Abstract
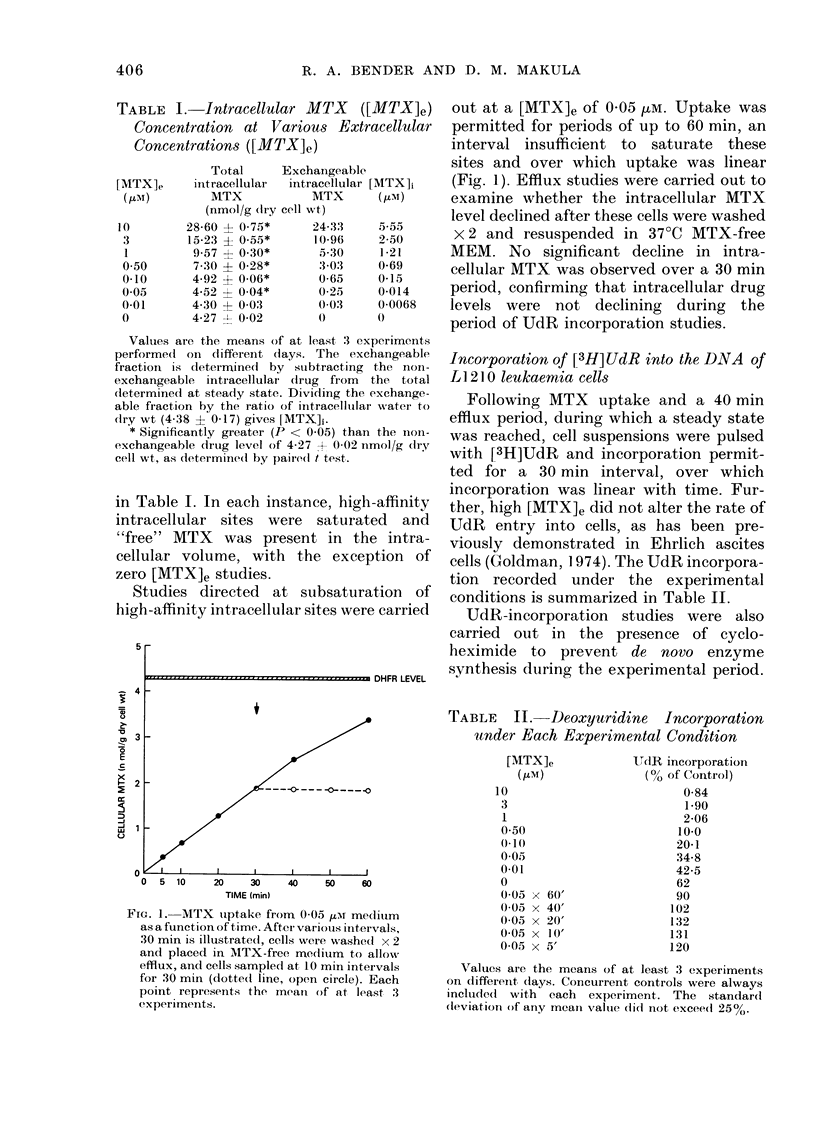
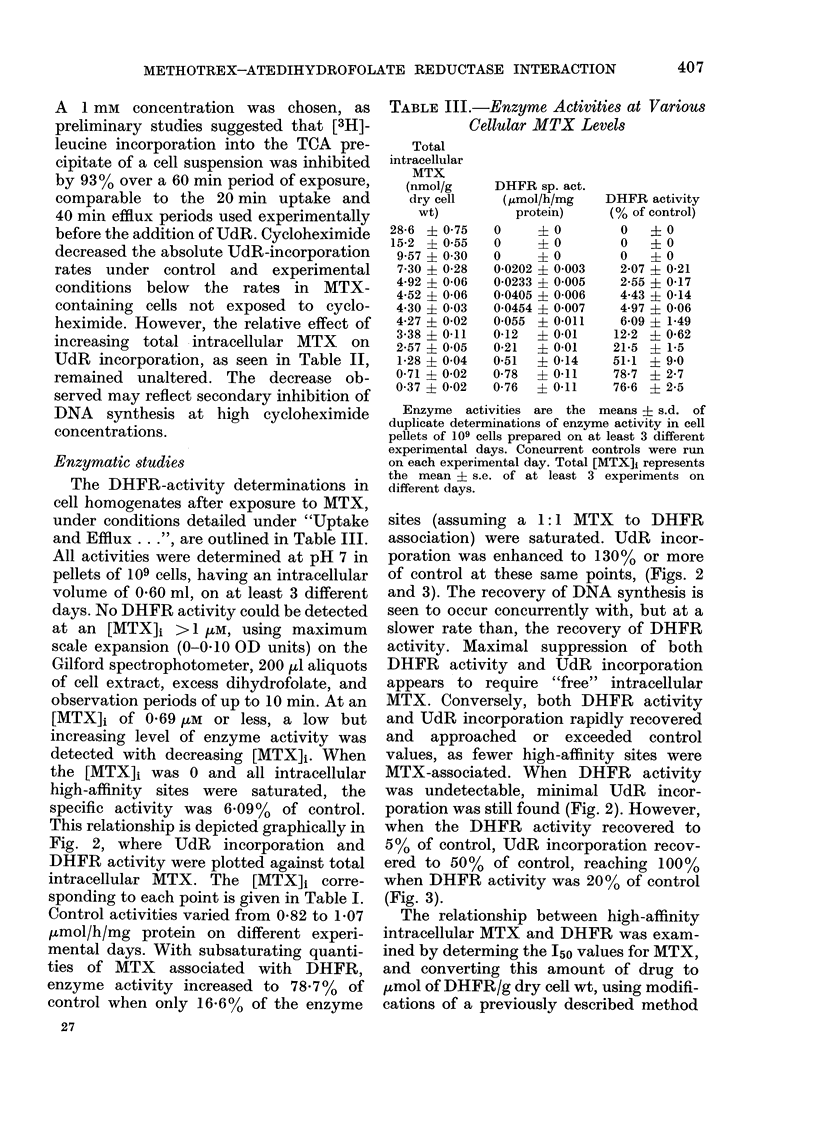
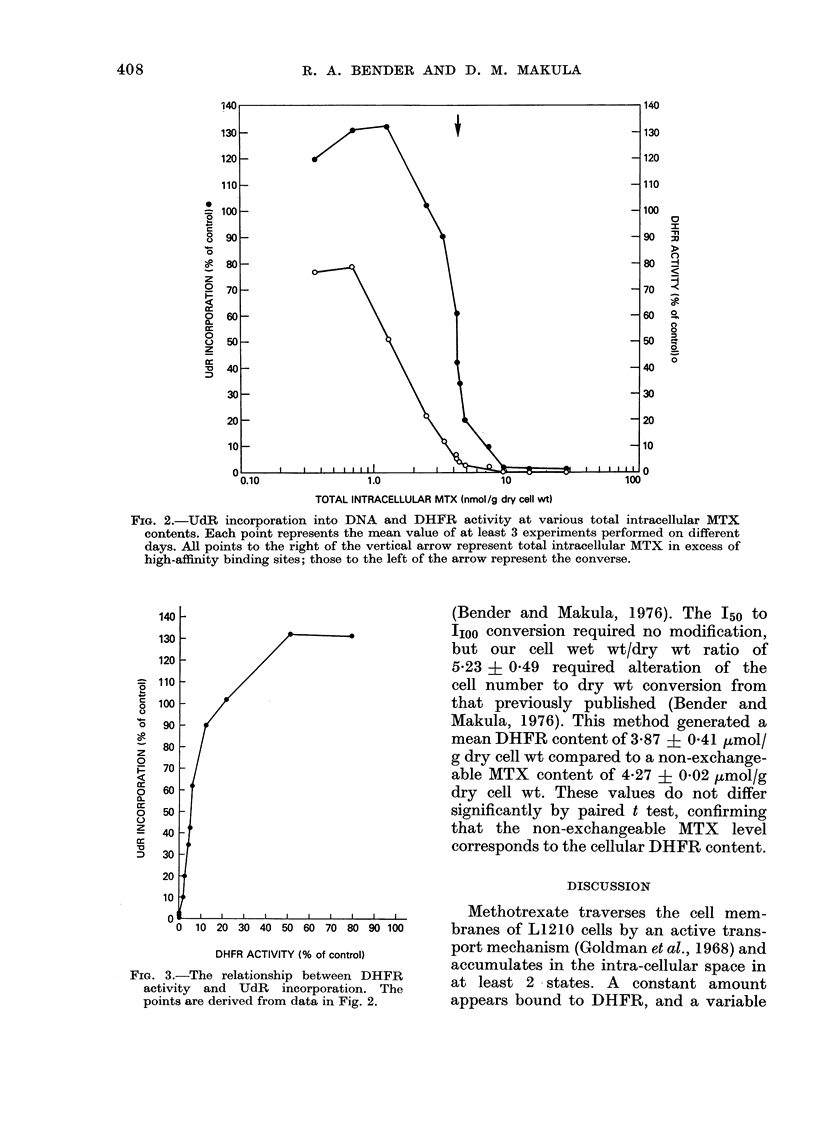
L1210 leukaemia cells were preloaded with [3H methotrexate] (MTX) to saturate high-affinity intracellular sites, and were then incubated with [3H]MTX to determine the steady-state intracellular MTX concentrations at extracellular concentrations ranging from 10 μM to zero. In addition, incubations to generate incomplete saturation of high-affinity intracellular MTX-binding sites were also carried out. Following determination of the total intracellular MTX, cells were pulsed with deoxyuridine (UdR) and its incorporation into DNA examined to assess the role of exchangeable and bound intracellular MTX on DNA synthesis. Further, cell pellets were disrupted and dihydrofolate reductase (DHFR) activity determined under each experimental condition. Extracellular MTX concentrations in excess of 1 μM depressed UdR incorporation to <2% of control, but incorporation rapidly recovered to 62% of control at the point of MTX-DHFR equivalence, and exceeded control values when all high-affinity sites were not saturated. DHFR activity was undetectable at extracellular MTX concentrations >0.50 μM, and never exceeded 6.09% of control at the “equivalence point” where all high-affinity sites were saturated. When less than 10% of potential inhibitor sites were occupied, enzyme activity increased rapidly, but never reached control. However, 5% of the DHFR activity was sufficient to permit UdR incorporation to continue at 50% of control levels, and UdR incorporation returned to control levels at 20% of the DHFR activity. The relationship between cellular MTX content and DNA synthesis or DHFR activity is sigmoid, suggesting a reversible interaction between enzyme and inhibitor. This lends support to the notion that “free” intracellular MTX is necessary for a maximal antitumour effect, and may explain its role in “high-dose” MTX therapy in man.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BERTINO J. R., BOOTH B. A., BIEBER A. L., CASHMORE A., SARTORELLI A. C. STUDIES ON THE INHIBITION OF DIHYDROFOLATE REDUCTASE BY THE FOLATE ANTAGONISTS. J Biol Chem. 1964 Feb;239:479–485. [PubMed] [Google Scholar]
- BERTINO J. R. THE MECHANISM OF ACTION OF THE FOLATE ANTAGONISTS IN MAN. Cancer Res. 1963 Sep;23:1286–1306. [PubMed] [Google Scholar]
- Bender R. A., Makulu D. R. Relationship between intracellular dihydrofolate reductase and tightly bound intracellular methotrexate in human neoplastic cells. Biochem Pharmacol. 1976 Apr 15;25(8):975–976. doi: 10.1016/0006-2952(76)90324-5. [DOI] [PubMed] [Google Scholar]
- Borsa J., Whitmore G. F. Studies relating to the mode of action of methotrexate. II. Studies on sites of action in L-cells in vitro. Mol Pharmacol. 1969 Jul;5(4):303–317. [PubMed] [Google Scholar]
- Dedrick R. L., Zaharko D. S., Bender R. A., Bleyer W. A., Lutz R. J. Pharmacokinetic considerations on resistance to anticancer drugs. Cancer Chemother Rep. 1975 Jul-Aug;59(4):795–804. [PubMed] [Google Scholar]
- Goldman I. D., Fyfe M. J. The mechanism of action of methotrexate. II. Augmentation by vincristine of inhibition of deoxyribonucleic aic synthesis by methotrexate in Ehrlich ascites tumor cells. Mol Pharmacol. 1974 Mar;10(2):275–282. [PubMed] [Google Scholar]
- Goldman I. D., Lichtenstein N. S., Oliverio V. T. Carrier-mediated transport of the folic acid analogue, methotrexate, in the L1210 leukemia cell. J Biol Chem. 1968 Oct 10;243(19):5007–5017. [PubMed] [Google Scholar]
- Goldman I. D. The mechanism of action of methotrexate. I. Interaction with a low-affinity intracellular site required for maximum inhibition of deoxyribonucleic acid synthesis in L-cell mouse fibroblasts. Mol Pharmacol. 1974 Mar;10(2):257–274. [PubMed] [Google Scholar]
- Hänggi U. J., Littlefield J. W. Isolation and characterization of the multiple forms of dihydrofolate reductase from methotrexate-resistant hamster cells. J Biol Chem. 1974 Mar 10;249(5):1390–1397. [PubMed] [Google Scholar]
- Jaffe N., Frei E., 3rd, Traggis D., Bishop Y. Adjuvant methotrexate and citrovorum-factor treatment of osteogenic sarcoma. N Engl J Med. 1974 Nov 7;291(19):994–997. doi: 10.1056/NEJM197411072911902. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Margolis S., Philips F. S., Sternberg S. S. The cytotoxicity of methotrexate in mouse small intestine in relation to inhibition of folic acid reductase and of DNA synthesis. Cancer Res. 1971 Dec;31(12):2037–2046. [PubMed] [Google Scholar]
- Mitchell M. S., Wawro N. W., DeConti R. C., Kaplan S. R., Papac R., Bertino J. R. Effectiveness of high-dose infusions of methotrexate followed by leucovorin in carcinoma of the head and neck. Cancer Res. 1968 Jun;28(6):1088–1094. [PubMed] [Google Scholar]
- Perkins J. P., Hillcoat B. L., Bertino J. R. Dihydrofolate reductase from a resistant subline of the L1210 lymphoma. Purification and properties. J Biol Chem. 1967 Oct 25;242(20):4771–4776. [PubMed] [Google Scholar]
- Roberts D., Wodinsky I. On the poor correlation between the inhibition by methotrexate of dihydrofolate reductase and of deoxynucleoside incorporation into DNA. Cancer Res. 1968 Oct;28(10):1955–1962. [PubMed] [Google Scholar]
- White J. C., Goldman I. D. Mechanism of action of methotrexate. IV. Free intracellular methotrexate required to suppress dihydrofolate reduction to tetrahydrofolate by Ehrlich ascites tumor cells in vitro. Mol Pharmacol. 1976 Sep;12(5):711–719. [PubMed] [Google Scholar]


