Abstract
Developing B cells must pass a series of checkpoints that are regulated by membrane-bound Igμ through the Igα-Igβ signal transducers. To determine how Igμ expression affects B cell development and Ab selection in humans we analyzed Ig gene rearrangements in pro-B cells from two patients who are unable to produce Igμ proteins. We find that Igμ expression does not affect VH, D, or JH segment usage and is not required for human Igκ and Igλ recombination or expression. However, the heavy and light chains found in pro-B cells differed from those in peripheral B cells in that they showed unusually long CDR3s. In addition, the Igκ repertoire in Igμ-deficient pro-B cells was skewed to downstream Jκs and upstream Vκs, consistent with persistent secondary V(D)J rearrangements. Thus, Igμ expression is not required for secondary V(D)J recombination in pro-B cells. However, B cell receptor expression shapes the Ab repertoire in humans and is essential for selection against Ab’s with long CDR3s.
Introduction
Expression of immunoglobulin heavy chains (Igμ) in pro-B cells induces pre-B cell development by assembly of a pre-B cell receptor (pre-BCR), which is a complex of Igμ, surrogate light chains (ψL), and two signal-transducing proteins, Igα and Igβ (1–6). Pre-BCR expression induces proliferative expansion and downregulation of recombinase-activating genes (RAG1 and RAG2), thereby ensuring allelic exclusion while selecting clones of cells with productive VDJH rearrangements (7). Mutations in mIgμ, ψL, or Igα/Igβ genes that disrupt pre-BCR assembly impede B cell development at the pro-B cell stage in mice and humans (8–15).
Pre-BCR assembly is dependent on interaction between VH variable regions and ψL, and in the mouse the IgH repertoire is selected in part on the basis of this interaction (16–18). It has been proposed that VH domains that pair well with ψL are positively selected at the pre-B cell stage, whereas VH domains that pair poorly with ψL are not. However, selection differs in mouse fetal and adult B cell development such that VHs that are counterselected in the adult are prominent in the fetal repertoire (18).
In contrast to mice, there is no apparent difference in the VH repertoire between pro-B, pre-B, or fetal B cells and adult mature B cells in the human (19–30). However, there is selection for IgH genes during human B cell development as determined by the length of the third complementary determining region (CDR3). Pro- and pre-B cells from adult bone marrow have longer CDR3s than mature B cells (31–33).
Following successful IgH assembly and pre-BCR expression, V(D)J recombination is targeted to the light chain (IgL) genes (34–38). Those cells that produce in-frame IgL chains test their newly synthesized Igs for self-reactivity. In the mouse, B cells that produce self-reactive receptors are either deleted or arrested in development and undergo receptor editing (39–45). In contrast, less is known about receptor selection and the role of the BCR in regulating B cell development in humans. Here we report on the role of the BCR in Ig repertoire selection in two patients with different mutations in the Igμ gene that impairs BCR assembly.
Methods
Patient samples and cell preparation.
Bone marrow samples were obtained from two Igμ-deficient patients with either a homozygous cytidine insertion in the Igμ gene (Igμ–/–) or with a homozygous deletion of the Igμ locus (IgμΔ) (see Results) (13, 46, 47) (C. Schiff, unpublished observations). Samples were obtained when the IgμΔ patient was 2 years old, the Igμ–/– patient was 4 years old, and her Igμ+/– brother (control) was 9 months old. The parents gave informed consent for this study. Bone marrow mononuclear cells were isolated by Ficoll gradients and CD34+CD19+ pro-B cells were sorted on a FACSVantage after labeling with FITC anti-CD34 and phycoerythrin anti-CD19 mAb’s (Beckman Coulter, Brea, California, USA).
RNA and RT-PCR.
Total RNA was extracted from 104–105 purified cells using TRIzol Reagent (Life Technologies Inc., Rockville, Maryland, USA). RNA was reverse transcribed with Superscript II (Life Technologies Inc.) according to the manufacturer’s instructions. For RT-PCR reactions, cDNA was amplified for 25 (actin), 35 (VH-Cμ), or 38 (Vκ-Cκ) cycles of 30 seconds at 94°C, 30 seconds at 60°C, and 30 seconds at 72°C, or for 40 cycles (Vλ-Cλ) of 30 seconds at 94°C, 30 seconds at 55°C, and 30 seconds at 72°C, with a final 10-minute extension at 72°C using Hot Star Taq DNA polymerase (QIAGEN Inc., Valencia, California, USA) and the following primers: Vλ consensus sense, 5′GGG(G/A)TC(T/C)CTGA(C/T/G)CG(A/C/G)TTCTCTGG(C/G)TCC3′; Cλ antisense, 5′CACAC(T/C)AGTGTGGCCTTGTTGGCTTG3′. VH1, VH3, VH4, Cμ, Vκ consensus and Cκ primers were described previously (48, 49). RT-PCR products were analyzed on 2% agarose gels and visualized by adding 0.3 pmol of 32PdATP to the PCR reaction.
Cloning and sequencing.
PCR products were gel-purified (Qiaquick; QIAGEN Inc.) and cloned into TA vectors (Invitrogen, Carlsbad, California, USA). Double-stranded DNA sequences were obtained using antisense Cμ, Cκ, or Cλ primers and Dye Terminator Cycle Sequencing (Applied Biosystems, Foster City, California, USA). Sequences were analyzed by comparison with Ig basic alignment search tool (BLAST). IgH CDR3 length was determined by counting amino acid residues between positions 94 and 102 (conserved tryptophan in all JH segments) and D segments were identified following the criteria of Corbett et al. (50). Igκ and Igλ CDR3 length included amino acids between conserved cystein 88 and the phenylalanine residue embedded in Jκ or Jλ (51). Nontemplate (N) nucleotides (52) found at Vκ-Jκ or Vλ-Jλ junctions were counted while template-dependent palindromic (P) nucleotides (53) were excluded. Differences in gene distribution were analyzed with χ2 tests (Cochran-Mantel-Haenszel test) adjusted by the Bonferroni method for multiple testing, and they were considered significant when P values were less than or equal to 0.05.
Results
IgH and IgL transcription is independent of Igμ expression.
Two patients with agammaglobulinemia and IgH mutations were studied. Igμ–/– has a cytidine insertion in the CH1 exon of the Igμ gene that leads to a frameshift and the inability to produce Igμ products (13, 46, 47). IgμΔ has a deletion in the Ig locus from 3′ of the diversity (D) region to Igγ2, with all junction (J) segments and Igμ, Igδ, Igγ3, and Igγ1 genes missing (C. Schiff, unpublished observations).
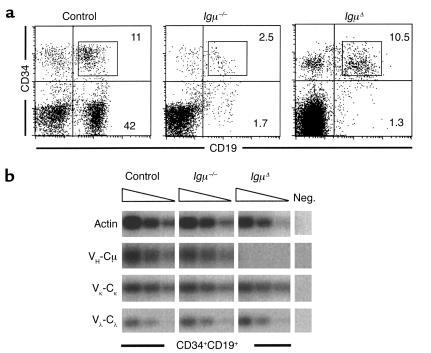
Flow cytometric analysis of bone marrow from Igμ–/– and IgμΔ patients revealed that in both cases B cell differentiation was arrested at the CD34+CD19+ pro-B cell stage (Figure 1a) (12, 13, 46, 47). To characterize Ig expression in Igμ-deficient pro-B cells, transcripts for heavy and light chain genes were amplified by semiquantitative RT-PCR from sorted CD34+CD19+ pro-B cells from Igμ–/–, IgμΔ, and a control sibling (Figure 1). As expected, VH-Cμ mRNA was missing in IgμΔ pro-B cells where the entire Igμ locus was deleted (Figure 1b). In contrast, Igμ–/– and control pro-B cells showed similar levels of VH-Cμ transcripts revealing that the absence of Igμ protein does not affect the Igμ gene expression in humans (Figure 1b).
Figure 1.
Immunoglobulin rearrangements in human pro-B cells. (a) B cell precursors in sibling control (left), Igμ–/– (middle), and IgμΔ (right) bone marrow. CD34+CD19+ pro-B cells from control and both Igμ-deficient patients were sorted as gated. (b) Heavy and light chain Ig gene expression in human pro-B cells. RNA from FACS-sorted CD34+CD19+ pro-B cells from both Igμ-deficient patients and control was analyzed by semiquantitative RT-PCR using 5′ consensus VH, Vκ, or Vλ and 3′ Cμ, Cκ, or Cλ primers, respectively, and visualized by 32PdATP incorporation. “Neg.” denotes a negative control without cDNA for RT-PCR reactions. Actin RT-PCR was used as mRNA loading control. Serial fivefold dilutions of cDNA are shown.
Light chain gene transcripts were found at similar levels in sorted control or Igμ-deficient pro-B cells (Figure 1b), but they were not amplified from Igμ–/– total bone marrow cells that contain few pro-B cells (46, 47). We conclude that light chain genes can be recombined and expressed in the absence of Igμ in human pro-B cells.
Igμ-independent VH, D, and JH gene segment usage.
To determine whether Igμ expression is required for VH, D, or JH segment selection, IgH genes from the three major VH families, VH1, VH3, and VH4, were cloned and sequenced. VH, D, and JH repertoire analysis revealed no statistically significant differences between Igμ–/– pro-B cells, control pro-B cells, and peripheral B cells (19, 20, 26–30) (see supplemental data 1, www.jci.org/cgi/content/full/108/06/879/DC1). Of 27 D genes in humans, only the D2-2 gene segment was over-represented in Igμ–/– and control pro-B cells (P = 0.02) (31, 50) (see supplemental data 2, www.jci.org/cgi/content/full/108/06/879/DC2). Thus, the pre-BCR is not essential for VH selection, and intrinsic genetic factors are responsible for specific VH, D, and JH gene usage in human B cells.
IgH CDR3 selection by Igμ.
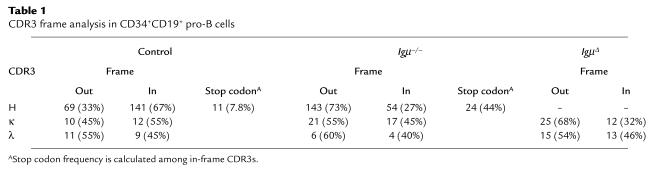
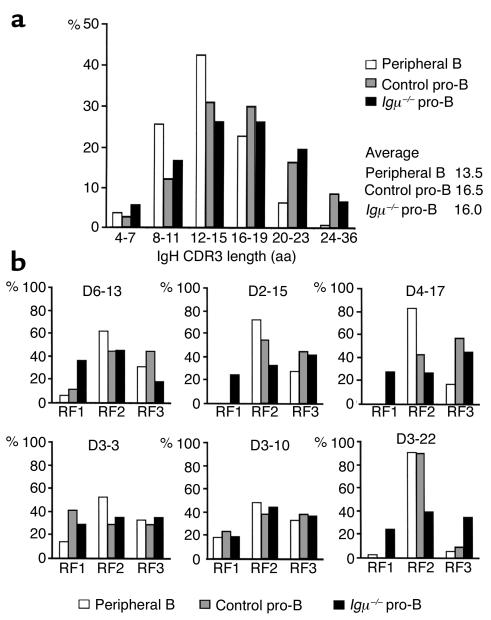
To determine whether Igμ expression influences CDR3 selection, CDR3 length and amino acid composition were analyzed in Igμ–/– pro-B cells and compared with control pro-B and peripheral B cells. About two-thirds of IgH genes were out of frame in Igμ–/– pro-B cells, confirming the absence of Igμ-mediated selection in these cells (Table 1) (20). In contrast, two-thirds of IgH CDR3s were found to be in-frame in control CD34+CD19+ precursor cells, suggesting early Igμ-mediated positive selection of a subpopulation of CD34+CD19+ cells that express Igμ (Table 1). We compared IgH genes expressed by Igμ–/– pro-B cells to the in-frame IgH genes expressed by control CD34+CD19+ cells and found an average CDR3 length of 16.0 and 16.5 amino acids, respectively, whereas peripheral B cells showed an average CDR3 length of 13.5 amino acids (Figure 2a). D-D fusions that increase the length of CDR3 were found in about 2% (4 of 198) of the IgH sequences from Igμ–/– pro-B cells and in 2.6% (3 of 117) of those from control CD34+CD19+ cells, but were absent in peripheral B cells (49, 50) (data not shown). We conclude that IgH CDR3 length is not selected in early B cell precursors and that long CDR3s and D-D fusions are counterselected during late stages of B cell development.
Table 1.
CDR3 frame analysis in CD34+CD19+ pro-B cells
Figure 2.
IgH CDR3 characteristics in pro-B cells. (a) VHDJH CDR3 length in 350 peripheral B cell (white bars), 117 in-frame control pro-B (gray bars), and 197 Igμ–/– (black bars) individual sequences. CDR3 length in amino acids (aa) is indicated below. The average CDR3 length for peripheral B, control pro-B, and Igμ–/– pro-B cells was 13.5, 16.5, and 16.0 amino acids, respectively. (b) D reading frame usage in IgH CDR3s from peripheral B, control, and Igμ–/– pro-B cells. The three RF uses reported by Corbett et al. (50) for some commonly used D gene segments are represented. D3-3 and D3-10 encode no intragenic stop codons whereas D6-13, D2-15, D4-17, and D3-22 sequences using RF1 display stop codons.
D segments can be used in three different reading frames, but in humans, RF1 tends to encode stop codons, RF2 usually encodes glycine residues, and hydrophilic amino acids and RF3 is biased to encode hydrophobic sequences (50). D segments in RF1 are normally under-represented in peripheral B cells and were counterselected in control CD34+CD19+ cells (Figure 2b, top and bottom row, and Table 1). In contrast, there was neither RF selection nor stop codon counter-selection in Igμ–/– pro-B cells (Figure 2b, top and bottom row, and Table 1). D3-3 or D3-10 genes that do not contain intragenic stop codons in RF1 were used in all three RFs in Igμ–/–, control CD34+CD19+, or peripheral B cells (Figure 2b, middle row). In addition, hydrophilic (RF2) and hydrophobic (RF3) Ds were used equally in Igμ–/– and control CD34+CD19+ cells whereas RF2 was favored in peripheral B cells (Figure 2b) (31, 50). However, D3-22 RF usage was already selected in control CD34+CD19+ cells since RF3 was clearly counterselected (and/or RF2 positively selected) when functional Igμ chains were generated (Figure 2b, bottom row). Thus, IgH CDR3s containing stop codons are counterselected in early B cell precursors whereas CDR3s with hydrophilic or hydrophobic RF are not.
Ongoing Igκ recombination in Igμ-deficient pro-B cells.
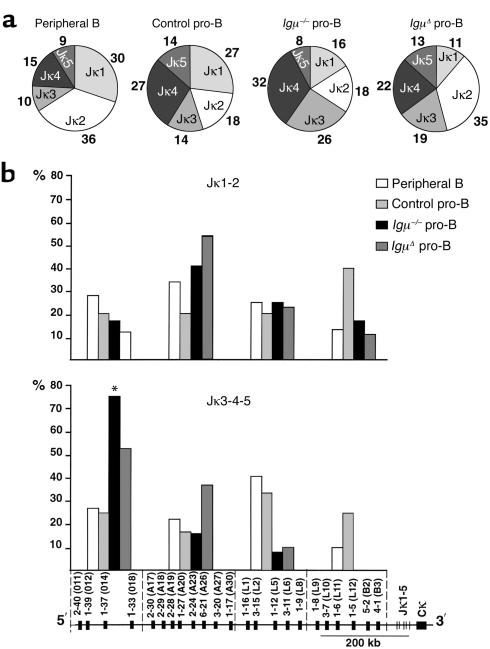
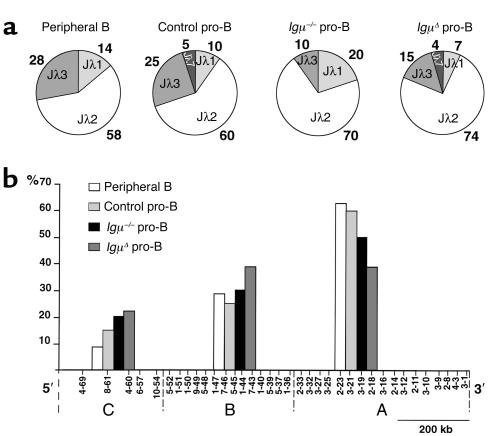
To characterize light chain gene expression in human pro-B cells, we amplified and sequenced Igκ mRNAs from Igμ–/– and IgμΔ pro-B cells and compared them with those of control pro-B and peripheral B cells (49). We found that Igκ mRNAs expressed in Igμ–/– and IgμΔ pro-B cells showed decreased Jκ1 and increased downstream Jκ3 usage when compared with normal B cell controls (Figure 3a). In addition, Jκ3-4-5 segments from Igμ–/– and IgμΔ pro-B cells were preferentially combined with upstream Vκs (P = 0.005, Figure 3b) whereas there was no such bias in the Jκ3-4-5 Igκ mRNAs from normal pro-B cell and peripheral B cell controls. In contrast, there was no bias in Vκ gene usage for Igκ genes using Jκ1-2 segments in patients and controls (Figure 3b). We conclude that in the absence of Igμ there is a shift in the Igκ repertoire to downstream Jκs and upstream Vκs consistent with secondary Igκ rearrangement in pro-B cells.
Figure 3.
Igκ light chain repertoire in pro-B cells. (a) Jκ usage in 108 peripheral B, 22 control pro-B, 38 Igμ–/–, and 37 IgμΔ pro-B VκJκ individual sequences. Percentages of Jκ usage are indicated. (b) Vκ usage in upstream Jκ1 and Jκ2 (Jκ1-2; top) and downstream Jκ3, Jκ4, and Jκ5 (Jκ3-4-5; bottom) rearrangements of peripheral B cells (white bars), control pro-B (light gray bars), Igμ–/– (black bars), and IgμΔ (darkgray bars) pro-B cells. The Vκ genes are subdivided in four groups on the locus (84). The percentages of each Vκ group are indicated on the y axis. *Statistically significant difference (P < 0.001).
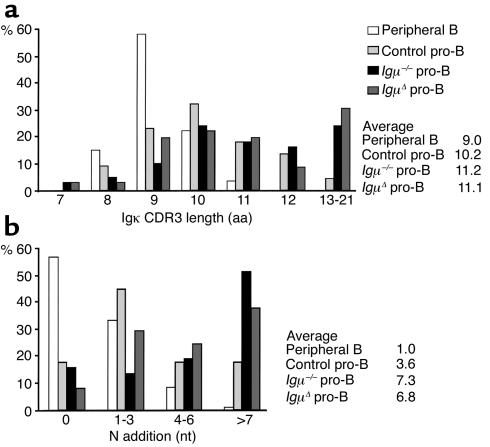
Igκ CDR3 analysis revealed that the ratio of productive to nonproductive Vκ-Jκ joints in human pro-B cells was similar to that reported for mIgμ-deficient mouse (μMT) pro-B cells (54). Igκ CDR3 length was increased in all pro-B cell samples when compared with peripheral B cells (Table 1 and Figure 4a). We found that Igκ CDR3s from control pro-B cells and from the patients had an average of 10.2, 11.2, or 11.1 amino acids whereas Igκ CDR3 from peripheral B cells were only 9.0 amino acids long (Figure 4a). The increased CDR3 length in pro-B cells was due to terminal deoxynucleotidyl transferase-catalyzed (TdT-catalyzed) addition of template-independent (N) nucleotides and not to template-dependent P nucleotides (Figure 4b). On average, 3.6, 7.3, and 6.8 nucleotides were added by TdT to Igκ gene CDR3s in control, Igμ–/–, and IgμΔ pro-B cells whereas only 1 N nucleotide was found in Igκ genes expressed by peripheral B cells (Figure 4b). We conclude that pro-B cells produce Igκ genes with unusually long CDR3s.
Figure 4.
Igκ CDR3 characteristics in pro-B cells. (a) Igκ CDR3 length in amino acids and (b) N nucleotide (nt) addition in peripheral B cells (white bars), control pro-B (light gray bars), Igμ–/– (black bars), and IgμΔ (dark gray bars) pro-B cells. For determination of N nucleotide addition, P nucleotides were not included. The average CDR3 length and N nucleotide addition for peripheral B, control pro-B, Igμ–/–, and IgμΔ pro-B cells was 9.0, 10.2, 11.2, and 11.1 amino acids and 1.0, 3.6, 7.3, and 6.8 nucleotides, respectively.
Igλ repertoire of Igμ-deficient pro-B cells.
To determine whether Igλ genes expressed in Igμ-deficient pro-B cells displayed features similar to Igκ genes we analyzed the Igλ repertoire. Although the human Igλ locus should allow deletional replacement of VJλs by secondary recombination (55), we found no significant increases in either downstream Jλ or distal Vλ segment usage in Igμ–/– and IgμΔ pro-B cells. In fact, there was a decrease in downstream Jλ3 segment usage (56, 57) (Figure 5, a and b).
Figure 5.
Igλ light chain repertoire in pro-B cells. (a) Jλ usage in 163 peripheral B, 20 control pro-B, 10 Igμ–/–, and 28 IgμΔ pro-B VλJλ individual sequences. Percentages of Jλ usage are indicated. (b) Vλ usage in peripheral B cells (white bars), control pro-B (light gray bars), Igμ–/– (black bars), and IgμΔ (dark gray bars) pro-B cells. The Vλ locus is shown clustered into three groups — A, B, and C — of Vλ genes (84). The percentages of each Vλ group are indicated on the y axis.
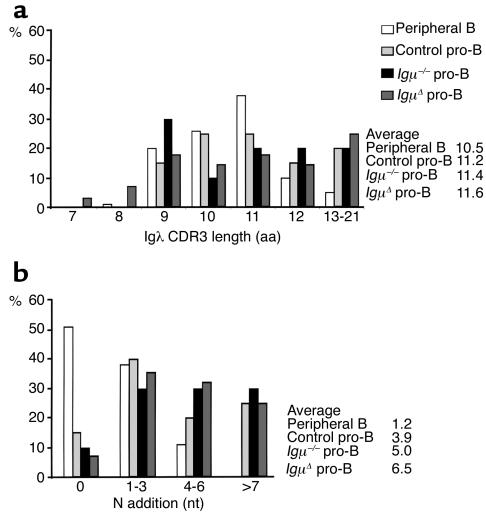
Igλ genes expressed in control, Igμ–/–, and IgμΔ pro-B cells resembled Igκ genes in that they showed a similar ratio of in-frame and out-of-frame sequences and long CDR3s, resulting from addition of N nucleotides by TdT (Figure 6, a and b, and Table 1). The average number of N nucleotides in Igλ CDR3s from control, Igμ–/–, and IgμΔ pro-B cells was 3.9, 5.0, and 6.5 as compared with 1.2 for mature B cells (Figure 6b). We conclude that the Igλ genes expressed by Igμ–/– and IgμΔ pro-B cells differ from Igκ genes in that they show no signs of secondary recombination, but they resemble Igκ genes in that they display long CDR3s with extensive N addition.
Figure 6.
Igλ CDR3 characteristics in pro-B cells. (a) Igλ CDR3 length in amino acids and (b) N nucleotide addition in peripheral B cells (white bars), control pro-B (light gray bars), Igμ–/– (black bars), and IgμΔ (dark gray bars) pro-B cells. The average CDR3 length and N nucleotide addition for peripheral B, control pro-B, Igμ–/–, and IgμΔ pro-B cells was 10.5, 11.2, 11.4, and 11.6 amino acids and 1.2, 3.9, 5.0, and 6.5 nucleotides, respectively.
Discussion
The absence of Igμ in Igμ–/– and IgμΔ patients provided an opportunity to study the role of Igμ in selecting the Ab repertoire in humans. We found no significant differences in the Ig heavy chain gene VH, D, or JH repertoire between Ig-deficient pro-B cells, control pro-B cells, and normal peripheral B cells. These findings are in agreement with cell-sorting experiments in which normal pro-B, pre-B, and immature B cell repertoires were compared with that of peripheral B cells (19–21, 31). Thus, VH, D, and JH segment usage in humans is independent of Igμ expression and is likely to be a function of intrinsic genetic elements controlling VH, D, or JH gene accessibility and recombination. In contrast, IgH CDR3 length appears to be selected throughout B cell development starting with CD34–CD19+IgM– pre-B cells (33). Our analysis of Igμ–/– and control CD34+CD19+ cells showed that Igμ expression is not involved in IgH CDR3 length and hydrophilic RF selection in the human at the pro-B cell stage. However, in-frame IgH genes without stop codons were enriched in control CD34+CD19+ B cell progenitors, suggesting that an efficient selection process driven by pre-BCRs operates in normal CD34+CD19+ B cell precursors. These Igμ-positive B cell precursors displaying surface pro-B cell markers are likely to be in transition to the pre-B cell stage and equivalent to the mouse C′ early pre-B cell fraction of Hardy’s classification (58). By analogy to Igμ or Igβ knockout mice, the C′ early pre-B cell fraction is missing in Igμ–/– CD34+CD19+ precursor B cells and results in a decrease of in-frame Igμ rearrangements when compared with normal CD34+CD19+ precursor B cells (10, 59).
In the mouse, ψL has been implicated in IgH repertoire selection by virtue of pairing with some but not all VH domains (16, 18). Analysis of the VH repertoire in λ5–/– mice showed that the normal repertoire shift seen between the pro-B and the pre-B cell stage was absent (16). However, differences in pairing efficiency between ψL and IgH are not likely to influence the selection against long or hydrophobic CDR3s in humans because these features are prevalent in control CD34+CD19+ cells, in immature B cells that have passed ψL selection, and in B cells that express ψLs in the periphery (31, 49). A more likely explanation for selection against long and hydrophobic IgH CDR3s is that these features are associated with self-reactivity and might also interfere directly with IgH and IgL pairing (31, 49, 60, 61). Immature B cells displaying such Ab’s therefore would be silenced by deletion or receptor editing, or alternatively, would fail to be positively selected in the mature B cell compartment (39, 40, 42–44, 62–64).
In the mouse, two Igμ-mediated mechanisms account for selection against self-reactive or poorly pairing Ab’s during B cell development, receptor editing and deletion (39–44). Editing makes a major contribution to the Ab repertoire in mice: up to 25% of all Ab’s are produced by editing, but the role of deletion in repertoire selection is not known (65). Our experiments suggest that Igμ-mediated selection also makes a large contribution to shaping the human Ab repertoire. The selection against IgHs with long or hydrophobic CDR3s found in pro-B cells would require loss of at least 20–25% of all heavy chains.
In both mouse and human B cells, V(D)J recombination is generally ordered, starting with IgH rearrangement in pro-B cells followed by IgL rearrangement in pre-B cells (66, 67). However, analysis of mouse Igμ mutants and normal pro-B cells showed that IgL genes can recombine before IgH in pro-B cells (54, 68–71). Our experiments show that human control and Igμ-deficient pro-B cells are similar to their mouse counterparts in that they undergo Igκ and Igλ gene rearrangements. These results are in agreement with the finding of rare IgL chain gene recombination in normal human pro-B cells and in Epstein-Barr virus–transformed fetal B cell precursors (67, 72). Thus, IgL rearrangement in the human is similar to the mouse in that it is not strictly dependent on Igμ expression or pre-B cell development.
Up to 50% of human light chains in normal peripheral B cells show N nucleotide addition, but Igκ or Igλ CDR3s are never as long as 11 or 13 amino acids, respectively (49, 73–76). Igκ or Igλ genes found in control and Igμ-deficient pro-B cells differ from those found in normal peripheral B cells in that they show extensive N nucleotide addition associated with long IgL CDR3s that can reach up to 21 amino acids. Thus, long IgL CDR3s are produced in early developing B cells but they appear to be incompatible with B cell development and are deleted from the mature peripheral B cell repertoire.
Igμ-deficient pro-B cells express Igκ genes that display a bias to 3′ Jκs and 5′ Vκs consistent with persistent V(D)J recombination. However, the bias to 3′ Jκs was incomplete since there was no significant increase in Jκ5, the most downstream Jκ segment. In addition, there was no bias to downstream Jλs despite a genomic configuration that allows secondary rearrangements (55, 77, 78). We speculate that in the absence of Igμ, human pro-B cells undergo several rounds of recombination on Igκ but do not survive long enough to allow extensive secondary recombination. The absence of secondary recombination on Igλ in Igμ-deficient pro-B cells may result from a delayed recombination of this locus when compared with Igκ (79, 80). Alternatively, secondary recombination may be less efficient for Igλ than Igκ.
Secondary Igκ recombination is prominent in Igμ-deficient pro-B cells yet not in normal control pro-B cells. Secondary recombination in Igμ-deficient pro-B cells therefore appears to be a default mechanism in the absence of Igμ expression, and termination of secondary recombination in developing B cells requires BCR signaling. We speculate that the regulation of Ig light chain gene recombination during B cell development resembles that of T cell receptor-α (TCR-α) chains during T cell development in that recombination is terminated by a yet to be determined positive selection signal transduced by the BCR (81). Developing B cells remain in the pre-B cell compartment for a few hours whereas developing T cells remain in the CD4+CD8+ double-positive compartment for 3–4 days (65, 82, 83). This kinetic difference may explain why Ig light chains are allelic excluded in B cells whereas TCR-α chains in T cells are not.
Supplementary Material
Acknowledgments
We thank M.J. Shlomchik and T.-A. Yang for comments on the manuscript and A. Deville, B. Lemmers, and J. Castro e Melo for help with bone marrow samples.
References
- 1.Pillai S, Baltimore D. Formation of disulfide-linked μ2ω2 tetramers in pre-B cells by the 18k ω-immunoglobulin light chain. Nature. 1987;329:172–174. doi: 10.1038/329172a0. [DOI] [PubMed] [Google Scholar]
- 2.Kerr WG, Cooper MD, Feng L, Burrows PD, Hendershot LM. Mu heavy chains can associate with a pseudo-light chain complex (ΨL) in human pre-B cell lines. Int Immunol. 1989;1:355–361. doi: 10.1093/intimm/1.4.355. [DOI] [PubMed] [Google Scholar]
- 3.Karasuyama H, Kudo A, Melchers F. The proteins encoded by the VpreB and lambda 5 pre-B cell-specific genes can associate with each other and with mu heavy chain. J Exp Med. 1990;172:969–972. doi: 10.1084/jem.172.3.969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tsubata T, Reth M. The products of pre-B cell-specific genes (lambda 5 and VpreB) and the immunoglobulin mu chain form a complex that is transported onto the cell surface. J Exp Med. 1990;172:973–976. doi: 10.1084/jem.172.3.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hombach J, Leclercq L, Radbruch A, Rajewsky K, Reth M. A novel 34-kd protein co-isolated with the IgM molecule in surface IgM-expressing cells. EMBO J. 1988;7:3451–3456. doi: 10.1002/j.1460-2075.1988.tb03219.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hermanson GG, Eisenberg D, Kincade PW, Wall R. B29: a member of the immunoglobulin gene superfamily exclusively expressed on B-lineage cells. Proc Natl Acad Sci USA. 1988;85:6890–6894. doi: 10.1073/pnas.85.18.6890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grawunder U, et al. Down-regulation of RAG1 and RAG2 gene expression in preB cells after functional immunoglobulin heavy chain rearrangement. Immunity. 1995;3:601–608. doi: 10.1016/1074-7613(95)90131-0. [DOI] [PubMed] [Google Scholar]
- 8.Kitamura D, Roes J, Kuhn R, Rajewsky K. A B cell deficient mouse by targeted disruption of the membrane exons of the immunoglobulin μ chain gene. Nature. 1991;350:423–426. doi: 10.1038/350423a0. [DOI] [PubMed] [Google Scholar]
- 9.Kitamura D, et al. A critical role of λ5 protein in B cell development. Cell. 1992;69:823–831. doi: 10.1016/0092-8674(92)90293-l. [DOI] [PubMed] [Google Scholar]
- 10.Gong S, Nussenzweig MC. Regulation of an early developmental checkpoint in the B cell pathway by Ig beta. Science. 1996;272:411–414. doi: 10.1126/science.272.5260.411. [DOI] [PubMed] [Google Scholar]
- 11.Mundt C, Licence S, Shimizu T, Melchers F, Martensson IL. Loss of Precursor B Cell Expansion but Not Allelic Exclusion in VpreB1/VpreB2 Double-deficient Mice. J Exp Med. 2001;193:435–446. doi: 10.1084/jem.193.4.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yel L, et al. Mutations in the mu heavy-chain gene in patients with agammaglobulinemia. N Engl J Med. 1996;335:1486–1493. doi: 10.1056/NEJM199611143352003. [DOI] [PubMed] [Google Scholar]
- 13.Schiff C, Lemmers B, Deville A, Fougereau M, Meffre E. Autosomal primary immunodeficiencies affecting human bone marrow B cell differentiation. Immunol Rev. 2000;178:91–98. doi: 10.1034/j.1600-065x.2000.17804.x. [DOI] [PubMed] [Google Scholar]
- 14.Minegishi Y, et al. Mutations in the human lambda5/14.1 gene result in B cell deficiency and agammaglobulinemia. J Exp Med. 1998;187:71–77. doi: 10.1084/jem.187.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Minegishi Y, et al. Mutations in Igalpha (CD79a) result in a complete block in B-cell development. J Clin Invest. 1999;104:1115–1121. doi: 10.1172/JCI7696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.ten Boekel E, Melchers F, Rolink AG. Changes in the V(H) gene repertoire of developing precursor B lymphocytes in mouse bone marrow mediated by the pre-B cell receptor. Immunity. 1997;7:357–368. doi: 10.1016/s1074-7613(00)80357-x. [DOI] [PubMed] [Google Scholar]
- 17.ten Boekel E, Melchers F, Rolink AG. Precursor B cells showing H chain allelic inclusion display allelic exclusion at the level of pre-B cell receptor surface expression. Immunity. 1998;8:199–207. doi: 10.1016/s1074-7613(00)80472-0. [DOI] [PubMed] [Google Scholar]
- 18.Wasserman R, et al. A novel mechanism for B cell repertoire maturation based on response by B cell precursors to pre-B receptor assembly. J Exp Med. 1998;187:259–264. doi: 10.1084/jem.187.2.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kraj P, et al. The human heavy chain Ig V region gene repertoire is biased at all stages of B cell ontogeny, including early pre-B cells. J Immunol. 1997;158:5824–5832. [PubMed] [Google Scholar]
- 20.Rao SP, et al. Biased VH gene usage in early lineage human B cells: evidence for preferential Ig gene rearrangement in the absence of selection. J Immunol. 1999;163:2732–2740. [PubMed] [Google Scholar]
- 21.Milili M, Schiff C, Fougereau M, Tonnelle C. The VDJ repertoire expressed in human preB cells reflects the selection of bona fide heavy chains. Eur J Immunol. 1996;26:63–69. doi: 10.1002/eji.1830260110. [DOI] [PubMed] [Google Scholar]
- 22.Schroeder HW, Jr, Hillson JL, Perlmutter RM. Early restriction of the human antibody repertoire. Science. 1987;238:791–793. doi: 10.1126/science.3118465. [DOI] [PubMed] [Google Scholar]
- 23.Schroeder HW, Jr, Wang JY. Preferential utilization of conserved immunoglobulin heavy chain variable gene segments during human fetal life. Proc Natl Acad Sci USA. 1990;87:6146–6150. doi: 10.1073/pnas.87.16.6146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hillson JL, et al. Emerging human B cell repertoire. Influence of developmental stage and interindividual variation. J Immunol. 1992;149:3741–3752. [PubMed] [Google Scholar]
- 25.Cuisinier AM, Gauthier L, Boubli L, Fougereau M, Tonnelle C. Mechanisms that generate human immunoglobulin diversity operate from the 8th week of gestation in fetal liver. Eur J Immunol. 1993;23:110–118. doi: 10.1002/eji.1830230118. [DOI] [PubMed] [Google Scholar]
- 26.Pascual V, Capra JD. Human immunoglobulin heavy-chain variable region genes: organization, polymorphism, and expression. Adv Immunol. 1991;49:1–74. doi: 10.1016/s0065-2776(08)60774-9. [DOI] [PubMed] [Google Scholar]
- 27.Huang C, Stewart AK, Schwartz RS, Stollar BD. Immunoglobulin heavy chain gene expression in peripheral blood B lymphocytes. J Clin Invest. 1992;89:1331–1343. doi: 10.1172/JCI115719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Milner EC, Hufnagle WO, Glas AM, Suzuki I, Alexander C. Polymorphism and utilization of human VH Genes. Ann N Y Acad Sci. 1995;764:50–61. doi: 10.1111/j.1749-6632.1995.tb55806.x. [DOI] [PubMed] [Google Scholar]
- 29.Brezinschek HP, Brezinschek RI, Lipsky PE. Analysis of the heavy chain repertoire of human peripheral B cells using single-cell polymerase chain reaction. J Immunol. 1995;155:19–202. [PubMed] [Google Scholar]
- 30.Brezinschek HP, et al. Analysis of the human VH gene repertoire. Differential effects of selection and somatic hypermutation on human peripheral CD5(+)/IgM+ and CD5(-)/IgM+ B cells. J Clin Invest. 1997;99:2488–2501. doi: 10.1172/JCI119433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Raaphorst FM, Raman CS, Tami J, Fischbach M, Sanz I. Human Ig heavy chain CDR3 regions in adult bone marrow pre-B cells display an adult phenotype of diversity: evidence for structural selection of DH amino acid sequences. Int Immunol. 1997;9:1503–1515. doi: 10.1093/intimm/9.10.1503. [DOI] [PubMed] [Google Scholar]
- 32.Dorner T, et al. Analysis of the frequency and pattern of somatic mutations within nonproductively rearranged human variable heavy chain genes. J Immunol. 1997;158:2779–2789. [PubMed] [Google Scholar]
- 33.Shiokawa S, et al. IgM heavy chain complementarity-determining region 3 diversity is constrained by genetic and somatic mechanisms until two months after birth. J Immunol. 1999;162:6060–6070. [PubMed] [Google Scholar]
- 34.Alt FW, et al. Ordered rearrangement of immunoglobulin heavy chain variable region segments. EMBO J. 1984;3:1209–1219. doi: 10.1002/j.1460-2075.1984.tb01955.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Reth M, Petrac E, Wiese P, Lobel L, Alt FW. Activation of V kappa gene rearrangement in pre-B cells follows the expression of membrane-bound immunoglobulin heavy chains. EMBO J. 1987;6:3299–3305. doi: 10.1002/j.1460-2075.1987.tb02649.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schlissel MS, Baltimore D. Activation of immunoglobulin kappa gene rearrangement correlates with induction of germline kappa gene transcription. Cell. 1989;58:1001–1007. doi: 10.1016/0092-8674(89)90951-3. [DOI] [PubMed] [Google Scholar]
- 37.Iglesias A, Kopf M, Williams GS, Buhler B, Kohler G. Molecular requirements for the mu-induced light chain gene rearrangement in pre-B cells. EMBO J. 1991;10:2147–2155. doi: 10.1002/j.1460-2075.1991.tb07749.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tsubata T, Tsubata R, Reth M. Crosslinking of the cell surface immunoglobulin (mu-surrogate light chains complex) on pre-B cells induces activation of V gene rearrangements at the immunoglobulin kappa locus. Int Immunol. 1992;4:637–641. doi: 10.1093/intimm/4.6.637. [DOI] [PubMed] [Google Scholar]
- 39.Nemazee DA, Bürki K. Clonal deletion of B lymphocytes in a transgenic mouse bearing anti-MHC class I antibody genes. Nature. 1989;337:562–566. doi: 10.1038/337562a0. [DOI] [PubMed] [Google Scholar]
- 40.Hartley SB, et al. Elimination from peripheral lymphoid tissues of self-reactive B lymphocytes recognizing membrane-bound antigens. Nature. 1991;353:765–769. doi: 10.1038/353765a0. [DOI] [PubMed] [Google Scholar]
- 41.Okamoto M, et al. A transgenic model of autoimmune hemolytic anemia. J Exp Med. 1992;175:71–79. doi: 10.1084/jem.175.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tiegs SL, Russell DM, Nemazee D. Receptor editing in self-reactive bone marrow B cells. J Exp Med. 1993;177:1009–1020. doi: 10.1084/jem.177.4.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gay D, Saunders T, Camper S, Weigert M. Receptor editing: an approach by autoreactive B cells to escape tolerance. J Exp Med. 1993;177:999–1008. doi: 10.1084/jem.177.4.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Radic MZ, Erickson J, Litwin S, Weigert M. B lymphocytes may escape tolerance by revising their antigen receptors. J Exp Med. 1993;177:1165–1173. doi: 10.1084/jem.177.4.1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yamagami T, et al. Four of five RAG-expressing JCkappa–/– small pre-BII cells have no L chain gene rearrangements: detection by high-efficiency single cell PCR. Immunity. 1999;11:309–316. doi: 10.1016/s1074-7613(00)80106-5. [DOI] [PubMed] [Google Scholar]
- 46.Meffre E, et al. A human non-XLA immunodeficiency disease characterized by blockage of B cell development at an early proB cell stage. J Clin Invest. 1996;98:1519–1526. doi: 10.1172/JCI118943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Meffre E, et al. A non-XLA primary deficiency causes the earliest known defect of B cell differentiation in humans: a comparison with an XLA case. Immunol Lett. 1997;57:93–99. doi: 10.1016/s0165-2478(97)00052-7. [DOI] [PubMed] [Google Scholar]
- 48.Fais F, et al. Chronic lymphocytic leukemia B cells express restricted sets of mutated and unmutated antigen receptors. J Clin Invest. 1998;102:1515–1525. doi: 10.1172/JCI3009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Meffre E, et al. Circulating human B cells that express surrogate light chains and edited receptors. Nat Immunol. 2000;1:207–213. doi: 10.1038/79739. [DOI] [PubMed] [Google Scholar]
- 50.Corbett SJ, Tomlinson IM, Sonnhammer ELL, Buck D, Winter G. Sequence of the human immunoglobulin diversity (D) segment locus: a systematic analysis provides no evidence for the use of DIR segments, inverted D segments, “minor” D segments or D-D recombination. J Mol Biol. 1997;270:587–597. doi: 10.1006/jmbi.1997.1141. [DOI] [PubMed] [Google Scholar]
- 51.Kabat, E.A., Wu, T.T., Perry, H.M., Gottesman, K.S., and Foeller, C. 1991. Sequences of proteins of immunological interest. US Department of Health and Human Services. Bethesda, Maryland, USA. 2387 pp.
- 52.Desiderio SV, et al. Insertion of N regions into heavy-chain genes is correlated with expression of terminal deoxytransferase in B cells. Nature. 1984;311:752–755. doi: 10.1038/311752a0. [DOI] [PubMed] [Google Scholar]
- 53.Lafaille JJ, DeCloux A, Bonneville M, Takagaki Y, Tonegawa S. Junctional sequences of T cell receptor gamma delta genes: implications for gamma delta T cell lineages and for a novel intermediate of V-(D)-J joining. Cell. 1989;59:859–870. doi: 10.1016/0092-8674(89)90609-0. [DOI] [PubMed] [Google Scholar]
- 54.Bentolila LA, et al. Extensive junctional diversity in Ig light chain genes from early B cell progenitors of mu MT mice. J Immunol. 1999;162:2123–2128. [PubMed] [Google Scholar]
- 55.Vasicek TJ, Leder P. Structure and expression of the human immunoglobulin lambda genes. J Exp Med. 1990;172:609–620. doi: 10.1084/jem.172.2.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Frippiat JP, et al. Organization of the human immunoglobulin lambda light-chain locus on chromosome 22q11.2. Hum Mol Genet. 1995;4:983–991. doi: 10.1093/hmg/4.6.983. [DOI] [PubMed] [Google Scholar]
- 57.Williams SC, et al. Sequence and evolution of the human germline V lambda repertoire. J Mol Biol. 1996;264:220–232. doi: 10.1006/jmbi.1996.0636. [DOI] [PubMed] [Google Scholar]
- 58.Hardy RR, Carmack CE, Shinton SA, Kemp JD, Hayakawa K. Resolution and characterization of pro-B and pre-pro-B cell stages in normal mouse bone marrow. J Exp Med. 1991;172:1213–1225. doi: 10.1084/jem.173.5.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Reichlin A, et al. B cell development is arrested at the immature b cell stage in mice carrying a mutation in the cytoplasmic domain of immunoglobulin beta. J Exp Med. 2001;193:13–24. doi: 10.1084/jem.193.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Klonowski KD, Primiano LL, Monestier M. Atypical VH-D-JH rearrangements in newborn autoimmune MRL mice. J Immunol. 1999;162:1566–1572. [PubMed] [Google Scholar]
- 61.Schroeder HW, Kirkham PM. Marriage, divorce, and promiscuity in human B cells. Nat Immunol. 2000;1:187–188. doi: 10.1038/79717. [DOI] [PubMed] [Google Scholar]
- 62.Sandel PC, Monroe JG. Negative selection of immature B cells by receptor editing or deletion is determined by site of antigen encounter. Immunity. 1999;10:289–299. doi: 10.1016/s1074-7613(00)80029-1. [DOI] [PubMed] [Google Scholar]
- 63.Gu H, Tarlinton D, Muller W, Rajewsky K, Forster I. Most peripheral B cells in mice are ligand selected. J Exp Med. 1991;173:1357–1371. doi: 10.1084/jem.173.6.1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Levine MH, et al. A B-cell receptor-specific selection step governs immature to mature B cell differentiation. Proc Natl Acad Sci USA. 2000;97:2743–2748. doi: 10.1073/pnas.050552997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Casellas R, et al. Contribution of receptor editing to the antibody repertoire. Science. 2001;291:1541–1544. doi: 10.1126/science.1056600. [DOI] [PubMed] [Google Scholar]
- 66.Blackwell TK, et al. Isolation of scid pre-B cells that rearrange kappa light chain genes: formation of normal signal and abnormal coding joins. EMBO J. 1989;8:735–742. doi: 10.1002/j.1460-2075.1989.tb03433.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ghia P, et al. Ordering of human bone marrow B lymphocyte precursors by single-cell polymerase chain reaction analyses of the rearrangement status of the immunoglobulin H and L chain loci. J Exp Med. 1996;184:2217–2229. doi: 10.1084/jem.184.6.2217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ehlich A, et al. Immunoglobulin heavy and light chain genes rearrange independently at early stages of B cell development. Cell. 1993;72:695–704. doi: 10.1016/0092-8674(93)90398-a. [DOI] [PubMed] [Google Scholar]
- 69.Chen J, et al. Immunoglobulin gene rearrangement in B cell deficient mice generated by targeted deletion of the JH locus. Int Immunol. 1993;5:647–656. doi: 10.1093/intimm/5.6.647. [DOI] [PubMed] [Google Scholar]
- 70.Grawunder U, Haasner D, Melchers F, Rolink A. Rearrangement and expression of kappa light chain genes can occur without mu heavy chain expression during differentiation of pre-B cells. Int Immunol. 1993;5:1609–1618. doi: 10.1093/intimm/5.12.1609. [DOI] [PubMed] [Google Scholar]
- 71.Novobrantseva TI, et al. Rearrangement and expression of immunoglobulin light chain genes can precede heavy chain expression during normal B cell development in mice. J Exp Med. 1999;189:75–88. doi: 10.1084/jem.189.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kubagawa H, Cooper MD, Carroll AJ, Burrows PD. Light-chain gene expression before heavy-chain gene rearrangement in pre-B cells transformed by Epstein-Barr virus. Proc Natl Acad Sci USA. 1989;86:2356–2360. doi: 10.1073/pnas.86.7.2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Klein R, Jaenichen R, Zachau HG. Expressed human immunoglobulin kappa genes and their hypermutation. Eur J Immunol. 1993;23:3248–3262. doi: 10.1002/eji.1830231231. [DOI] [PubMed] [Google Scholar]
- 74.Klein U, Kuppers R, Rajewsky K. Human IgM+IgD+ B cells, the major B cell subset in the peripheral blood, express V kappa genes with no or little somatic mutation throughout life. Eur J Immunol. 1993;23:3272–3277. doi: 10.1002/eji.1830231232. [DOI] [PubMed] [Google Scholar]
- 75.Fischer M, Klein U, Kuppers R. Molecular single-cell analysis reveals that CD5-positive peripheral blood B cells in healthy humans are characterized by rearranged Vkappa genes lacking somatic mutation. J Clin Invest. 1997;100:1667–1676. doi: 10.1172/JCI119691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Foster SJ, Brezinschek HP, Brezinschek RI, Lipsky PE. Molecular mechanisms and selective influences that shape the kappa gene repertoire of IgM+ B cells. J Clin Invest. 1997;99:1614–1627. doi: 10.1172/JCI119324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Berinstein N, Levy S, Levy R. Activation of an excluded immunoglobulin allele in a human B lymphoma cell line. Science. 1989;244:337–339. doi: 10.1126/science.2496466. [DOI] [PubMed] [Google Scholar]
- 78.Stiernholm NB, Berinstein NL. Up-regulated recombination-activating gene expression in sIg- variants of a human mature B cell line undergoing secondary Ig lambda rearrangements in cell culture. Eur J Immunol. 1993;23:1501–1507. doi: 10.1002/eji.1830230716. [DOI] [PubMed] [Google Scholar]
- 79.Hieter PA, Korsmeyer SJ, Waldmann TA, Leder P. Human immunoglobulin kappa light-chain genes are deleted or rearranged in lambda-producing B cells. Nature. 1981;290:368–372. doi: 10.1038/290368a0. [DOI] [PubMed] [Google Scholar]
- 80.Korsmeyer SJ, et al. Developmental hierarchy of immunoglobulin gene rearrangements in human leukemic pre-B-cells. Proc Natl Acad Sci USA. 1981;78:7096–7100. doi: 10.1073/pnas.78.11.7096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Borgulya P, Kishi H, Uematsu Y, Boehmer HV. Exclusion and inclusion of a and b T cell receptor alleles. Cell. 1992;69:529–537. doi: 10.1016/0092-8674(92)90453-j. [DOI] [PubMed] [Google Scholar]
- 82.Huesmann M, Scott B, Kisielow P, von Boehmer H. Kinetics and efficacy of positive selection in the thymus of normal and T cell receptor transgenic mice. Cell. 1991;66:533–540. doi: 10.1016/0092-8674(81)90016-7. [DOI] [PubMed] [Google Scholar]
- 83.Malissen M, et al. A T cell clone expresses two T cell receptor alpha genes but uses one alpha beta heterodimer for allorecognition and self MHC-restricted antigen recognition. Cell. 1988;55:49–59. doi: 10.1016/0092-8674(88)90008-6. [DOI] [PubMed] [Google Scholar]
- 84.Lefranc MP. Locus maps and genomic repertoire of the human Ig genes. The Immunologist. 2000;8:80–87. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.