Upon primary immunization, antigen-specific T cells proliferate, generating a large number of effector cells that migrate to peripheral tissues to fight pathogens. Some of these primed T cells develop into memory cells, which confer immediate protection as well as the capacity to mount a more rapid and effective secondary immune response. Understanding the multiple facets of immunological memory and its relationship to protection remains a central issue in immunology (1, 2).
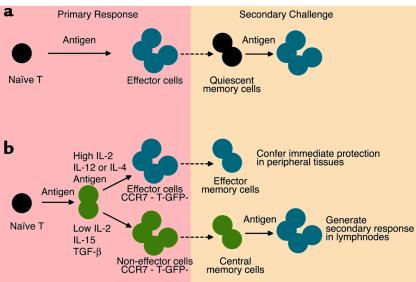
Memory T cells have been traditionally viewed as survivors of effector cells that revert to a quiescent state (Figure 1a). However, memory T cells have been shown to be heterogenous and to comprise at least two subsets, endowed with different migratory capacity and effector function (3–5). Cells of the first subset resemble the effector cells generated in the primary response in that they lack the lymph node–homing receptors L-selectin and CCR7 and express receptors for migration into inflamed tissues. Upon re-encounter with antigen, these “effector memory T cells” (TEM) can rapidly produce IFN-γ or IL-4 or release pre-stored perforin. Cells of the second subset express L-selectin and CCR7 as naive T cells and lack immediate effector function. These “central memory T cells” (TCM) have a low activation threshold and, upon restimulation in secondary lymphoid organs, proliferate and differentiate to effectors (6).
Figure 1.
Pathways for memory T cell generation. (a) According to the most conventional model, memory T cells are derived from effector cells that revert to a quiescent state. (b) According to the new model of progressive T cell differentiation, the duration of antigenic stimulation and the type and amount of cytokines present during priming lead either to fully differentiated effector cells that home to peripheral tissues (blue) or to intermediate cells that are devoid of effector function and home to lymph nodes (green). In the system used by Manjunath et al. (7), these two cell types can be identified according to the differential expression of the T-GFP marker transgene and the lymph node–homing receptor CCR7. Both cell types are maintained in the memory pool (dotted arrows) and, upon secondary challenge, mediate immediate protection in nonlymphoid tissues or secondary responses in lymph nodes.
An outstanding question with broad basic and practical implication relates to the signals that drive the formation of the two types of memory T cells. In this issue of the JCI, Manjunath et al. show that cytokines can modulate the differentiation of CD8+ T cells (7). Taking advantage of an earlier serendipitous discovery — that a green fluorescent protein reporter gene (T-GFP) is expressed in naive and short-term activated T cells is silent in terminally differentiated effector cells (8) — the authors have defined conditions for the expansion of almost pure populations of TCM or TEM (7). In these transgenic mice GFP expression can be conveniently used to monitor T cell differentiation.
Now, Manjunath et al. have generated naive T cells from T-GFP mice carrying a class I–restricted T cell receptor (TCR) that recognizes a specific viral peptide (7). They stimulated these cells in vitro with antigen for 2 days and expanded them in the presence of different cytokines. When cultured in high doses of IL-2 (CD8IL-2), the cells become large blasts, express high levels of activation markers, lose expression of GFP and the chemokine receptor CCR7, and acquire the capacity to produce IFN-γ and to kill target cells. In contrast, the same cells cultured in the presence of IL-15 (CD8IL-15), or of low doses of IL-2, become small, retain GFP and CCR7 expression, and fail to acquire cytotoxic function, although they acquire IFN-γ–producing capacity. Importantly, after adoptive transfer, CD8IL-15 cells survive for several weeks and, upon antigen rechallenge, mount a secondary response that is comparable to that mediated by endogenously generated memory cells.
The migratory properties of CD8IL-2 and CD8IL-5 are described in an elegant study by the same group, now in press in The Journal of Experimental Medicine (9). This study shows that central memory-like CD8IL-15 cells home avidly to lymphoid organs, where they mediate rapid recall responses; these cells are only moderately efficient at homing to sites of inflammation. Conversely, CD8IL-2 effector-like T cells accumulate in inflamed tissues but are excluded from lymph nodes and Peyer’s patches. Together the two papers (7, 9) indicate that the amount and quality of cytokines can precisely determine the differentiation of CD8+ T cells, altering their effector function and their migratory capacity. Whereas high doses of IL-2 drive terminal differentiation to tissue-homing effector cells, IL-15 preserves the proliferating cells’ ability to home to lymph nodes and maintains the cells in an intermediate state of differentiation characteristic of central memory cells.
Studies on CD4+ T cells have previously supported the model of progressive T cell differentiation shown in Figure 1b (10). For CD4+ T cells the differentiation process is controlled by the interplay between duration of TCR stimulation and cytokines. Thus, while a prolonged TCR stimulation in the presence of IL-12 or IL-4 promotes terminal differentiation to effector Th1 or Th2 cells respectively, a short TCR stimulation and TGF-β preserve the cells in a central memory-like stage (6, 11–13). Thus, the above studies, together with that of Manjunath et al. (7), point to the same conclusion, namely that for both CD4+ and CD8+ T cells terminal differentiation is not a necessary consequence of T cell activation. The generation of intermediates that persist as central memory cells provides the immune system with a strategic reserve of highly sensitive cells that can be rapidly recruited in secondary immune responses to generate large numbers of potent effector cells.
In light of these findings, it will be important to learn whether cytokines and TCR stimulation have the same relative importance in vivo as in this cell culture model. In vivo, access of T cells to antigen-carrying dendritic cells is a stochastic and competitive process, and dendritic cell numbers can vary widely during T cell priming (13, 14). Furthermore, IL-2 is produced only by antigen-stimulated T cells and is therefore strictly dependent on sustained antigenic stimulation, whereas IL-15 is constitutively produced by stromal cells. Thus, while under defined culture conditions, such as those used by Manjunath et al. (7), highly homogenous populations can be obtained, it is inevitable that in vivo the antigen-specific proliferating T cells will receive different levels of stimulation and will consequently generate both effector cells and intermediates. We have recently found that in humans most expanded memory T cell clones comprise both effector memory and central memory cells (F. Sallusto, unpublished results), indicating that the heterogeneity generated in the primary response is transferred into the memory pool and raising questions concerning the homeostatic mechanisms responsible for their long-term maintenance (15).
The capacity to generate virtually pure populations of effector memory and central memory cells and to track them using GFP should help resolve long-standing questions such as the role of persisting antigen and the relationship between memory and protection under different experimental and pathological conditions. These findings have practical implications, too, since they pave the way to the selective reconstitution of memory T cell pools for adoptive immunotherapy.
Footnotes
See the related article beginning on page 871.
References
- 1.Ahmed R, Gray D. Immunological memory and protective immunity: understanding their relation. Science. 1996;272:54–60. doi: 10.1126/science.272.5258.54. [DOI] [PubMed] [Google Scholar]
- 2.Zinkernagel RM, et al. On immunological memory. Annu Rev Immunol. 1996;14:333–367. doi: 10.1146/annurev.immunol.14.1.333. [DOI] [PubMed] [Google Scholar]
- 3.Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401:708–712. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- 4.Reinhardt RL, Khoruts A, Merica R, Zell T, Jenkins MK. Visualizing the generation of memory CD4 T cells in the whole body. Nature. 2001;410:101–105. doi: 10.1038/35065111. [DOI] [PubMed] [Google Scholar]
- 5.Masopust D, Vezys V, Marzo AL, Lefrancois L. Preferential localization of effector memory cells in nonlymphoid tissue. Science. 2001;291:2413–2417. doi: 10.1126/science.1058867. [DOI] [PubMed] [Google Scholar]
- 6.Iezzi G, Scheidegger D, Lanzavecchia A. Migration and function of antigen-primed nonpolarized T lymphocytes in vivo. J Exp Med. 2001;193:987–994. doi: 10.1084/jem.193.8.987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Manjunath N, et al. Effector differentiation is not prerequisite for generation of memory cytotoxic T lymphocytes. J Clin Invest. 2001;108:871–878. doi: 10.1172/JCI13296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Manjunath N, et al. A transgenic mouse model to analyze CD8(+) effector T cell differentiation in vivo. Proc Natl Acad Sci USA. 1999;96:13932–13937. doi: 10.1073/pnas.96.24.13932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weninger, W., Crowley, M.A., Manjunath, N., and von Andrian, U.H. 2001. Migratory properties of naive, effector and memory CD8+ T cells. J. Exp. Med. In press. [DOI] [PMC free article] [PubMed]
- 10.Lanzavecchia A, Sallusto F. Dynamics of T lymphocyte responses: intermediates, effectors and memory cells. Science. 2000;290:92–97. doi: 10.1126/science.290.5489.92. [DOI] [PubMed] [Google Scholar]
- 11.Sad S, Mosmann TR. Single IL-2-secreting precursor CD4 T cell can develop into either Th1 or Th2 cytokine secretion phenotype. J Immunol. 1994;153:3514–3522. [PubMed] [Google Scholar]
- 12.Iezzi G, Scotet E, Scheidegger D, Lanzavecchia A. The interplay between the duration of TCR and cytokine signalling determines T cell polarization. Eur J Immunol. 1999;29:4092–4101. doi: 10.1002/(SICI)1521-4141(199912)29:12<4092::AID-IMMU4092>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 13.Langenkamp A, Messi M, Lanzavecchia A, Sallusto F. Kinetics of dendritic cell activation: impact on priming of Th1, Th2 and nonpolarized T cells. Nat Immunol. 2000;1:311–316. doi: 10.1038/79758. [DOI] [PubMed] [Google Scholar]
- 14.Kedl RM, et al. T cells compete for access to antigen-bearing antigen-presenting cells. J Exp Med. 2000;192:1105–1113. doi: 10.1084/jem.192.8.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tough DF, Sun S, Zhang X, Sprent J. Stimulation of naive and memory T cells by cytokines. Immunol Rev. 1999;170:39–47. doi: 10.1111/j.1600-065x.1999.tb01327.x. [DOI] [PubMed] [Google Scholar]