 10.1146/annurev.immunol.18.1.767. [DOI] [PubMed] [Google Scholar]
- 3.Constant SL, Bottomly K. Induction of Th1 and Th2 CD4+ T cell responses: the alternative approaches. Annu Rev Immunol. 1997;15:297–322. doi: 10.1146/annurev.immunol.15.1.297. [DOI] [PubMed] [Google Scholar]
- 4.Chang M, et al. Molecular cloning and functional characterization of a novel CC chemokine, stimulated T cell chemotactic protein (STCP-1) that specifically acts on activated T lymphocytes. J Biol Chem. 1997;272:25229–25237. doi: 10.1074/jbc.272.40.25229. [DOI] [PubMed] [Google Scholar]
- 5.Godiska R, et al. Human macrophage-derived chemokine (MDC), a novel chemoattractant for monocytes, monocyte-derived dendritic cells, and natural killer cells. J Exp Med. 1997;185:1595–1604. doi: 10.1084/jem.185.9.1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zlotnik A, Yoshie O. Chemokines: a new classification system and their role in immunity. Immunity. 2000;12:121–127. doi: 10.1016/s1074-7613(00)80165-x. [DOI] [PubMed] [Google Scholar]
- 7.Andrew DP, et al. STCP-1 (MDC) CC chemokine acts specifically on chronically activated Th2 lymphocytes and is produced by monocytes on stimulation with Th2 cytokines IL-4 and IL-13. J Immunol. 1998;161:5027–5038. [PubMed] [Google Scholar]
- 8.Bonecchi R, et al. Differential expression of chemokine receptors and chemotactic responsiveness of type 1 T helper cells (Th1s) and Th2s. J Exp Med. 1998;187:129–134. doi: 10.1084/jem.187.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Imai T, et al. Macrophage-derived chemokine is a functional ligand for the CC chemokine receptor 4. J Biol Chem. 1998;273:1764–1768. doi: 10.1074/jbc.273.3.1764. [DOI] [PubMed] [Google Scholar]
- 10.Sallusto F, Lenig D, Mackay CR, Lanzavecchia A. Flexible programs of chemokine receptor expression on human polarized T helper 1 and 2 lymphocytes. J Exp Med. 1998;187:875–883. doi: 10.1084/jem.187.6.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cyster JG. Chemokines and cell migration in secondary lymphoid organs. Science. 1999;286:2098–2102. doi: 10.1126/science.286.5447.2098. [DOI] [PubMed] [Google Scholar]
- 12.Imai T, et al. The T cell-directed CC chemokine TARC is a highly specific biological ligand for CC chemokine receptor 4. J Biol Chem. 1997;272:15036–15042. doi: 10.1074/jbc.272.23.15036. [DOI] [PubMed] [Google Scholar]
- 13.Imai T, et al. Selective recruitment of CCR4-bearing Th2 cells toward antigen-presenting cells by the CC chemokines thymus and activation-regulated chemokine and macrophage-derived chemokine. Int Immunol. 1999;11:81–88. doi: 10.1093/intimm/11.1.81. [DOI] [PubMed] [Google Scholar]
- 14.Rossi D, Zlotnik A. The biology of chemokines and their receptors. Annu Rev Immunol. 2000;18:217–242. doi: 10.1146/annurev.immunol.18.1.217. [DOI] [PubMed] [Google Scholar]
- 15.Sallusto F, Mackay CR, Lanzavecchia A. The role of chemokine receptors in primary, effector, and memory immune responses. Annu Rev Immunol. 2000;18:593–620. doi: 10.1146/annurev.immunol.18.1.593. [DOI] [PubMed] [Google Scholar]
- 16.Tang HL, Cyster JG. Chemokine Up-regulation and activated T cell attraction by maturing dendritic cells. Science. 1999;284:819–822. doi: 10.1126/science.284.5415.819. [DOI] [PubMed] [Google Scholar]
- 17.Chantry D, et al. Macrophage-derived chemokine is localized to thymic medullary epithelial cells and is a chemoattractant for CD3(+), CD4(+), CD8(low) thymocytes. Blood. 1999;94:1890–1898. [PubMed] [Google Scholar]
- 18.Schaniel C, et al. Activated murine B lymphocytes and dendritic cells produce a novel CC chemokine which acts selectively on activated T cells. J Exp Med. 1998;188:451–463. doi: 10.1084/jem.188.3.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gonzalo JA, et al. Mouse monocyte-derived chemokine is involved in airway hyperreactivity and lung inflammation. J Immunol. 1999;163:403–411. [PubMed] [Google Scholar]
- 20.Hersh J, Crystal RG, Bewig B. Modulation of gene expression after replication-deficient, recombinant adenovirus-mediated gene transfer by the product of a second adenovirus vector. Gene Ther. 1995;2:124–131. [PubMed] [Google Scholar]
- 21.Crystal RG, et al. Administration of an adenovirus containing the human CFTR cDNA to the respiratory tract of individuals with cystic fibrosis. Nat Genet. 1994;8:42–51. doi: 10.1038/ng0994-42. [DOI] [PubMed] [Google Scholar]
- 22.Rosenfeld MA, et al. Adenovirus-mediated transfer of a recombinant alpha 1-antitrypsin gene to the lung epithelium in vivo. Science. 1991;252:431–434. doi: 10.1126/science.2017680. [DOI] [PubMed] [Google Scholar]
- 23.Rosenfeld MA, et al. In vivo transfer of the human cystic fibrosis transmembrane conductance regulator gene to the airway epithelium. Cell. 1992;68:143–155. doi: 10.1016/0092-8674(92)90213-v. [DOI] [PubMed] [Google Scholar]
- 24.Kikuchi T, Moore MAS, Crystal RG. Dendritic cells modified to express CD40 ligand elicit therapeutic immunity against preexisting murine tumors. Blood. 2000;96:91–99. [PubMed] [Google Scholar]
- 25.Song W, et al. Dendritic cells genetically modified with an adenovirus vector encoding the cDNA for a model antigen induce protective and therapeutic antitumor immunity. J Exp Med. 1997;186:1247–1256. doi: 10.1084/jem.186.8.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chvatchko Y, et al. A key role for CC chemokine receptor 4 in lipopolysaccharide-induced endotoxic shock. J Exp Med. 2000;191:1755–1764. doi: 10.1084/jem.191.10.1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Inngjerdingen M, Damaj B, Maghazachi AA. Human NK cells express CC chemokine receptors 4 and 8 and respond to thymus and activation-regulated chemokine, macrophage-derived chemokine, and I-309. J Immunol. 2000;164:4048–4054. doi: 10.4049/jimmunol.164.8.4048. [DOI] [PubMed] [Google Scholar]
- 28.Kanazawa N, et al. Fractalkine and macrophage-derived chemokine: T cell-attracting chemokines expressed in T cell area dendritic cells. Eur J Immunol. 1999;29:1925–1932. doi: 10.1002/(SICI)1521-4141(199906)29:06<1925::AID-IMMU1925>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 29.Kikuchi T, Worgall S, Singh R, Moore MAS, Crystal RG. Dendritic cells genetically modified to express CD40 ligand and pulsed with antigen can initiate antigen-specific humoral immunity independent of CD4+ T cells. Nat Med. 2000;10:1154–1159. doi: 10.1038/80498. [DOI] [PubMed] [Google Scholar]
- 30.Starke JR, Edwards MS, Langston C, Baker CJ. A mouse model of chronic pulmonary infection with Pseudomonas aeruginosa and Pseudomonas cepacia. Pediatr Res. 1987;22:698–702. doi: 10.1203/00006450-198712000-00017. [DOI] [PubMed] [Google Scholar]
- 31.Delves PJ, Roitt IM. The immune system. N Engl J Med. 2000;343:108–117. doi: 10.1056/NEJM200007133430207. [DOI] [PubMed] [Google Scholar]
- 32.Struyf S, et al. Enhanced anti-HIV-1 activity and altered chemotactic potency of NH2-terminally processed macrophage-derived chemokine (MDC) imply an additional MDC receptor. J Immunol. 1998;161:2672–2675. [PubMed] [Google Scholar]
- 33.Pal R, et al. Inhibition of HIV-1 infection by the beta-chemokine MDC. Science. 1997;278:695–698. doi: 10.1126/science.278.5338.695. [DOI] [PubMed] [Google Scholar]
- 34.Schaniel C, et al. Three chemokines with potential functions in T lymphocyte-independent and -dependent B lymphocyte stimulation. Eur J Immunol. 1999;29:2934–2947. doi: 10.1002/(SICI)1521-4141(199909)29:09<2934::AID-IMMU2934>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 35.Matsukawa A, et al. Pivotal role of the CC chemokine, macrophage-derived chemokine, in the innate immune response. J Immunol. 2000;164:5362–5368. doi: 10.4049/jimmunol.164.10.5362. [DOI] [PubMed] [Google Scholar]
- 36.Romagnani S. Biology of human TH1 and TH2 cells. J Clin Immunol. 1995;15:121–129. doi: 10.1007/BF01543103. [DOI] [PubMed] [Google Scholar]
- 37.Galli G, et al. Macrophage-derived chemokine production by activated human T cells in vitro and in vivo: preferential association with the production of type 2 cytokines. Eur J Immunol. 2000;30:204–210. doi: 10.1002/1521-4141(200001)30:1<204::AID-IMMU204>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 38.Vestergaard C, et al. Overproduction of Th2-specific chemokines in NC/Nga mice exhibiting atopic dermatitis-like lesions. J Clin Invest. 1999;104:1097–1105. doi: 10.1172/JCI7613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Anderson WF. Human gene therapy. Nature. 1998;392:25–30. doi: 10.1038/32058. [DOI] [PubMed] [Google Scholar]
- 40.Crystal RG. Transfer of genes to humans: early lessons and obstacles to success. Science. 1995;270:404–410. doi: 10.1126/science.270.5235.404. [DOI] [PubMed] [Google Scholar]
- 41.Wilson JM. Adenoviruses as gene-delivery vehicles. N Engl J Med. 1996;334:1185–1187. doi: 10.1056/NEJM199605023341809. [DOI] [PubMed] [Google Scholar]
- 42.Li H, Llera A, Malchiodi EL, Mariuzza RA. The structural basis of T cell activation by superantigens. Annu Rev Immunol. 1999;17:435–466. doi: 10.1146/annurev.immunol.17.1.435. [DOI] [PubMed] [Google Scholar]
- 43.Lin YS, et al. Requirement of I-E molecule for thymocyte apoptosis induced by staphylococcal enterotoxin B in vivo. Cell Immunol. 1999;193:71–79. doi: 10.1006/cimm.1998.1442. [DOI] [PubMed] [Google Scholar]
- 44.Matsumoto T, et al. Effect of immunization with Pseudomonas aeruginosa on gut-derived sepsis in mice. J Med Microbiol. 1998;47:295–301. doi: 10.1099/00222615-47-4-295. [DOI] [PubMed] [Google Scholar]
- 45.Kersten CM, McCluskey RT, Shaw Warren H, Kurnick JT. Responses of human T cells to dominant discrete protein antigens of Escherichia coli and Pseudomonas aeruginosa. Scand J Immunol. 1994;40:151–157. doi: 10.1111/j.1365-3083.1994.tb03444.x. [DOI] [PubMed] [Google Scholar]