Abstract
The mitogen-activated protein kinase (MAPK) pathway regulates growth and survival of many cell types, and its constitutive activation has been implicated in the pathogenesis of a variety of malignancies. In this study we demonstrate that small-molecule MEK inhibitors (PD98059 and PD184352) profoundly impair cell growth and survival of acute myeloid leukemia (AML) cell lines and primary samples with constitutive MAPK activation. These agents abrogate the clonogenicity of leukemic cells but have minimal effects on normal hematopoietic progenitors. MEK blockade also results in sensitization to spontaneous and drug-induced apoptosis. At a molecular level, these effects correlate with modulation of the expression of cyclin-dependent kinase inhibitors (p27Kip1 and p21Waf1/CIP1) and antiapoptotic proteins of the inhibitor of apoptosis proteins (IAP) and Bcl-2 families. Interruption of constitutive MEK/MAPK signaling therefore represents a promising therapeutic strategy in AML.
Introduction
In acute myeloid leukemia (AML), hematopoietic stem cells fail to differentiate normally, resulting in the expansion and accumulation of clonal cells arrested at various stages of development (1). Genetic alterations affecting the function of transcription factors that regulate myeloid maturation play a central role in leukemogenesis (2). In most cases, however, additional genetic alterations are necessary for the leukemic transformation to occur (3, 4). In particular, aberrant activation of the kinase-based signal transduction pathways that normally translate extracellular stimuli into appropriate homeostatic responses can powerfully contribute to leukemogenesis by enabling leukemic cells to grow autonomously and escape programmed cell death (5).
The mitogen-activated protein kinase (MAPK) pathway is a key integration point along the signal transduction cascade that links diverse extracellular stimuli to proliferation, differentiation, and survival (6). Constitutive activation of the MAPK pathway drives the oncogenic transformation of normal fibroblasts and is commonly detected in human cancers resulting from a variety of genetic alterations, from ras mutations to the overexpression of growth factor receptors (6, 7). Inappropriate MAPK activation may also play a role in the leukemic transformation of myeloid cells. Activation of upstream kinases (MAPK kinase, also known as MEK), downregulation of phosphatases, and overexpression of p44/42MAPK (also known as extracellular signal–regulated kinase 1/2, hereafter referred to as MAPK) itself result in constitutive MAPK activation in most AML cases (8, 9). Internal tandem duplications of the Flt3 receptor also result in constitutive MAPK activation and the autonomous growth of myeloid cell lines and primary AML samples (10). Direct evidence for a role of the MAPK pathway in leukemic transformation comes from the findings that a conditionally active form of MEK1 converts hematopoietic cells to cytokine independence (11, 12) and that the efficient induction of a myeloproliferative phenotype by TEL-TRKC fusion variants depends critically on their ability to activate the MAPK pathway (13).
Recent data from our group indicate that constitutive MAPK phosphorylation is an independent predictor of poor response to chemotherapy and shorter survival in AML patients (S.M. Kornblau, unpublished observations). Here we demonstrate that disruption of the MEK/MAPK module has profound functional consequences in AML cell lines and primary AML samples, while it is relatively sparing of normal hematopoietic progenitors, thus providing the preclinical rationale for the development of MAPK-targeted therapeutic strategies in AML.
Methods
Cell lines, primary samples, and cell cultures.
AML cell lines were cultured under standard conditions (14). Bone marrow and peripheral blood samples were obtained from AML patients and healthy donors after informed consent, according to institutional guidelines. Mononuclear cells were purified by Ficoll-Hypaque (Sigma Chemical Co., St. Louis, Missouri, USA) density-gradient centrifugation. CD34+ cells were separated to greater than 95% purity by positive-selection magnetic-bead sorting using a VarioMACS device (Miltenyi Biotec, Auburn, California, USA). Cell lines were harvested in log-phase growth and exposed to the MEK inhibitors PD98059 (2′-amino-3′-methoxyflavone; Calbiochem-Novabiochem Corp., La Jolla, California, USA) (15, 16) and PD184352 (2-[2-chloro-4-iodo-phenylamino]-N-cyclopropylmethoxy-3,4-difluoro-benzamide) (17), or to a matched concentration of vehicle (DMSO). In combination experiments, cells were preincubated with PD98059 for 1 hour prior to the addition of all-trans-retinoic acid (ATRA). In other experiments, cells were seeded at 5 × 105 cells/ml and exposed to 1-β-D-arabinofuranosylcytosine (Ara-C) for 4 hours at 37°C. Ara-C was then removed and cells were plated at 1 × 105 cells/ml in the presence of either DMSO or PD98059. In all experiments, cell viability was evaluated by triplicate counting of trypan blue dye–excluding cells under a light microscope. AML blast and normal bone marrow progenitor colony assays were performed as previously described (18).
Cell cycle and apoptosis analysis.
Cells were fixed in ice-cold ethanol (70% vol/vol) and stained with propidium iodide (PI) solution (25 μg/ml PI, 180 U/ml RNase, 0.1% Triton X-100, and 30 mg/ml polyethylene glycol in 4 mM citrate buffer, pH 7.8; Sigma Chemical Co.). The DNA content was determined using a FACScan flow cytometer (Becton Dickinson Immunocytometry Systems, San Jose, California, USA). Cell cycle distribution was analyzed using ModFit LT software (Verity Software House Inc., Topsham, Maine, USA). Cells with a hypodiploid DNA content (< 2n, > 0.2n) were counted as apoptotic. For annexin V binding studies, cells were washed twice with binding buffer (10 mM HEPES, 140 mM NaCl, and 5 mM CaCl2 at pH 7.4; Sigma Chemical Co.) and incubated with a 1:500 solution of FITC-conjugated annexin V (Roche Diagnostic Corp., Indianapolis, Indiana, USA) for 15 minutes at room temperature. Stained cells were analyzed by flow cytometry, while membrane integrity was simultaneously assessed by PI exclusion.
Western blot analysis and in vitro kinase assay.
For phosphorylation studies, cells were lysed for 10 minutes on ice in a solution containing 10 mM NaF, 1 mM Na3VO4, 150 mM NaCl, 1 mM MgCl2, 1 mM CaCl2, 0.1% NaN3, 10 mM iodoacetamide, 3 mM PMSF, and 1% Triton X-100, supplemented with a protease inhibitor cocktail (Roche Diagnostic Corp.). Equal amounts of proteins were subjected to SDS-PAGE, transferred to Hybond-P membranes (Amersham Pharmacia Biotech, Little Chalfont, England), and immunoblotted with an mAb specific for T202/Y204 doubly-phosphorylated p44/42MAPK (Cell Signaling Technology Inc., Beverly, Massachusetts, USA). Blots were then stripped and reprobed with a polyclonal Ab recognizing p42MAPK (Santa Cruz Biotechnology Inc., Santa Cruz, California, USA). Survivin immunoblotting was performed as previously described (14). For proteins related to cell cycle and apoptosis, the following primary Ab’s were used: anti–phospho-p27Kip1 (T187) rabbit polyclonal Ab (Zymed Laboratories Inc., San Francisco, California, USA), anti-p27Kip1 mAb, anti–Mcl-1 mAb (both from PharMingen, San Diego, California, USA), anti–Waf-1 mAb, anti–Bcl-XL mAb (both from Oncogene Research Products, Cambridge, Massachusetts, USA), and anti–Bcl-2 mAb (DAKO Corp., Carpinteria, California, USA). MAPK enzymatic activity was assessed by immunoprecipitation and in vitro kinase assay using the p44/42 MAP Kinase Assay Kit (Cell Signaling Technology Inc.).
Quantitative real-time RT-PCR.
OCI-AML3 cells were treated with either DMSO or PD98059, and RNA was isolated with TRIzol solution (Life Technologies Inc., Grand Island, New York, USA). One microgram of total RNA was reverse transcribed by AMV reverse transcriptase (Roche Diagnostic Corp.) at 42°C for 1 hour. PCR amplification reaction mixtures (25 μl) contained cDNA, p21Waf1/CIP1 (5′-CGCTAATGGCGGGCTG-3′) or p27Kip1 (5′-GAGGACACGCATTTGGTGG-3′) forward primers, p21Waf1/CIP1 (5′-CGGTGACAAAGTCGAAGTTCC-3′) or p27Kip1 (5′-GCTCCGCTAACCCCGTCT-3′) reverse primers, p21Waf1/CIP1 (5′-ATCCAGGAGGCCCGTGAGCGA-3′) or p27Kip1 (5′-CCCAAAGACTGATCCGTCGGACAGC-3′) probes, and TaqMan Universal PCR Master Mix (PE Applied Biosystems, Foster City, California, USA). β2-microglobulin (β2-m) coamplified with p21Waf1/CIP1 and p27Kip1 was included as internal control for normalization of the variable content of cDNA in each sample (forward primer 5′-AGCTGTGCTCGCGCTACTCT-3′, reverse primer 5′-TTGACTTTCCATTCTCTGCTGG-3′, probe 5′-TCTTTCTGGCCTGGAGGGCATCC-3′). Thermal cycle conditions included holding the reactions at 50°C for 2 minutes and at 95°C for 10 minutes, and cycling for 40 cycles between 95°C for 15 seconds and 60°C for 1 minute. Results were collected and analyzed with an ABI Prism 7700 Sequence Detection System (PE Applied Biosystems) as follows: the PCR cycle number that generated the first fluorescence signal above a threshold (threshold cycle, CT; 10 SDs above the mean fluorescence generated during the baseline cycles) was determined, and a comparative CT method was then used to measure relative gene expression. The following formula was used to calculate the relative amount of the transcript of interest in the treated sample (X) and the control sample (Y), both normalized to an endogenous reference (β2-m): 2–ΔΔCT, where ΔCT is the difference in CT between the gene of interest and β2-m, and ΔΔCT for sample X = ΔCT(X) – ΔCT(Y).
Results
MEK blockade inhibits the growth of AML cell lines and primary AML samples.
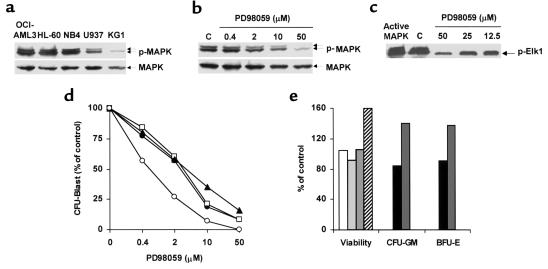
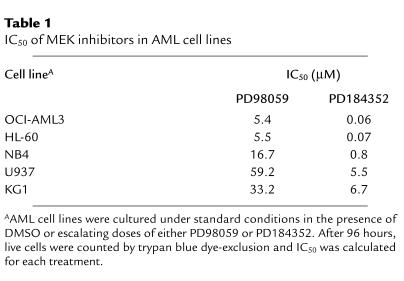
We analyzed MAPK expression and phosphorylation status in freshly isolated, unstimulated blasts from 186 patients with newly diagnosed AML. Constitutive MAPK phosphorylation was detectable in 138 samples (74%), indicating that this pathway is deregulated in most AML patients (S.M. Kornblau, unpublished observations). We therefore investigated the functional consequences of the pharmacological disruption of the MEK/MAPK module using the selective inhibitor of MEK activation PD98059. Among the AML cell lines tested, OCI-AML3, HL-60, and NB4 showed high steady-state levels of doubly phosphorylated (i.e., active) MAPK (Figure 1a). MAPK activation appeared to be constitutive in that comparably high levels of MAPK phosphorylation were detectable even after 48 hours of serum starvation (data not shown). As shown in Figure 1, b and c, PD98059 inhibited MAPK phosphorylation and enzymatic activity downstream of MEK in OCI-AML3 cells in a dose-dependent manner. MEK blockade resulted in a profound, dose-dependent growth inhibition in OCI-AML3 and HL-60 cells, and a less extensive inhibition in NB4 cells (Table 1). Conversely, U937 and KG1 cells, which showed low levels of constitutive MAPK activation (Figure 1a), proved relatively resistant (Table 1).
Figure 1.
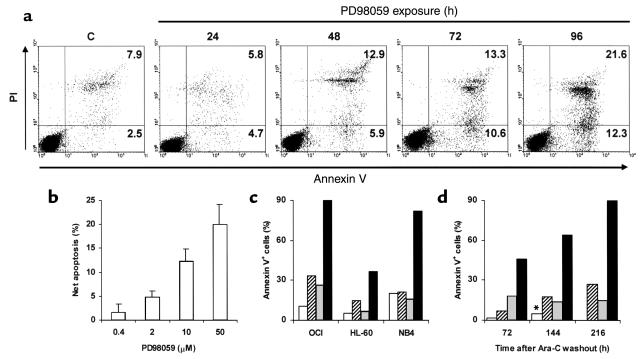
MEK blockade inhibits the growth of leukemic, but not normal, hematopoietic cells. (a) AML cell lines were assessed for MAPK phosphorylation by Western blot. (b) OCI-AML3 cells were exposed to DMSO (C) or PD98059 at the indicated doses for 6 hours. MAPK phosphorylation was then assessed by Western blot. (c) OCI-AML3 cells were treated as in b. MAPK enzymatic activity was measured by the ability to phosphorylate the specific substrate Elk1 in an in vitro kinase assay. (d) Mononuclear cells from patients 5 (filled circles), 9 (open squares), 10 (filled triangles), and 11 (open circles) were grown in colony assays in the presence of DMSO or PD98059 at the indicated doses. Results show AML blast colonies in the PD98059-treated group, expressed as a percentage of the colonies in the DMSO-treated group, and represent the average of quadruplicate cultures (SEM was constantly less than 10%). (e) MACS-sorted CD34+ cells from healthy donors were cultured in the presence of DMSO or 20 μM PD98059. Viability was then assessed by trypan blue dye exclusion. Results show viable cells in the PD98059-treated group, expressed as a percentage of the viable cells in the DMSO-treated group (four bars at left; each bar represents a single donor). Bone marrow cells from healthy donors were grown in colony assays in the presence of DMSO or 50 μM PD98059. Results show CFU-GM and burst-forming units–erythroid (BRU-E) in the PD98059-treated group, expressed as a percentage of the colonies in the DMSO-treated group (four bars at right; two donors).
Table 1.
IC50 of MEK inhibitors in AML cell lines
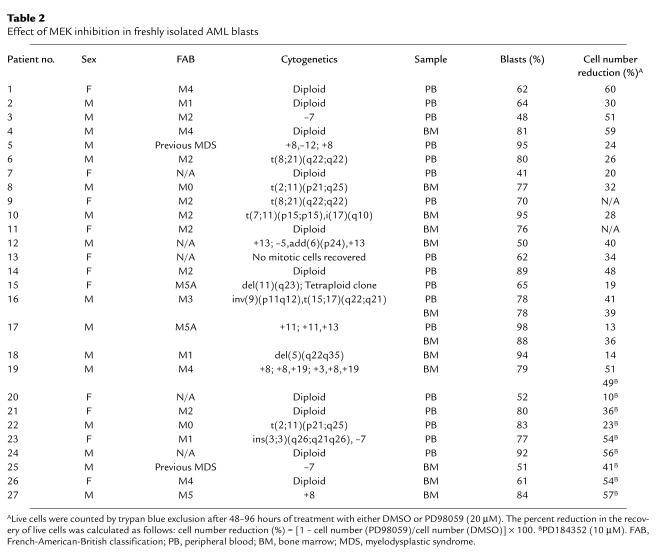
In primary AML samples, PD98059 (20 μM) caused a greater than 25% decrease in the recovery of viable cells after short-term in vitro culture in 14 of 19 samples tested (median reduction in sensitive samples, 40%; range, 26–60%) (Table 2). MEK blockade by PD98059 profoundly affected primary AML cell growth and viability at the progenitor cell level, as shown by the striking dose-dependent decrease in the number of AML blast colonies observed in all four samples tested (Figure 1d). Conversely, no reduction in either cell viability or clonogenic potential was observed in response to MEK blockade in CD34-selected progenitors (n = 4) and bone marrow cells (n = 2) from healthy donors (Figure 1e).
Table 2.
Effect of MEK inhibition in freshly isolated AML blasts
Collectively, these findings indicate that disruption of the MEK/MAPK module selectively inhibits the growth of AML cell lines and primary samples, but not that of normal hematopoietic progenitors.
MEK blockade inhibits cell cycle progression.
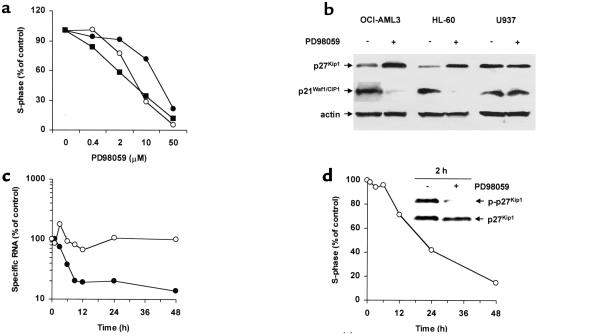
Consistent with the observed inhibition of cell growth, PD98059 caused a dose- and time-dependent inhibition of G1/S transition, with accumulation of cells in the G1 phase in MEK inhibition–sensitive cell lines and primary samples (Figure 2, a and d). Conversely, the resistant cell lines U937 and KG1 showed a slight inhibition of cell cycle progression only at the highest dose (data not shown). Western blot analysis showed that MEK inhibition–induced cell cycle arrest was accompanied by downregulation of p21Waf1/CIP1 and upregulation of p27Kip1 in sensitive cell lines (OCI-AML3 and HL-60), but not in resistant cell lines (U937) (Figure 2b). Levels of p21Waf1/CIP1 RNA decreased strikingly in response to PD98059 in a time-dependent fashion (Figure 2c), suggesting the possibility of direct transcriptional regulation (19). Conversely, p27Kip1 RNA levels remained relatively constant following exposure to PD98059. However, T187 phosphorylation, a posttranslational modification that targets p27Kip1 for proteasome-mediated degradation (20), was already decreased by PD98059 after 2 hours, suggesting that protein stabilization occurs before changes in cell cycle distribution are detectable (Figure 2d).
Figure 2.
MEK inhibition causes cell cycle arrest and modulates p27Kip1 and p21Waf1/CIP1 expression. (a) OCI-AML3 (open circles), HL-60 (filled circles), and freshly isolated leukemic blasts from patient 3 (filled squares) were cultured for 48 hours in the presence of DMSO or PD98059 at the indicated concentrations, and then stained for DNA content. Results are expressed as percentage of S-phase cells in the DMSO-treated group. Cell line results are representative of one of three independent experiments. (b) OCI-AML3, HL-60, and U937 cells were cultured for 24 hours in the presence of DMSO or PD98059 (20 μM), and subjected to Western blot analysis with mAb’s specific for the indicated cell cycle–regulating proteins. Results are representative of one of three independent experiments. (c) OCI-AML3 cells were cultured for the indicated times in the presence of DMSO or PD98059 (20 μM), and subjected to quantitative real-time PCR analysis of p21Waf1/CIP1 (filled circles) and p27Kip1 (open circles) RNA transcripts. Results are expressed as percentage of specific RNA transcripts in the DMSO-treated group and are representative of one of two independent experiments. (d) OCI-AML3 cells were cultured for the indicated times in the presence of DMSO or PD98059 (20 μM) and assessed for DNA content. Results are expressed as percentage of S-phase cells in the DMSO-treated group. Two hours after the addition of PD98059, cells were lysed, and the phosphorylation status of p27 Kip1 was analyzed by Western blot using T187-phosphospecific Ab’s (p-p27Kip1).
MEK inhibition induces apoptosis in AML cell lines and primary AML samples.
Consistent with the reported reversibility of MEK inhibition, removal of PD98059 from the culture medium promptly restored cell cycle progression and growth in HL-60 and NB4 cells (data not shown). By contrast, OCI-AML3 cells showed sustained growth impairment even 96 hours after PD98059 removal (data not shown). Since the MEK/MAPK pathway is considered a key regulator of apoptotic cell death (21, 22), we tested the hypothesis that sustained growth impairment might be due to induction of apoptosis. Treatment of OCI-AML3 cells with 20 μM PD98059 caused a time-dependent increase in the percentage of annexin V–binding cells that was detectable within 48 hours, and reached maximum levels after 96 hours (Figure 3a). Phosphatidylserine exposure on the outer leaflet of the plasma membrane was accompanied by caspase-3 activation and a decrease in the DNA content to hypodiploid levels (data not shown). A slight increase in apoptosis was also induced by PD98059 in HL-60 cells, but not in the other cell lines tested (data not shown). A net increase in the proportion of apoptotic cells of more than 10%, as detected by annexin V binding and hypodiploid DNA content, was observed in 11 of 18 primary AML samples after exposure to 20 μM PD98059 (median net apoptosis increase in sensitive samples, 13%; range, 10–23%; data not shown). The dose dependency of PD98059-induced apoptosis in MEK inhibition–sensitive samples (n = 3) is shown in Figure 3b. Conversely, no induction of apoptosis, but rather a slight protection from spontaneous cell death in cytokine-free cultures, was observed in normal CD34+ progenitor cells from healthy donors following treatment with PD98059 (20 μM; n = 4) (data not shown).
Figure 3.
MEK inhibition sensitizes to spontaneous and drug-induced apoptosis. (a) OCI-AML3 cells were cultured in the presence of DMSO or PD98059 (20 μM) and stained for annexin V binding at the indicated times. Matched DMSO control at 96 hours is shown (C). Results are representative of one of three independent experiments. (b) Primary AML blasts were cultured in the presence of DMSO or PD98059 at the indicated doses for 96 hours. Apoptosis was then measured as percentage of cells with hypodiploid DNA content. Results are expressed as the net apoptosis induction (percentage of apoptosis in treated cells minus percentage of apoptosis in DMSO-treated cells) and represent the mean ± SD of the results obtained in three different patient samples (patients 3, 14, and 16). (c) AML cell lines were cultured for 96 hours in the presence of DMSO (white bars), 20 μM PD98059 (hatched bars), 1 μM ATRA (gray bars), or a combination of PD98059 and ATRA (black bars), and stained for annexin V binding. Results are representative of one of three independent experiments. (d) HL-60 cells were exposed to 10 μM Ara-C, washed, and cultured in the presence of DMSO (gray bars) or 20 μM PD98059 (black bars). White bars and hatched bars represent DMSO- and PD98059-treated HL-60 cells, respectively, in the absence of prior exposure to Ara-C. At the indicated times, cells were stained for annexin V binding. *DMSO-treated cultures were discontinued after 144 hours due to overgrowth. Results are representative of one of three independent experiments.
MEK inhibition sensitizes AML cells to retinoid- and chemotherapy-induced apoptosis.
PD98059 (20 μM) also potently sensitized AML cell lines with constitutive MAPK activation (OCI-AML3, NB4, HL-60), but not those with low steady-state levels of MAPK activity (U937, KG1), to ATRA-induced apoptosis (Figure 3c). Apoptosis was not caused by increased differentiation; on the contrary, ATRA-induced differentiation was partially blocked by simultaneous MEK inhibition, as previously reported (23). A similar degree of sensitization was observed in response to the pan-RA receptor agonist 9-cis-RA, but not to RAR- or RXR-selective ligands, suggesting that this proapoptotic effect requires simultaneous activation of both the RAR and RXR pathways (data not shown). Moreover, when HL-60 cells were exposed to clinically relevant concentrations (10 μM) of Ara-C for short periods (4 hours), the subsequent exposure to PD98059 (20 μM) greatly potentiated drug-induced apoptosis (Figure 3d), and completely prevented regrowth of the leukemic cells for up to 9 days. This effect was strictly sequence dependent, in that pretreatment with PD98059 did not sensitize leukemic cells to, but rather slightly protected them from Ara-C–induced killing (data not shown). Similar results were obtained in OCI-AML3 and NB4 cells, whereas no such effect was observed in U937 and KG1 cells (data not shown).
MEK inhibition downregulates antiapoptotic proteins of the “inhibitor of apoptosis protein” and Bcl-2 families.
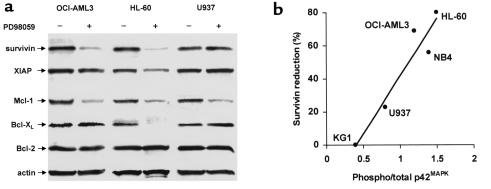
In an attempt to elucidate the molecular mechanisms underlying the lowering of the apoptotic threshold in response to MEK blockade, we examined whether PD98059 affected the expression of antiapoptotic proteins of the “inhibitor of apoptosis protein” (IAP) and Bcl-2 families. We have recently reported that survivin expression is regulated through MAPK-dependent mechanisms in OCI-AML3 cells (14). Here we show that survivin levels were significantly decreased by treatment with 20 μM PD98059 in the MEK inhibition–sensitive cell lines (OCI-AML3 and HL-60), but not in the resistant cell lines (U937; Figure 4a). Overall, a strong, statistically significant correlation was observed between constitutive MAPK activation and the reduction in survivin levels in response to MEK inhibition (R2 = 0.9104, P = 0.012; Figure 4b). Antiapoptotic protein XIAP levels were only slightly affected. PD98059 also significantly decreased Mcl-1 expression, whereas no changes in Bcl-2 and Bcl-XL levels were detected, with the notable exception of HL-60 cells, in which Bcl-XL became undetectable within 48 hours (Figure 4a).
Figure 4.
MEK inhibition downregulates antiapoptotic proteins of the IAP and Bcl-2 families. (a) OCI-AML3, HL-60, and U937 cells were cultured under standard conditions for 48 hours in the presence of DMSO or PD98059 (20 μM), and then subjected to Western blot analysis with Ab’s specific for the indicated antiapoptotic proteins of IAP (survivin, XIAP) and Bcl-2 (Mcl-1, Bcl-XL, Bcl-2) families. Results are representative of one of three independent experiments. (b) The MAPK phosphorylation status of AML cell lines was assessed by Western blot analysis. The ratio of phosphorylated to total p42MAPK (Phospho/total p42MAPK) was then plotted against the percent reduction in survivin expression observed in the corresponding cell lines after PD98059 treatment, and regression analysis was performed (R2 = 0.9104, P = 0.012). Results are representative of one of three independent experiments.
Effects of the novel MEK inhibitor PD184352 in AML cell lines and primary samples.
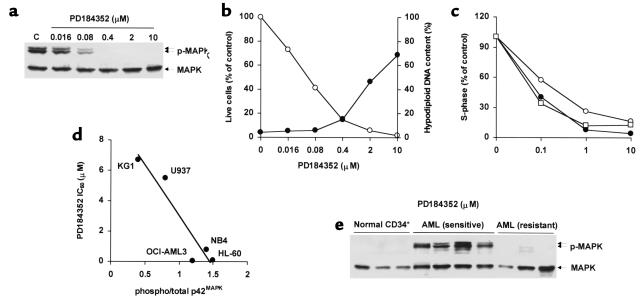
Sebolt-Leopold et al. have recently reported that the novel, orally bioavailable MEK inhibitor PD184352 successfully suppresses the growth of colon tumors in vivo without causing overt toxicity (17). We therefore tested its effects in AML cell lines and primary AML samples. Consistent with results reported in different cellular models (17, 24), PD184352 inhibited MAPK phosphorylation and cell growth at nanomolar concentrations in sensitive cell lines (Figure 5, a and b; Table 1). In primary AML samples, PD184352 (10 μM) caused a greater than 25% decrease in the recovery of viable cells in seven of nine samples tested (median, 54%; range, 36–57%; Table 2). The pattern of sensitivity to the growth-inhibiting effects of PD184352 was superimposable on that observed with PD98059 in primary samples tested for response to both MEK inhibitors (n = 5; Table 2 and data not shown). PD184352 caused G1 arrest (Figure 5c), and at higher doses (1–10 μM), induced apoptotic cell death in cells with constitutive MAPK activation (OCI-AML3, HL-60, NB4), but not in those with low steady-state levels of MAPK activity (U937, KG1) (Figure 5b and data not shown). A net increase of more than 10% in the proportion of apoptotic cells was also observed in seven of nine primary AML samples (median, 17%; range 11–72%) after exposure to 10 μM PD184352 (data not shown). In contrast, no reduction in cell viability or increase in apoptosis was observed in normal CD34+ progenitor cells from healthy donors (n = 3) following exposure to 10 μM PD184352 (data not shown).
Figure 5.
Effect of PD184352 on AML cell lines and primary samples. (a) OCI-AML3 cells were exposed to DMSO (C) or PD184352 for 6 hours and analyzed for MAPK phosphorylation. (b) OCI-AML3 cells were cultured in the presence of DMSO or PD184352. After 96 hours, cells were assessed for viability (open circles) and DNA content (filled circles). Results show viable cells in the PD98059-treated group, expressed as percentage of the viable cells in the DMSO-treated group, and percentage of cells with a hypodiploid DNA content, respectively, and are representative of one of three independent experiments. (c) OCI-AML3 (open circles), HL-60 (filled circles), and leukemic blasts from patient 19 (open squares) were cultured in the presence of DMSO or PD184352 for 48 hours and stained for DNA content. Results are expressed as percentage of S-phase cells in the DMSO-treated group. Cell line results are representative of one of three independent experiments. (d) The MAPK phosphorylation status of AML cell lines was assessed by Western blot. The ratio of phosphorylated to total p42MAPK was then plotted against the IC50 of PD184352 for the corresponding cell lines, and regression analysis was performed (R2 = 0.8933, P = 0.015). (e) CD34+ cells from healthy donors (n = 3) and blasts from AML patients (n = 7) were analyzed for phosphorylated MAPK by Western blot. Patient samples showing a greater than 25% decrease (from patients 14, 19, 21, and 24) and a less than 25% decrease (from patients 17, 20, and 22) in cell viability after in vitro exposure to MEK inhibitors are labeled as sensitive and resistant, respectively.
Interestingly, a strong, statistically significant correlation between steady-state levels of active MAPK and sensitivity to the growth-inhibiting effect of PD184352 was observed in AML cell lines (R2 = 0.8933, P = 0.015; Figure 5d). Likewise, MEK inhibition–sensitive primary AML samples (n = 4) showed high levels of constitutive MAPK phosphorylation, whereas MAPK phosphorylation was barely detectable in resistant samples (n = 3, P = 0.01) and in normal CD34+ progenitors (Figure 5e).
Discussion
Here we provide evidence that small-molecule inhibitors of the MEK/MAPK pathway profoundly affect the growth of AML cell lines and primary AML samples with constitutive MAPK activation, but have little effect on cells with low steady-state MAPK activity. Cell growth was inhibited as a result of cell cycle arrest and induction of apoptosis, and resulted in the abrogation of leukemic, but not normal, hematopoietic cell clonogenicity. In addition, MEK inhibition profoundly sensitized leukemic cells to the proapoptotic effects of retinoids and chemotherapy.
Consistent with the proposed role of the RAS/MAPK pathway in the regulation of G1/S transition (25), both MEK inhibitors caused cell cycle arrest in sensitive AML cell lines and primary samples. Constitutive activation of the MEK/MAPK pathway results in decreased expression of the cyclin-dependent kinase inhibitor p27Kip1 in different cell types, and recent data show that MAPK regulates p27Kip1 turnover at a posttranslational level (26, 27). Our data demonstrate that, in AML cell lines with constitutive MAPK activation, p27Kip1 turnover is similarly regulated through MAPK-dependent mechanisms. Likewise, the observed decrease in p21Waf1/CIP1 levels in response to MEK inhibition is consistent with the reported ability of MAPK to transcriptionally induce p21Waf1/CIP1 expression (19). While downregulation of p21Waf1/CIP1 argues against its involvement in the observed inhibition of cell cycle progression, decreased levels of expression may play a role in the MEK inhibition–induced sensitization to the proapoptotic effects of retinoids and chemotherapy (28, 29). The potential clinical relevance of these findings is highlighted by recent reports that both low levels of p27Kip1 and high levels of p21Waf1/CIP1 confer a poor clinical outcome to AML patients (30, 31).
The evidence presented here suggests that MEK blockade alters the apoptotic balance by reducing the expression of both upstream (Mcl-1, Bcl-XL) and downstream (survivin) antiapoptotic proteins in cells that rely heavily on MAPK activation for their growth and survival. Although the relative importance of each of these players in the MEK inhibition–mediated lowering of the apoptotic threshold deserves further investigation, changes in survivin expression show the best correlation with the functional response to disruption of the MEK/MAPK module. These findings are consistent with recent observations showing that the MEK/MAPK pathway protects against apoptosis at the level of cytosolic caspase activation, without interfering with the release of cytochrome c from mitochondria, strongly suggesting the involvement of an IAP-like molecule (32). Despite the above-described molecular changes, MEK inhibitors, at doses close to the IC50 for MAPK enzymatic activity, have cytostatic rather than cytotoxic effects, and higher doses are required to trigger apoptosis. However, MEK blockade sets the stage for increased sensitivity to other apoptotic stimuli, such as retinoids and DNA-damaging agents. Although MAPK may regulate Bcl-2 antiapoptotic activity at a posttranslational level (33), we show here that MEK inhibition does not affect its protein expression; therefore, in the absence of additional cellular stresses, above-threshold levels of Bcl-2 can maintain cell viability even in the presence of low levels of other antiapoptotic players (such as Mcl-1 and survivin). This might in turn explain the proapoptotic synergism observed for the combination of ATRA treatment and MEK inhibition. In fact, ATRA efficiently decreases Bcl-2 expression (34, 35), whereas MEK inhibition downregulates downstream caspase inhibitors such as survivin, resulting in the simultaneous inhibition of complementary survival pathways, with synergistic effects on cell viability. This hypothesis is supported by our recent observation that simultaneous targeting of the MAPK and Bcl-2 pathways results in the synergistic induction of apoptosis in AML cells (M. Milella, unpublished observations).
Based on its ability to lower the apoptotic threshold, disruption of the MEK/MAPK module has been proposed as a novel approach to the chemosensitization of cancer cells (36). Although our findings support this concept, they differ from previously reported results in two clinically relevant aspects. First, we found that only cell lines with constitutive activation of the MAPK pathway were sensitized to Ara-C–induced apoptosis, suggesting that the observed effect may depend on intrinsic rather than Ara-C–stimulated MAPK activity, as previously suggested (37). Second, as recently reported for paclitaxel (38), the Ara-C–potentiating effect was strictly sequence dependent. Ara-C followed by PD98059 substantially potentiated Ara-C–induced apoptosis, completely abrogating the ability of leukemic cells to recover from the cytotoxic insult, whereas the reverse sequence had a slight protective effect. The reduced efficacy of the combination of PD98059 followed by Ara-C may be related to the inhibition of cell cycle progression, which would result in decreased incorporation of Ara-C, and has potential implications for the design of clinical trials using MEK-targeted therapies as chemosensitizers.
The usefulness of mechanism-targeted therapeutic strategies, such as MEK inhibition, will ultimately depend on the ability to prospectively select those patients most likely to benefit from a specific intervention. Recent studies have shown that constitutive deregulation of specific pathways is the mechanism responsible for the sensitivity of cancer cells to signal-transduction inhibitors (39–41). In agreement with recent findings in different tumor models (17, 42), we observed a significant correlation between constitutive MAPK phosphorylation and sensitivity to MEK blockade–induced growth inhibition in both AML cell lines and primary AML samples. Along these lines, the lack of constitutive MAPK activation in normal hematopoietic progenitors (ref. 10 and Figure 5e) may explain their relative resistance to MEK inhibition, as observed here and in other recently published studies (43, 44), providing the basis for a selective antileukemic effect.
Our overall results strongly support the hypothesis that disruption of the MEK/MAPK module, alone or in combination with retinoids and/or chemotherapy, may represent an effective and relatively specific therapeutic strategy for AML. The availability of orally active MEK inhibitors that have little or no systemic toxicity, such as PD184352, will allow further exploration of this approach in animal models and in human clinical trials.
Acknowledgments
This work was supported in part by grants from NIH (PO1 CA-55164, PO1 CA-49639, and CA-16672) and the Stringer Professorship for Cancer Treatment and Research (to M. Andreeff), and by an American Cancer Society International Fellowship for Beginning Investigators (to M. Milella). The authors wish to thank Judith S. Sebolt-Leopold (Department of Cell Biology, Parke-Davis Pharmaceutical Research) for kindly providing the MEK inhibitor PD184352, and Teresa McQueen for the separation of normal CD34+ hematopoietic progenitors.
References
- 1.Lowenberg B, Downing JR, Burnett A. Acute myeloid leukemia. N Engl J Med. 1999;341:1051–1062. doi: 10.1056/NEJM199909303411407. [DOI] [PubMed] [Google Scholar]
- 2.Look AT. Oncogenic transcription factors in the human acute leukemias. Science. 1997;278:1059–1064. doi: 10.1126/science.278.5340.1059. [DOI] [PubMed] [Google Scholar]
- 3.Castilla LH, et al. The fusion gene Cbfb-MYH11 blocks myeloid differentiation and predisposes mice to acute myelomonocytic leukaemia. Nat Genet. 1999;23:144–146. doi: 10.1038/13776. [DOI] [PubMed] [Google Scholar]
- 4.Rhoades KL, et al. Analysis of the role of AML1-ETO in leukemogenesis, using an inducible transgenic mouse model. Blood. 2000;96:2108–2115. [PubMed] [Google Scholar]
- 5.Dash A, Gilliland DG. Molecular genetics of acute myeloid leukaemia. Baillieres Best Pract Res Clin Haematol. 2001;14:49–64. doi: 10.1053/beha.2000.0115. [DOI] [PubMed] [Google Scholar]
- 6.Lewis TS, Shapiro PS, Ahn NG. Signal transduction through MAP kinase cascades. Adv Cancer Res. 1998;74:49–139. doi: 10.1016/s0065-230x(08)60765-4. [DOI] [PubMed] [Google Scholar]
- 7.Hunter T. Oncoprotein networks. Cell. 1997;88:333–346. doi: 10.1016/s0092-8674(00)81872-3. [DOI] [PubMed] [Google Scholar]
- 8.Kim SC, et al. Constitutive activation of extracellular signal-regulated kinase in human acute leukemias: combined role of activation of MEK, hyperexpression of extracellular signal-regulated kinase, and downregulation of a phosphatase, PAC1. Blood. 1999;93:3893–3899. [PubMed] [Google Scholar]
- 9.Towatari M, et al. Constitutive activation of mitogen-activated protein kinase pathway in acute leukemia cells. Leukemia. 1997;11:479–484. doi: 10.1038/sj.leu.2400617. [DOI] [PubMed] [Google Scholar]
- 10.Hayakawa F, et al. Tandem-duplicated Flt3 constitutively activates STAT5 and MAP kinase and introduces autonomous cell growth in IL-3-dependent cell lines. Oncogene. 2000;19:624–631. doi: 10.1038/sj.onc.1203354. [DOI] [PubMed] [Google Scholar]
- 11.Blalock WL, et al. A conditionally-active form of MEK1 results in autocrine transformation of human and mouse hematopoietic cells. Oncogene. 2000;19:526–536. doi: 10.1038/sj.onc.1203337. [DOI] [PubMed] [Google Scholar]
- 12.Blalock WL, et al. Combined effects of aberrant MEK1 activity and BCL2 overexpression on relieving the cytokine dependency of human and murine hematopoietic cells. Leukemia. 2000;14:1080–1096. doi: 10.1038/sj.leu.2401793. [DOI] [PubMed] [Google Scholar]
- 13.Liu Q, et al. Signal transduction and transforming properties of the TEL-TRKC fusions associated with t(12;15)(p13;q25) in congenital fibrosarcoma and acute myelogenous leukemia. EMBO J. 2000;19:1827–1838. doi: 10.1093/emboj/19.8.1827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carter BZ, Milella M, Altieri DC, Andreeff M. Cytokine-regulated expression of survivin in myeloid leukemia. Blood. 2001;97:2784–2790. doi: 10.1182/blood.v97.9.2784. [DOI] [PubMed] [Google Scholar]
- 15.Dudley DT, Pang L, Decker SJ, Bridges AJ, Saltiel AR. A synthetic inhibitor of the mitogen-activated protein kinase cascade. Proc Natl Acad Sci USA. 1995;92:7686–7689. doi: 10.1073/pnas.92.17.7686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alessi DR, Cuenda A, Cohen P, Dudley DT, Saltiel AR. PD 098059 is a specific inhibitor of the activation of mitogen-activated protein kinase kinase in vitro and in vivo. J Biol Chem. 1995;270:27489–27494. doi: 10.1074/jbc.270.46.27489. [DOI] [PubMed] [Google Scholar]
- 17.Sebolt-Leopold, et al. Blockade of the MAP kinase pathway suppresses growth of colon tumors in vivo. Nat Med. 1999;5:810–816. doi: 10.1038/10533. [DOI] [PubMed] [Google Scholar]
- 18.Estrov Z, et al. Effect of interleukin-1 beta converting enzyme inhibitor on acute myelogenous leukemia progenitor proliferation. Blood. 1995;86:4594–4602. [PubMed] [Google Scholar]
- 19.Liu Y, Martindale JL, Gorospe M, Holbrook NJ. Regulation of p21WAF1/CIP1 expression through mitogen-activated protein kinase signaling pathway. Cancer Res. 1996;56:31–35. [PubMed] [Google Scholar]
- 20.Slingerland J, Pagano M. Regulation of the cdk inhibitor p27 and its deregulation in cancer. J Cell Physiol. 2000;183:10–17. doi: 10.1002/(SICI)1097-4652(200004)183:1<10::AID-JCP2>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 21.Xia Z, Dickens M, Raingeaud J, Davis RJ, Greenberg ME. Opposing effects of ERK and JNK-p38 MAP kinases on apoptosis. Science. 1995;270:1326–1331. doi: 10.1126/science.270.5240.1326. [DOI] [PubMed] [Google Scholar]
- 22.Jarpe MB, et al. Anti-apoptotic versus pro-apoptotic signal transduction: checkpoints and stop signs along the road to death. Oncogene. 1998;17:1475–1482. doi: 10.1038/sj.onc.1202183. [DOI] [PubMed] [Google Scholar]
- 23.Yen A, Roberson MS, Varvayanis S, Lee AT. Retinoic acid induced mitogen-activated protein (MAP)/extracellular signal-regulated kinase (ERK) kinase-dependent MAP kinase activation needed to elicit HL-60 cell differentiation and growth arrest. Cancer Res. 1998;58:3163–3172. [PubMed] [Google Scholar]
- 24.Davies SP, Reddy H, Caivano M, Cohen P. Specificity and mechanism of action of some commonly used protein kinase inhibitors. Biochem J. 2000;351:95–105. doi: 10.1042/0264-6021:3510095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Malumbres M, Pellicer A. RAS pathways to cell cycle control and cell transformation. Front Biosci. 1998;3:d887–d912. doi: 10.2741/a331. [DOI] [PubMed] [Google Scholar]
- 26.Lenferink AE, et al. Blockade of the epidermal growth factor receptor tyrosine kinase suppresses tumorigenesis in MMTV/Neu + MMTV/TGF-alpha bigenic mice. Proc Natl Acad Sci USA. 2000;97:9609–9614. doi: 10.1073/pnas.160564197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang HY, Zhou BP, Hung MC, Lee MH. Oncogenic signals of HER-2/neu in regulating the stability of the cyclin-dependent kinase inhibitor p27. J Biol Chem. 2000;275:24735–24739. doi: 10.1074/jbc.C000147200. [DOI] [PubMed] [Google Scholar]
- 28.Wang Z, et al. Dysregulation of the cyclin-dependent kinase inhibitor p21WAF1/CIP1/MDA6 increases the susceptibility of human leukemia cells (U937) to 1-beta-D-arabinofuranosylcytosine-mediated mitochondrial dysfunction and apoptosis. Cancer Res. 1999;59:1259–1267. [PubMed] [Google Scholar]
- 29.Rosato RR, Wang Z, Gopalkrishnan RV, Fisher PB, Grant S. Evidence of a functional role for the cyclin-dependent kinase-inhibitor p21WAF1/CIP1/MDA6 in promoting differentiation and preventing mitochondrial dysfunction and apoptosis induced by sodium butyrate in human myelomonocytic leukemia cells (U937) Int J Oncol. 2001;19:181–191. doi: 10.3892/ijo.19.1.181. [DOI] [PubMed] [Google Scholar]
- 30.Yokozawa T, et al. Prognostic significance of the cell cycle inhibitor p27Kip1 in acute myeloid leukemia. Leukemia. 2000;14:28–33. doi: 10.1038/sj.leu.2401640. [DOI] [PubMed] [Google Scholar]
- 31.Zhang W, et al. High levels of constitutive WAF1/Cip1 protein are associated with chemoresistance in acute myelogenous leukemia. Clin Cancer Res. 1995;1:1051–1057. [PubMed] [Google Scholar]
- 32.Erhardt P, Schremser EJ, Cooper GM. B-Raf inhibits programmed cell death downstream of cytochrome c release from mitochondria by activating the MEK/Erk pathway. Mol Cell Biol. 1999;19:5308–5315. doi: 10.1128/mcb.19.8.5308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Deng X, Ruvolo P, Carr B, May WS., Jr Survival function of ERK1/2 as IL-3-activated, staurosporine-resistant Bcl2 kinases. Proc Natl Acad Sci USA. 2000;97:1578–1583. doi: 10.1073/pnas.97.4.1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Andreeff M, et al. Expression of bcl-2-related genes in normal and AML progenitors: changes induced by chemotherapy and retinoic acid. Leukemia. 1999;13:1881–1892. doi: 10.1038/sj.leu.2401573. [DOI] [PubMed] [Google Scholar]
- 35.Ketley NJ, Allen PD, Kelsey SM, Newland AC. Modulation of idarubicin-induced apoptosis in human acute myeloid leukemia blasts by all-trans retinoic acid, 1,25(OH)2 vitamin D3, and granulocyte-macrophage colony-stimulating factor. Blood. 1997;90:4578–4587. [PubMed] [Google Scholar]
- 36.Dent P, Grant S. Pharmacologic interruption of the mitogen-activated extracellular- regulated kinase/mitogen-activated protein kinase signal transduction pathway: potential role in promoting cytotoxic drug action. Clin Cancer Res. 2001;7:775–783. [PubMed] [Google Scholar]
- 37.Jarvis WD, et al. Evidence for involvement of mitogen-activated protein kinase, rather than stress-activated protein kinase, in potentiation of 1-beta-D- arabinofuranosylcytosine-induced apoptosis by interruption of protein kinase C signaling. Mol Pharmacol. 1998;54:844–856. doi: 10.1124/mol.54.5.844. [DOI] [PubMed] [Google Scholar]
- 38.Yu C, Wang S, Dent P, Grant S. Sequence-dependent potentiation of paclitaxel-mediated apoptosis in human leukemia cells by inhibitors of the mitogen-activated protein kinase kinase/mitogen-activated protein kinase pathway. Mol Pharmacol. 2001;60:143–154. doi: 10.1124/mol.60.1.143. [DOI] [PubMed] [Google Scholar]
- 39.Shayesteh L, et al. PIK3CA is implicated as an oncogene in ovarian cancer. Nat Genet. 1999;21:99–102. doi: 10.1038/5042. [DOI] [PubMed] [Google Scholar]
- 40.Druker BJ, et al. Effects of a selective inhibitor of the Abl tyrosine kinase on the growth of Bcr-Abl positive cells. Nat Med. 1996;2:561–566. doi: 10.1038/nm0596-561. [DOI] [PubMed] [Google Scholar]
- 41.Brognard J, Clark AS, Ni Y, Dennis PA. Akt/protein kinase B is constitutively active in non-small cell lung cancer cells and promotes cellular survival and resistance to chemotherapy and radiation. Cancer Res. 2001;61:3986–3997. [PubMed] [Google Scholar]
- 42.Hoshino R, Tanimura S, Watanabe K, Kataoka T, Kohno M. Blockade of the extracellular signal-regulated kinase pathway induces marked G1 cell cycle arrest and apoptosis in tumor cells in which the pathway is constitutively activated: Up-regulation of p27Kip1. J Biol Chem. 2000;276:2686–2692. doi: 10.1074/jbc.M006132200. [DOI] [PubMed] [Google Scholar]
- 43.Fichelson S, et al. Megakaryocyte growth and development factor-induced proliferation and differentiation are regulated by the mitogen-activated protein kinase pathway in primitive cord blood hematopoietic progenitors. Blood. 1999;94:1601–1613. [PubMed] [Google Scholar]
- 44.Morgan MA, Dolp O, Reuter CW. Cell-cycle-dependent activation of mitogen-activated protein kinase kinase (MEK-1/2) in myeloid leukemia cell lines and induction of growth inhibition and apoptosis by inhibitors of RAS signaling. Blood. 2001;97:1823–1834. doi: 10.1182/blood.v97.6.1823. [DOI] [PubMed] [Google Scholar]