Abstract
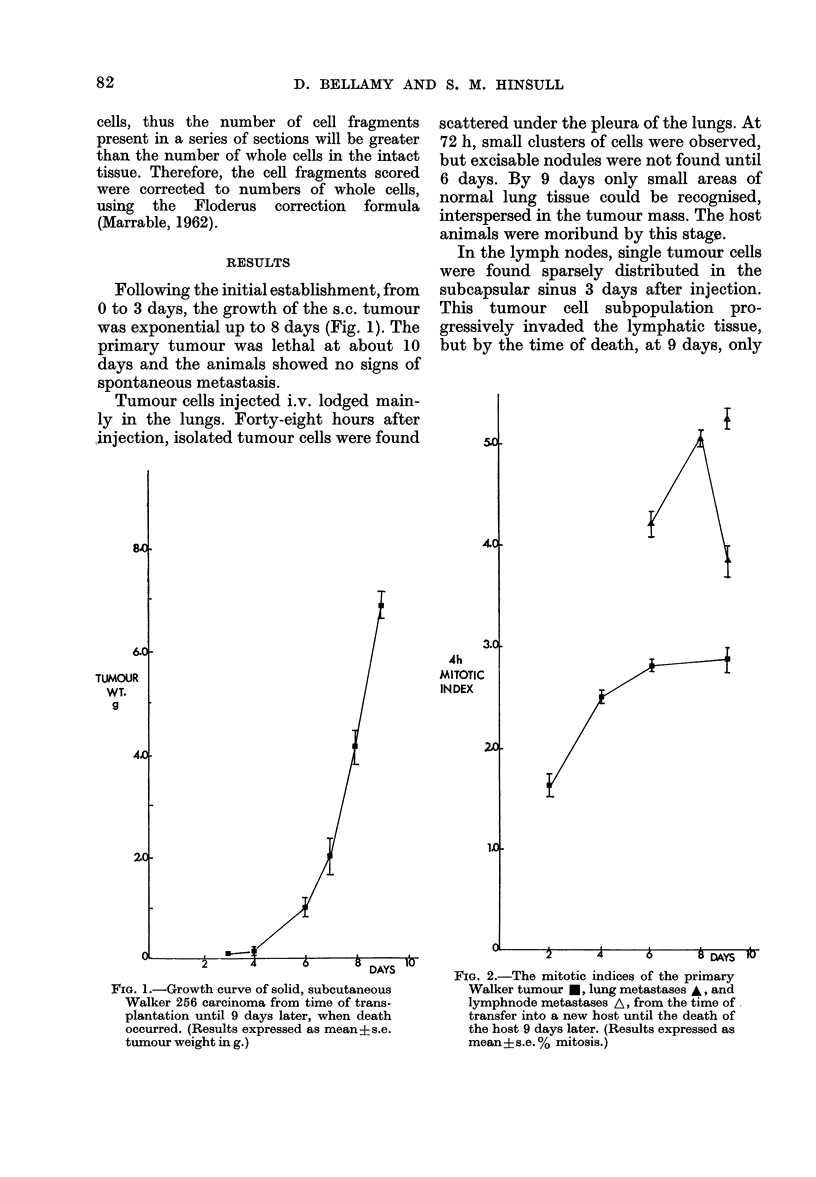
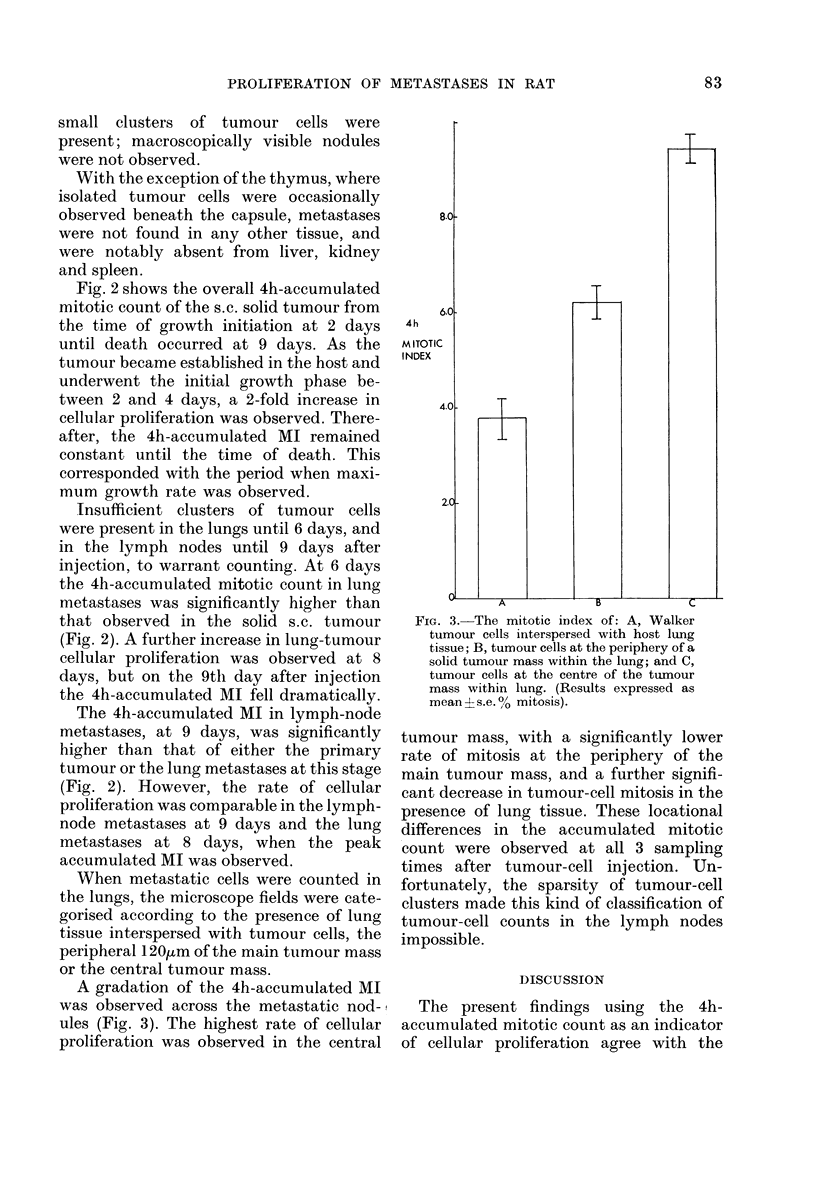
The growth of s.c. Walker 256 carcinoma was found to be independent of secondary growths induced by i.v. injection. Tumour cells injected i.v. lodged mainly in the lungs, with small clusters of cells in the lymph nodes. The rate of cellular proliferation of these secondary growths of Walker carcinoma was significantly higher than that observed in the s.c. tumour. In addition, host lung tissue was found to inhibit the development of metastases, and it is postulated that the host tissue may produce a diffusible inhibitor and that differences in the effectiveness of these humoral factors may account, in part, for locational differences in tumour growth patterns.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BASERGA R., KISIELESKI W. E., HALVORSEN K. A study on the establishment and growth of tumor metastases with tritiated thymidine. Cancer Res. 1960 Jul;20:910–917. [PubMed] [Google Scholar]
- BERTALANFFY F. D., LAU C. Rates of cell division of transplantable malignant rat tumors. Cancer Res. 1962 Jun;22:627–631. [PubMed] [Google Scholar]
- Billingham R. E., Silvers W. K. A biologist's reflections on dermatology. J Invest Dermatol. 1971 Oct;57(4):227–240. doi: 10.1111/1523-1747.ep12261543. [DOI] [PubMed] [Google Scholar]
- Bröyn Kinetics of cell proliferation and cell loss in the peripheral and central parts of Walker tumours growing in rats and nude mice. Virchows Arch B Cell Pathol. 1975 Jul 18;18(3):181–191. doi: 10.1007/BF02889246. [DOI] [PubMed] [Google Scholar]
- Bröyn T. The interaction between Walker tumour cells and mucosa cells in the lamina propria of gastric mucosa in rats. The tumour behaviour in previously X-irradiated mucosa compared to normal mucosa. Virchows Arch B Cell Pathol. 1975 Sep 29;19(1):27–36. doi: 10.1007/BF02889353. [DOI] [PubMed] [Google Scholar]
- Carr I., Norris P., McGinty F. Reverse diapedesis; the mechanism of invasion of lymphatic vessels by neoplastic cells. Experientia. 1975 May 15;31(5):590–591. doi: 10.1007/BF01932476. [DOI] [PubMed] [Google Scholar]
- Loewenstein W. R., Penn R. D. Intercellular communication and tissue growth. II. Tissue regeneration. J Cell Biol. 1967 May;33(2):235–242. doi: 10.1083/jcb.33.2.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saidel G. M., Liotta L. A., Kleinerman J. System dynamics of metastatic process from an implanted tumor. J Theor Biol. 1976 Feb;56(2):417–434. doi: 10.1016/s0022-5193(76)80083-5. [DOI] [PubMed] [Google Scholar]
- Simpson-Herren L., Sanford A. H., Holmquist J. P. Cell population kinetics of transplanted and metastatic Lewis lung carcinoma. Cell Tissue Kinet. 1974 Jul;7(4):349–361. doi: 10.1111/j.1365-2184.1974.tb00417.x. [DOI] [PubMed] [Google Scholar]
- Tannock I. F. Population kinetics of carcinoma cells, capillary endothelial cells, and fibroblasts in a transplanted mouse mammary tumor. Cancer Res. 1970 Oct;30(10):2470–2476. [PubMed] [Google Scholar]
- Tropé C. Different sensitivity to cytostatic drugs of primary tumor and metastasis of the Lewis carcinoma. Neoplasma. 1975;22(2):171–180. [PubMed] [Google Scholar]
- VAN SCOTT E. J., REINERTSON R. P. The modulating influence of stromal environment on epithelial cells studied in human autotransplants. J Invest Dermatol. 1961 Feb;36:109–131. [PubMed] [Google Scholar]


