Abstract
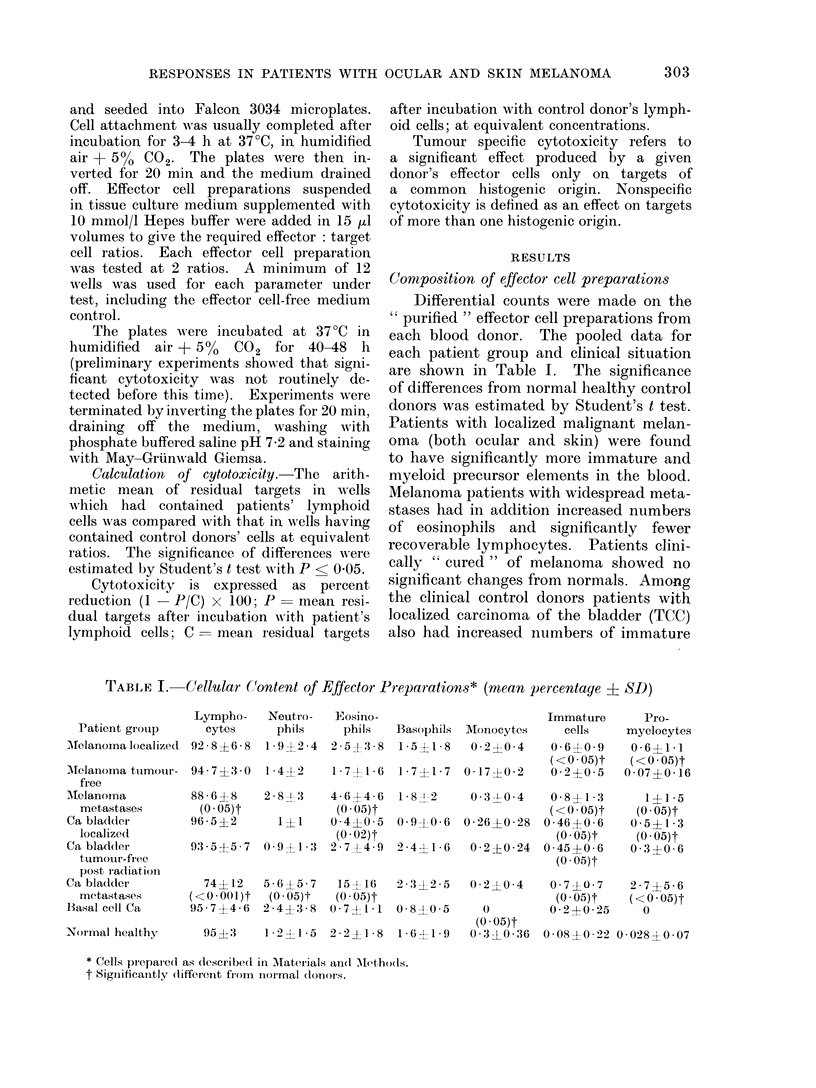
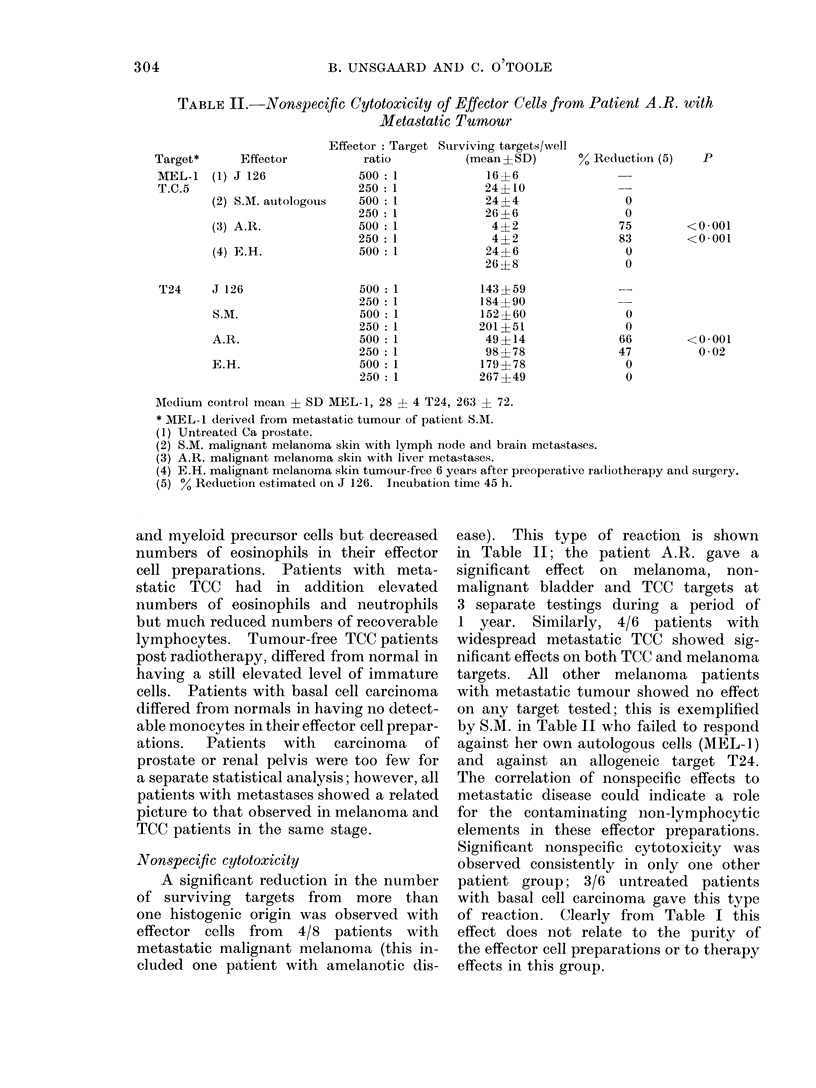
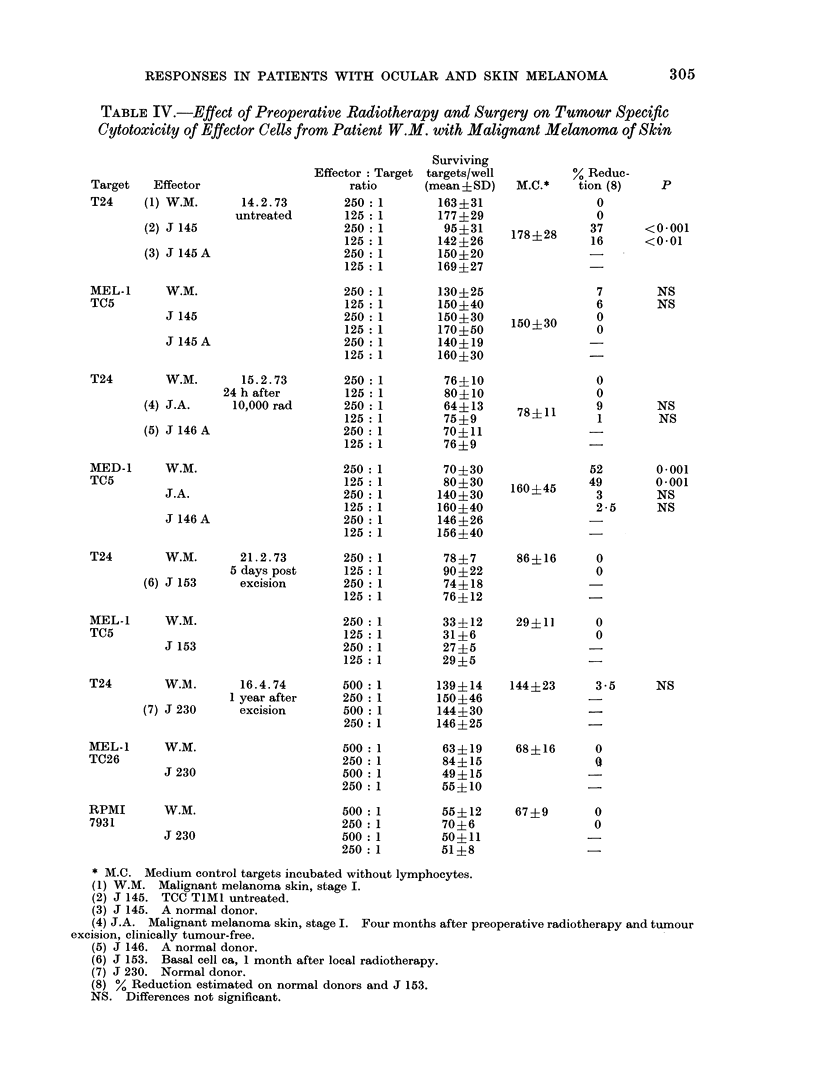
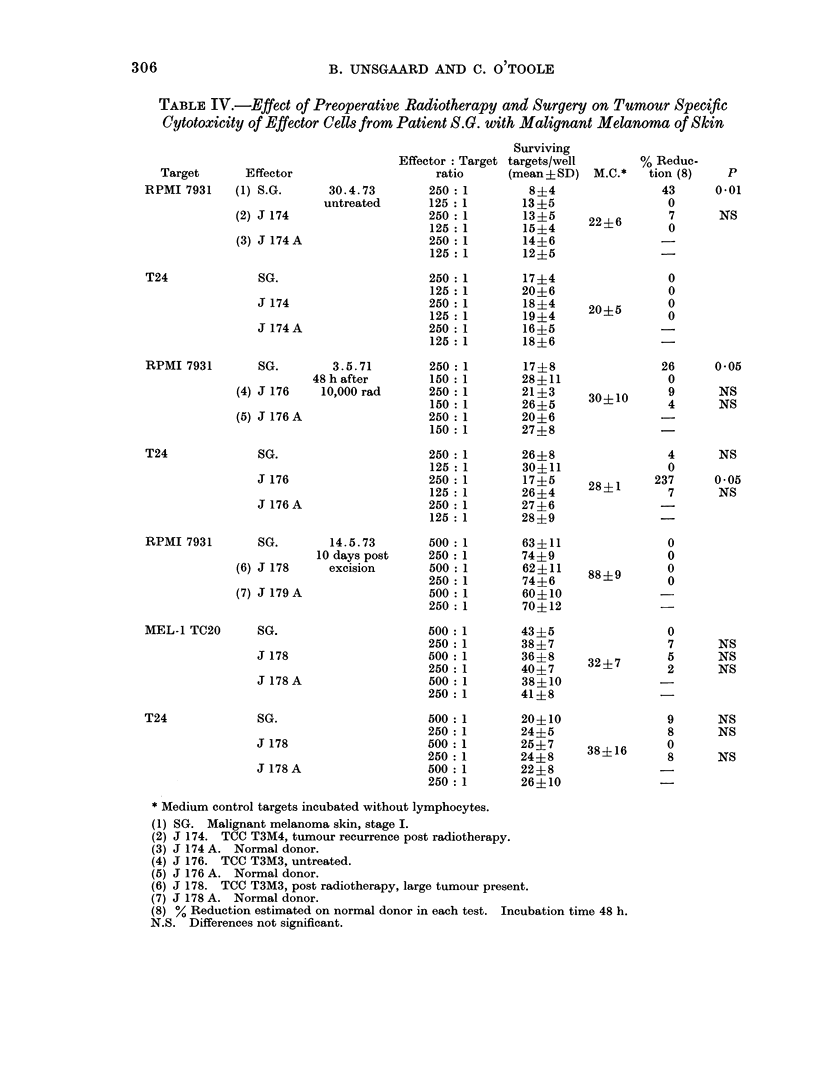
Using a microassay for cellular immunity, tumour specific cytotoxicity was detected in 2/5 cases of ocular melanoma and 1/3 cases of primary cutaneous melanoma before treatment. Reactivity was measured against allogeneic skin melanoma target cells in short or long term in vitro culture. Lymphoid cells from patients with disseminated cutaneous melanoma were either non-reactive (4/8 cases) or gave a nonspecific cytotoxicity on target cells of diverse histogenic origins. Among tumour-free patients tested after surgery, 0/2 patients with ocular tumour were non-reactive 3-4 months post surgery. After sugical excision of cutaneous melanoma 2/2 patients gave tumour specific reactions during the first month after surgery. After longer time intervals, from 5 months to 3 years, only 1/8 patients were reactive. Preoperative radiotherapy in a total skin dose of 10,000 rad produ-ed a transient tumour specific reaction 24 h after therapy in a single case. Following local tumour excision in patients given preoperative irradiation, 2 cases which had previously demonstrated tumour specific CMI lost reactivity. Among 14 tumour-free individuals tested only after preoperative radiotherapy and surgery, at intervals from 5 day to 13 years, a single case gave tumour specific CMI. Palliative irradiation in doses 4000-4960 rad to the inguinal or axillary lymph nodes was found to induce a generalized lymphopenia within 48 h after treatment. Lymphoid cell preparations from patients with localized melanoma contained significantly increased numbers of immature cells (lymphoblasts and myeloblasts) and myeloid precursor elements. Those prepared from patients with disseminated disease had in addition elevated levels of eosinophils but reduced numbers of recoverable lymphocytes.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bergkvist A., Ljungqvist A., Moberger G. Classification of bladder tumours based on the cellular pattern. Preliminary report of a clinical-pathological study of 300 cases with a minimum follow-up of eight years. Acta Chir Scand. 1965 Oct;130(4):371–378. [PubMed] [Google Scholar]
- Boyle W. An extension of the 51Cr-release assay for the estimation of mouse cytotoxins. Transplantation. 1968 Sep;6(6):761–764. doi: 10.1097/00007890-196809000-00002. [DOI] [PubMed] [Google Scholar]
- Bubeník J., Baresová M., Viklický V., Jakoubková J., Sainerová H., Donner J. Established cell line of urinary bladder carcinoma (T24) containing tumour-specific antigen. Int J Cancer. 1973 May;11(3):765–773. doi: 10.1002/ijc.2910110327. [DOI] [PubMed] [Google Scholar]
- COULSON A. S., CHALMERS D. G. SEPARATION OF VIABLE LYMPHOCYTES FROM HUMAN BLOOD. Lancet. 1964 Feb 29;1(7331):468–469. doi: 10.1016/s0140-6736(64)90799-8. [DOI] [PubMed] [Google Scholar]
- Chee C. A., Ilbery P. L., Rickinson A. B. Depression of lymphocyte replicating ability in radiotherapy patients. Br J Radiol. 1974 Jan;47(553):37–43. doi: 10.1259/0007-1285-47-553-37. [DOI] [PubMed] [Google Scholar]
- Currie G. A., Lejeune F., Fairley G. H. Immunization with irradiated tumour cells and specific lymphocyte cytotoxicity in malignant melanoma. Br Med J. 1971 May 8;2(5757):305–310. doi: 10.1136/bmj.2.5757.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doré C. F., Balfour B. M. A device for preparing cell spreads. Immunology. 1965 Oct;9(4):403–405. [PMC free article] [PubMed] [Google Scholar]
- Edelson P. J., Cohn Z. A. Peroxidase-mediated mammalian cell cytotoxicity. J Exp Med. 1973 Jul 1;138(1):318–323. doi: 10.1084/jem.138.1.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faucault J. P., Bernard J. P., Potier J. C., Lecacheux C. Cytotoxic lymphocytes in melanoma patients. Int J Cancer. 1972 May 15;9(3):567–576. doi: 10.1002/ijc.2910090313. [DOI] [PubMed] [Google Scholar]
- Federman J. L., Lewis M. G., Clark W. H. Tumor-associated antibodies to ocular and cutaneous malignant melanomas: negative interaction with normal choroidal melanocytes. J Natl Cancer Inst. 1974 Feb;52(2):587–589. doi: 10.1093/jnci/52.2.587. [DOI] [PubMed] [Google Scholar]
- Fossati G., Colnaghi M. I., Porta G. D., Cascinelli N., Veronesi U. Cellular and humoral immunity against human malignant melanoma. Int J Cancer. 1971 Sep 15;8(2):344–350. doi: 10.1002/ijc.2910080221. [DOI] [PubMed] [Google Scholar]
- GREENWALT T. J., GAJEWSKI M., McKENNA J. L. A new method for preparing buffy coat-poor blood. Transfusion. 1962 Jul-Aug;2:221–229. doi: 10.1111/j.1537-2995.1962.tb00228.x. [DOI] [PubMed] [Google Scholar]
- Hellström I., Hellström K. E. Some recent studies on cellular immunity to human melanomas. Fed Proc. 1973 Feb;32(2):156–159. [PubMed] [Google Scholar]
- Hellström I., Warner G. A., Hellström K. E., Sjögren H. O. Sequential studies on cell-mediated tumor immunity and blocking serum activity in ten patients with malignant melanoma. Int J Cancer. 1973 Mar 15;11(2):280–292. doi: 10.1002/ijc.2910110206. [DOI] [PubMed] [Google Scholar]
- Heppner G. H., Stolbach L., Byrne M., Cummings F. J., McDonough E., Calabresi P. Cell-mediated and serum blocking reactivity to tumor antigens in patients with malignant melanoma. Int J Cancer. 1973 Mar 15;11(2):245–260. doi: 10.1002/ijc.2910110202. [DOI] [PubMed] [Google Scholar]
- Jehn U. W., Nathanson L., Schwartz R. S., Skinner M. In vitro lymphocyte stimulation by a soluble antigen from malignant melanoma. N Engl J Med. 1970 Aug 13;283(7):329–333. doi: 10.1056/NEJM197008132830702. [DOI] [PubMed] [Google Scholar]
- Lewis M. G., Ikonopisov R. L., Nairn R. C., Phillips T. M., Fairley G. H., Bodenham D. C., Alexander P. Tumour-specific antibodies in human malignant melanoma and their relationship to the extent of the disease. Br Med J. 1969 Sep 6;3(5670):547–552. doi: 10.1136/bmj.3.5670.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morton D. L., Eilber F. R., Malmgren R. A. Immune factors in human cancer: malignant melanomas, skeletal and soft tissue sarcomas. Prog Exp Tumor Res. 1971;14:25–42. doi: 10.1159/000392269. [DOI] [PubMed] [Google Scholar]
- Morton D. L., Malmgren R. A., Holmes E. C., Ketcham A. S. Demonstration of antibodies against human malignant melanoma by immunofluorescence. Surgery. 1968 Jul;64(1):233–240. [PubMed] [Google Scholar]
- Nairn R. C., Nind A. P., Guli E. P., Davies D. J., Little J. H., Davis N. C., Whitehead R. H. Anti-tumor immunoreactivity in patients with malignant melanoma. Med J Aust. 1972 Feb 26;1(9):397–403. [PubMed] [Google Scholar]
- O'Toole C., Perlmann P., Unsgaard B., Almgård L. E., Johansson B., Moberger G., Edsmyr F. Cellular immunity to human urinary bladder carcinoma. II. EEffect of surgery and preoperative irradiation. Int J Cancer. 1972 Jul 15;10(1):92–98. doi: 10.1002/ijc.2910100112. [DOI] [PubMed] [Google Scholar]
- O'Toole C., Perlmann P., Unsgaard B., Moberger G., Edsmyr F. Cellular immunity to human urinary bladder carcinoma. I. Correlation to clinical stage and radiotherapy. Int J Cancer. 1972 Jul 15;10(1):77–91. doi: 10.1002/ijc.2910100111. [DOI] [PubMed] [Google Scholar]
- O'Toole C. Standardization of microcytotoxicity assay for cell-mediated immunity. Natl Cancer Inst Monogr. 1973 Jun;37:19–24. [PubMed] [Google Scholar]
- O'Toole C., Stejskal V., Perlmann P., Karlsson M. Lymphoid cells mediating tumor-specific cytotoxicity to carcinoma of the urinary bladder. Separation of the effector population using a surface marker. J Exp Med. 1974 Mar 1;139(3):457–466. doi: 10.1084/jem.139.3.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigby C. C., Franks L. M. A human tissue culture cell line from a transitional cell tumour of the urinary bladder: growth, chromosone pattern and ultrastructure. Br J Cancer. 1970 Dec;24(4):746–754. doi: 10.1038/bjc.1970.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stjernswärd J., Jondal M., Vánky F., Wigzell H., Sealy R. Lymphopenia and change in distribution of human B and T lymphocytes in peripheral blood induced by irradiation for mammary carcinoma. Lancet. 1972 Jun 24;1(7765):1352–1356. doi: 10.1016/s0140-6736(72)91091-4. [DOI] [PubMed] [Google Scholar]
- Takasugi M., Klein E. A microassay for cell-mediated immunity. Transplantation. 1970 Mar;9(3):219–227. doi: 10.1097/00007890-197003000-00005. [DOI] [PubMed] [Google Scholar]
- Thomas J. W., Lewis H. S., Yuen A. Effect of therapeutic irradiation on lymphocyte transformation in lung cancer. Cancer. 1971 May;27(5):1046–1050. doi: 10.1002/1097-0142(197105)27:5<1046::aid-cncr2820270507>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]


