Apoptosis, a genetically programmed cell death conserved throughout phylogeny, provides a counterbalance to mitosis in the regulation of tissue growth and homeostasis (1, 2). Interest in and knowledge of the mechanisms mediating the induction and execution of the death program have blossomed in the last decade and a half. Engulfment of apoptotic bodies and debris represents the denouement of the death program for most cells in multicellular organisms.
Macrophages and other cells manifesting their primitive phagocytic potential clear apoptotic bodies from tissues, preventing their lysis and the consequent release of toxic or immunogenic intracellular components. By inducing the release of mediators such as TGF-β, IL-10, prostaglandin E2 (PGE2), and others, clearance of apoptotic cells also sets up an anti-inflammatory milieu within the tissue (reviewed in refs. 3, 4). However, phagocytic clearance is not merely a silent process with respect to inflammation and immunity but is also actively anti-inflammatory. In spite of its critical importance to tissue and host homeostasis, clearance of apoptotic cells is poorly understood, and studies devoted to elucidating the mechanisms mediating recognition and engulfment have lagged behind those probing the induction and execution of the death process itself. This situation is finally changing as we begin to unravel the mechanisms mediating clearance of apoptotic cells.
Many of the macrophage receptors that contribute to apoptotic cell recognition are critical players in innate immunity. These include certain lectins and integrins, the class A and class B scavenger receptors (see Platt and Gordon, this Perspective series, ref. 5; and Krieger, this series, ref. 6), receptors for oxidized LDL, including CD68 and lectin-like oxidized LDL receptor-1 (LOX-1), some of the receptors for complement-derived proteins, and the endotoxin receptor CD14 (reviewed in ref. 7). Paradoxically, however, when microbial organisms or their products are engulfed via these receptors, inflammation results, and in many cases, acquired immunity is stimulated. In contrast, uptake of apoptotic cells is generally noninflammatory and thereby avoids activation of an acquired immune response. Our task is to understand and explain these diametrically opposed reactions to stimulation of the same receptors.
Phospholipid asymmetry in the plasma membrane
Macrophages and other phagocytes clearly discriminate between apoptotic cells and viable cells. Recognition occurs prior to the lysis of the dying cell, implying an early surface change. The most characteristic surface change associated with apoptosis is loss of phospholipid asymmetry and exposure of phosphatidylserine (reviewed in ref. 8), and this change is absolutely required for recognition and engulfment to occur (9). Plasma membrane asymmetry in viable cells is maintained by the activity of an aminophospholipid translocase, which is believed to be a 120-kDa Mg2+-dependent ATPase (reviewed in refs. 10, 11). This ATPase transports any phosphatidylserine (and, to a lesser extent, phosphatidylethanolamine) that may have reached the outer leaflet back to the inner leaflet of the plasma membrane. However, the rapid appearance of phosphatidylserine on the cell surface during cellular activation and during apoptosis owes more to the activation of a lipid-nonspecific membrane phospholipid scrambling activity, which moves phospholipids bidirectionally across the membrane, thereby increasing surface expression of phosphatidylserine (reviewed in ref. 12).
The exact nature of the proteins involved in lipid scrambling is not clear, but there are tantalizing clues. First, a human patient with a bleeding defect has been identified whose platelets could not initiate the coagulation cascade because they failed to trigger transmembrane phospholipid scrambling and therefore prevented phosphatidylserine from reaching the platelet surface. The laboratories of Wiedmer and Sims have identified and cloned a protein from red blood cells that can be reconstituted into artificial membranes and can transport a variety of phospholipids bidirectionally following stimulation with a calcium ionophore (reviewed in ref. 12). In our hands, scramblase activity associated with this protein can be regulated by intracellular calcium levels and by phosphorylation by protein kinase Cδ (PKCδ). Wiedmer et al. have recently reported that there are at least four homologues of the scramblase in the human and in the mouse, and it is not yet known exactly how each functions during apoptosis (12).
Nearly all cells undergoing apoptosis lose phospholipid asymmetry, the only known exceptions being certain tumor cell lines (9, 13). This finding has been so reliable that phosphatidylserine is commonly used as a marker for apoptosis (e.g., see ref. 14). However, it should be kept in mind that activated cells also lose phospholipid asymmetry, at least transiently. During cellular activation, the scramblase is activated, but because there is no corresponding downregulation of aminophospholipid translocase activity (15), any resulting asymmetry is soon rectified. We have shown that scramblase 1 is phosphorylated by PKCδ during cellular activation as well as during cell death, so phosphorylation per se does not appear to be the major difference between activation and death (15). Interestingly, PKCδ is cleaved during apoptosis by caspase-3, suggesting a plausible mechanism by which this kinase, and subsequently the scramblase, can become activated in a sustainable manner.
When apoptosis is induced, the aminophospholipid translocase activity is downregulated, at least in part by elevation of intracellular Ca2+ levels (reviewed in ref. 8). Oxidation of phosphatidylserine during apoptosis may alter its ability to act as a substrate for transportation back to the inner leaflet by the aminophospholipid translocase (16, 17). The net result is that phosphatidylserine transport from the outer to the inner leaflet is significantly decreased.
The identity of the proteins that normally mediate aminophospholipid translocase activity directionally into the inner leaflet remains somewhat controversial. Tang et al. cloned a 130-kDa P-type ATPase (ATPase type II) that can translocate phosphatidylserine in artificial membranes and that exhibits the characteristics expected of an authentic aminophospholipid translocase (18). However, when Siegmund and colleagues ablated the yeast homologue of this ATPase, they found no effect on phosphatidylserine uptake and distribution (19). Consistent with the possibility that more than one protein can serve as a phosphatidylserine translocase, Ding and colleagues have isolated four forms of ATPase II from the brain. These enzymes differ significantly with regard to ATPase activity and phospholipid selectivity, suggesting that regulation of membrane phosphatidylserine transport is complex (20).
To complicate matters further, the activity of the ABC1 transporter, which helps transfer cholesterol and phospholipids from cells to protein acceptors such as apoA-I, appears to facilitate phospholipid scrambling. Deletion of the transporter gene or downregulation of its product reduces exposure of phosphatidylserine during cellular activation and apoptosis (21, 22). It has also been reported that the enhancement of apoptotic cell engulfment by phagocytes expressing ABC1 can result from loss of phospholipid asymmetry in the macrophage as well as the target cell (21, 23). Indeed, ABC1 is found in macrophages engulfing apoptotic cells during embryonic limb bud remodeling (24), and inhibition of this transporter in macrophages dramatically inhibits their uptake of apoptotic cells in vitro and in vivo. Involvement of ABC1 as a promoter of phospholipid scrambling in target cell and phagocyte is appealing, as it mimics the requirement for ced-7 function in target and phagocyte in Caenorhabditis elegans (25). How this protein works to enhance phospholipid scrambling remains a fascinating question.
Other apoptotic cell surface determinants
Exactly how the small polar head group on this phospholipid triggers recognition and uptake is not well understood, but this process requires one or more phosphatidylserine-specific receptors (26). Loss of phospholipid asymmetry may also facilitate the exposure or formation of other recognition ligands. Staining of apoptotic cells with fluorescently labeled annexin V, which recognizes phosphatidylserine, often reveals aggregation of this lipid on the cell surface, perhaps supporting the notion of large recognition domains on membrane blebs (27). Christopher Gregory has termed these ACAMPs, for “apoptotic cell–associated molecular patterns” (28). The Rosens have elegantly demonstrated that these sites contain many of the cellular proteins known to induce autoantibody production in diseases such as systemic lupus erythematosus (27). Together with the established association between defective clearance and autoimmune disorders (29), these observations underscore the importance of clearance for proper regulation of anti-self immune responses.
Other surface changes promoting recognition of apoptotic cells remain to be identified, and it is very possible that some may be cell type–specific. Two groups of investigators have shown that carbohydrate groups are altered on the dying cell surface and that these changes can be recognized by phagocytes (30, 31). Sugar-lectin interactions are very appealing in this regard. Like the process of apoptotic cell engulfment, these interactions are evolutionarily conserved. Following an initial loss of phospholipid asymmetry, several different collectins — C1q (32, 33), the mannose-binding lectin (33), and surfactant protein A (34) among them — bind to the surface of apoptotic cells in an aggregated pattern reminiscent of the distribution of phosphatidylserine and enhance uptake. Mevorach, Elkon, and colleagues have shown that apoptotic cells can fix complement, and that this ability increases the efficiency of uptake by macrophages (35). It is likely that loss of phospholipid asymmetry must precede this change also, and it has been suggested that phosphatidylserine may activate complement directly (see Supplemental reading list, http://www.JCI.org/cgi/content/full/108/7/957/DC1., for references on this point).
As the apoptotic process evolves, membrane phospholipids become not only redistributed, but also oxidized. In particular, phosphatidylserine may be specifically targeted (16, 17). Chang and colleagues recently showed that oxidation of membrane phospholipids during apoptosis results in the development of a recognition ligand for macrophages, as uptake can be inhibited by the mAb’s that recognize oxidized forms of choline-containing phospholipids. The antibodies bind to the surface of apoptotic but not viable cells (36), and preliminary evidence suggests that the oxidized epitope appears on the cell surface only after phospholipid asymmetry is compromised (36). These oxidized phospholipids likely provide a ligand for recognition by scavenger receptors.
Evidence is mounting that the surface of the apoptotic cell becomes more complex as apoptosis proceeds (37). Early exposure of phosphatidylserine provides the early recognition signal, and other ligands accumulate as the process evolves. Specific cellular surface proteins may also trigger recognition by phagocytes. Moffat and colleagues have raised the possibility that ICAM-3 on lymphocytes undergoing apoptosis is altered qualitatively, perhaps as a result of a conformation change or the exposure of cryptic epitope, to provide a signal for engulfment by macrophages. Most intriguing, however, is the observation (reviewed in ref. 28) that recognition of altered ICAM-3 is independent of ICAM-3 binding integrins and is mediated instead by macrophage CD14. Gregory and colleagues suggest that apoptosis results in the exposure or generation of a carbohydrate group on ICAM-3, which could bind to CD14 in its capacity as a lectin (28).
Receptors mediating recognition and uptake of apoptotic cells
Many receptors have been reported to mediate the binding and uptake of apoptotic cells by macrophages, fibroblasts, hepatocytes, epithelial cells, and endothelial cells. For many of these cell types, the specific ligands remain a mystery. The first interaction, described by Duvall and colleagues, was the recognition of N-acetylglucosamine, N-acetylgalactosamine, and galactose groups on apoptotic lymphocytes by an uncharacterized macrophage lectin (31). Dini and colleagues refined these observations, showing that the hepatocyte asialoglycoprotein receptor mediated recognition of apoptotic hepatocytes by their viable neighbors (30). These authors have gone on to show the involvement of sugar-lectin interactions in recognition of apoptotic lymphocytes and hepatic cells by liver endothelial cells and Kupffer cells as well. An asialoglycoprotein receptor has been identified on macrophages (38) and likely contributes to uptake of apoptotic cells. In addition, Beppu, Eda, and colleagues have described the recognition of poly-N-acetyllactosaminyl groups on oxidized cells by macrophages, and they suggest that the same may be true for apoptotic cells (reviewed in ref. 39).
Savill and colleagues were the first to identify integrin and scavenger receptor involvement in recognition of dying cells (7). They showed that the αvβ3 vitronectin receptor cooperates with CD36 on human monocyte-derived macrophages to bind to thrombospondin, a soluble molecule that can provide a bridge to the apoptotic cell. Since that time, other scavenger receptors, including the CD36 relative SR-BI, scavenger receptor A, the oxLDL receptor CD68/macrosialin, and LOX-1 have been implicated in this process. The ability of scavenger receptors to mediate recognition of apoptotic cells is also widely distributed throughout phylogeny. Natalie Franc and colleagues have identified a receptor critical for uptake of cellular corpses in Drosophila melanogaster; this molecule, which they have creatively termed “croquemort,” is homologous to mammalian CD36 (40). In addition, Zheng Zhou et al. have recently shown that Ced-1, a protein involved in engulfment in C. elegans, is homologous to a newly described scavenger receptor in endothelial cells, termed SREC (41). Many of these receptors are known to bind to phosphatidylserine (reviewed in ref. 42), but they also bind to oxidized phospholipids, which may arise during apoptosis and provide additional ligands for recognition by phagocytes. In addition to the pattern recognition receptors, integrins other than αvβ3 have been implicated in apoptotic cell recognition. Dendritic cells (DCs) employ the αvβ5 vitronectin receptor, which appears to be coupled to the cell’s phagocytic machinery (43, 44). Others have implicated the β1 and the β2 (leukocyte) integrins as well, at least in modulating uptake if not in directly mediating it (reviewed in ref. 7). In addition, there are a number of phosphatidylserine-binding proteins that may act as a bridge between the phagocyte and the apoptotic cells, most notably β2GP1 and gas-6 (45, 46).
It is important to note that, while they can bind phosphatidylserine, the scavenger receptors and CD14 are not specific for this phospholipid. Because macrophage uptake of apoptotic cells is inhibited specifically (indeed stereospecifically) by phosphatidylinositol and two related phospholipids (glycerophosphorylserine and phosphoserine) (47), some other phosphatidylserine receptor must be involved. To identify this receptor, we screened mAb’s against whole phagocytes and used the resulting antibody to clone a novel gene present in humans, mice, D. melanogaster, and C. elegans. The gene product in mammals is found on the surface of all cells known to engulf apoptotic cells, including macrophages, DCs (our unpublished data), fibroblasts, epithelial cells, and endothelial cells (26). It is not present on circulating cells or cell lines mimicking them, such as red blood cells, lymphocytes, monocytes, and neutrophils. Moreover, when expressed in lymphocytes, this protein confers the ability to bind to and engulf apoptotic bodies and phosphatidylserine-expressing red blood cells (26). Clissold and Ponting have recently suggested that the phosphatidylserine receptor contains a JmjC domain similar to that found in the transcription factor jumonji. This finding places the receptor in a family of cupin metalloenzymes, regulators of chromatin structure that are found throughout the plant and animal kingdoms (48). It is not clear, however, whether the receptor retains the predicted activity, nor how such an enzymatic activity might relate to its function on the surface of the phagocyte. Our data suggest that engagement of this receptor by phosphatidylserine on apoptotic cells is essential for uptake by macrophages and fibroblasts in mammals (9); its role in clearance of apoptotic cells in D. melanogaster and C. elegans remains to be determined.
The most recent additions to the phagocyte armamentarium for corpse removal include the receptor tyrosine kinase MER (49) and CD91 (33). Mice deficient for MER expression show reduced clearance of apoptotic cells, and, like the C1q-deficient mouse (50), they develop an autoimmune disease similar to systemic lupus erythematosus (49). MER is known to bind to the product of the Gas6 gene, a soluble phosphatidylserine-binding protein (51) that has also been shown to be involved in apoptotic cell uptake (46). The interaction between phagocyte CD91 (the α2 macroglobulin receptor, or LRP; see Herz and Strickland, this Perspective series, ref. 52) and apoptotic cells is likely to be complex. Ogden and colleagues found that C1q and mannose-binding lectin bind to apoptotic cells (33). The collagenous tails of these molecules bind to calreticulin adhered to the phagocyte cell surface by CD91. Our interest in collectins was piqued by the observation of Korb and Ahearn that C1q binds to the surface of apoptotic keratinocytes (53). Furthermore, Botto et al. showed that mice deficient for C1q appear to have defective apoptotic cell clearance in the kidney prior to the onset of immune-mediated glomerulonephritis, supporting the hypothesis that clearance defects contribute to autoimmunity (50). Taylor and colleagues have also found that these animals had defective macrophage phagocytosis of apoptotic cells in the inflamed peritoneum (32). Our own preliminary data suggest that these animals may also have defective clearance of apoptotic cells in the inflamed lung, as well as in the mammary gland during postweaning involution. Lastly, surfactant protein A, another collectin, has been shown to be involved in apoptotic cell removal (34).
Mechanisms and consequences of the uptake of apoptotic debris
The mechanics of phagocytosis have been elucidated by genetic studies in the nematode C. elegans, in which at least seven engulfment genes have been implicated (54). Ced-2, ced-5, and ced-10 are homologous to the mammalian proteins CRK II, DOCK 180, and Rac (55, 56), all of which have been implicated in mammalian engulfment of apoptotic cells (44, 57). Furthermore, Albert and colleagues recently showed that stimulation of αvβ5 vitronectin receptor on DCs activates this pathway, and it is likely that other receptors will be found to activate this pathway (44). It is exciting to note also that ced-6 has a human homologue that is implicated in signaling for uptake in mammalian cells (58–61). Combining the power of the genetic studies in C. elegans and Drosophila with the recognition and biochemical studies in mammalian cells has allowed the study of apoptotic cell clearance to finally make significant progress.
Efficient engulfment of apoptotic cells is of paramount importance in vivo, in part because clearance of apoptotic cells prior to their lysis is critical for the resolution of inflammation. We are finally beginning to understand the mechanisms mediating resolution and how this affects tissue homeostasis and the acquired immune response. Uptake prior to lysis prevents the release of proinflammatory and immunogenic material and actively suppresses inflammation. When apoptotic cells are recognized by macrophages, production of proinflammatory mediators is downregulated through the action of TGF-β, PGE2, and other anti-inflammatory mediators (reviewed in refs. 3, 4). In other systems, IL-10 is released by either macrophages or the apoptotic cells themselves (62–64). Such anti-inflammatory mediators profoundly influence whether an acquired immune response will be activated.
Recent interest in how DCs respond to apoptotic cells has generated a flurry of investigations (recently reviewed in refs. 3, 4). DCs also phagocytose apoptotic cells, although not as efficiently as macrophages. The data on whether DCs can mature and present antigens derived from apoptotic cells are conflicting. Several publications suggest that DCs can phagocytose apoptotic tumor cells and present tumor-derived antigens via cross-presentation to CD8+ cytotoxic T cells and that the activated cytotoxic T lymphocytes can kill the tumor cells, at least in vitro (reviewed in refs. 3, 4). Rovere et al. have suggested that this outcome results from delayed clearance (65), which may reflect the relative inefficiency of apoptotic cell engulfment by DCs.
The observations that DCs can cross-present exogenous antigens from apoptotic cells to cytotoxic T lymphocytes form the basis for the generation of DC-based vaccines for the treatment of several cancers. However, the techniques used to induce apoptosis in this work (see refs. 3, 4 for specific citations) may actually generate a mix of apoptotic and necrotic cells. Furthermore, in some cases, the apoptotic cells were stressed by heat shock or infected with virus and may have produced additional stimulatory signals that could complicate the interpretation of the experiments. In the hands of some investigators, the binding and engulfment of pure apoptotic populations by apoptotic cell receptors on DCs is nonstimulatory, whereas binding/engulfment of necrotic cells, particularly those derived from tumors, strongly stimulates the DCs to mature and activate T cells (reviewed in refs. 3, 4, 66). A number of intracellular proteins, including heat shock proteins, have been reported to activate DCs, suggesting that cells must lyse before they can promote an immune response. In fact, Binder and colleagues have suggested that CD91 specifically is a sensor for necrotic cell death because it binds to gp96, one of the heat shock/stress proteins released when cells die necrotically (67). Others have shown that uptake of apoptotic cells does not result in DC maturation and antigen presentation but that necrotic cells, particularly tumor cells, can activate the response. Uptake of apoptotic cells may well be tolerogenic (66, 68), and apoptosis may thus provide a way by which tumor cells or even intracellular pathogens could avoid immune surveillance. In fact, Freire-de-Lima and colleagues have shown that uptake of apoptotic cells with trypanosomes can promote the intracellular growth of these parasites (69).
Explaining the anti-inflammatory effect of apoptosis
An old but scattered literature suggests an inhibitory effect of phosphatidylserine on lymphocyte and macrophage function. This lipid is apparently able to suppress the production of proinflammatory cytokines, such as IFN-γ and TNF-α, and of cytocidal products such as nitrous oxide (reviewed in ref. 4). The mAb used to clone the phosphatidylserine receptor has the interesting property of mimicking the effects of apoptotic cells on phagocytes, stimulating the production of TGF-β, and inhibiting that of TNF-α (26). Liposomes containing phosphatidylserine have the same effect.
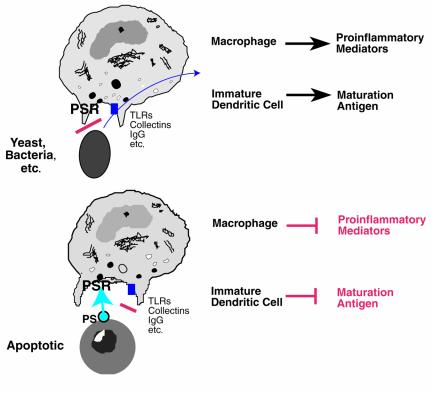
We are in the early days of understanding how the phosphatidylserine receptor works, but our preliminary data suggest that blocking its expression not only inhibits uptake of apoptotic cells but also interferes with release of TGF-β. Furthermore, only apoptotic cells that express phosphatidylserine are able to induce the release of TGF-β and the downregulation of proinflammatory cytokines. We therefore propose the model shown in Figure 1. We envision that uptake of microbes that fail to express phosphatidylserine on their surfaces will trigger a proinflammatory response because they activate CD91, CD14, integrins, toll-like receptors, and, if opsonized, immunoglobulin and complement receptors. In contrast, an apoptotic cell expresses phosphatidylserine externally and thus engages the phosphatidylserine receptor, providing a dominant anti-inflammatory signal.
Figure 1.
A hypothetical model for the role of a newly described receptor for phosphatidylserine (PS) (26) in modulating inflammation and immune response. Engulfment of microbial organisms that fail to express PS externally does not engage the PS receptor (PSR); instead, receptors are stimulated by the microbe or its products that transduce proinflammatory signals. These include receptors for immunoglobulin, complement, collectins, endotoxin, etc. In contrast, when apoptotic cells are recognized, they expose PS, thus engaging the PSR, which provides an anti-inflammatory signal. TLR, toll-like receptor.
Many aspects of this model and of phagocytosis in general remain to be explored. Even the identity of the receptors that support adhesion and those that signal the macrophage to begin engulfment has been difficult to determine. Because of the complexity of the apoptotic cell surface, it may be best to develop models in which only one receptor is triggered at a time. It is also unclear why there are so many receptors involved in apoptotic cell recognition and whether their roles are fully redundant. It seems equally possible that engulfment requires a series of receptor engagements or that a phagocytic synapse, analogous to the immunological synapse, forms between the phagocyte and its target. The roles of the various receptors will need to be evaluated in multiple tissues and in both noninflammatory and inflammatory models. Lastly, we must also critically dissect the mechanisms by which apoptotic cells regulate inflammation and the immune response, as the data at the present time seem to conflict. In some cases apoptotic cells appear to activate an immune response and in others they do not. We have firm evidence that triggering the phosphatidylserine receptor is anti-inflammatory, but the effects of other receptors need to be established. Clearance of apoptotic cells has finally hit its stride. Advances should be rapid and exciting from this point on.
Supplementary Material
References
- 1.Kerr JF, Wyllie AH, Currie AR. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer. 1972;26:239–257. doi: 10.1038/bjc.1972.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wyllie AH, Kerr JF, Currie AR. Cell death: the significance of apoptosis. Int Rev Cytol. 1980;68:251–306. doi: 10.1016/s0074-7696(08)62312-8. [DOI] [PubMed] [Google Scholar]
- 3.Henson PM, Bratton DL, Fadok VA. The phosphatidylserine receptor: a crucial molecular switch? Nat Rev Mol Cell Biol. 2001;2:627–633. doi: 10.1038/35085094. [DOI] [PubMed] [Google Scholar]
- 4.Fadok, V.A., and Chimini, G. 2001. The phagocytosis of apoptotic cells. Semin. Immunol. In press. [DOI] [PubMed]
- 5.Platt N, Gordon S. Is the class A macrophage scavenger receptor (SR-A) multifunctional? — The mouse’s tale. J Clin Invest. 2001;108:649–654. doi: 10.1172/JCI13903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Krieger M. Scavenger receptor class B type I is a multiligand HDL receptor that influences diverse physiologic systems. J Clin Invest. 2001;108:793–797. doi: 10.1172/JCI14011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Savill J, Fadok V. Corpse clearance defines the meaning of cell death. Nature. 2000;407:784–788. doi: 10.1038/35037722. [DOI] [PubMed] [Google Scholar]
- 8.Fadok VA, Bratton DL, Frasch SC, Warner ML, Henson PM. The role of phosphatidylserine in recognition of apoptotic cells by phagocytes see comments. Cell Death Differ. 1998;5:551–562. doi: 10.1038/sj.cdd.4400404. [DOI] [PubMed] [Google Scholar]
- 9.Fadok VA, de Cathelineau A, Daleke DL, Henson PM, Bratton DL. Loss of phospholipid asymmetry and surface exposure of phosphatidylserine is required for phagocytosis of apoptotic cells by macrophages and fibroblasts. J Biol Chem. 2001;276:1071–1077. doi: 10.1074/jbc.M003649200. [DOI] [PubMed] [Google Scholar]
- 10.Daleke DL, Lyles JV. Identification and purification of aminophospholipid flippases. Biochim Biophys Acta. 2000;1486:108–127. doi: 10.1016/s1388-1981(00)00052-4. [DOI] [PubMed] [Google Scholar]
- 11.Bevers EM, Comfurius P, Dekkers DW, Zwaal RF. Lipid translocation across the plasma membrane of mammalian cells. Biochim Biophys Acta. 1999;1439:317–330. doi: 10.1016/s1388-1981(99)00110-9. [DOI] [PubMed] [Google Scholar]
- 12.Sims PJ, Wiedmer T. Unraveling the mysteries of phospholipid scrambling. Thromb Haemost. 2001;86:266–275. [PubMed] [Google Scholar]
- 13.Fadeel B, et al. Phosphatidylserine exposure during apoptosis is a cell-type-specific event and does not correlate with plasma membrane phospholipid scramblase expression. Biochem Biophys Res Commun. 1999;266:504–511. doi: 10.1006/bbrc.1999.1820. [DOI] [PubMed] [Google Scholar]
- 14.Zhang G, Gurtu V, Kain SR, Yan G. Early detection of apoptosis using a fluorescent conjugate of annexin V. Biotechniques. 1997;23:525–531. doi: 10.2144/97233pf01. [DOI] [PubMed] [Google Scholar]
- 15.Frasch SC, et al. Regulation of phospholipid scramblase activity during apoptosis and cell activation by protein kinase Cdelta. J Biol Chem. 2000;275:23065–23073. doi: 10.1074/jbc.M003116200. [DOI] [PubMed] [Google Scholar]
- 16.Kagan VE, et al. Oxidative signaling pathway for externalization of plasma membrane phosphatidylserine. FEBS Lett. 2000;477:1–7. doi: 10.1016/s0014-5793(00)01707-5. [DOI] [PubMed] [Google Scholar]
- 17.Tyurina YY, et al. Phospholipid signaling in apoptosis: peroxidation and externalization of phosphatidylserine. Toxicology. 2000;148:93–101. doi: 10.1016/s0300-483x(00)00199-2. [DOI] [PubMed] [Google Scholar]
- 18.Tang X, Halleck MS, Schegel RA, Williamson P. A subfamily of P-type ATPases with aminophospholipid transporting activity. Science. 1996;272:1495–1497. doi: 10.1126/science.272.5267.1495. [DOI] [PubMed] [Google Scholar]
- 19.Siegmund A, et al. Loss of Drs2p does not abolish transfer of fluorescence-labeled phospholipids across the plasma membrane of Saccharomyces cerevisiae. J Biol Chem. 1998;273:34399–34405. doi: 10.1074/jbc.273.51.34399. [DOI] [PubMed] [Google Scholar]
- 20.Ding J, et al. Identification and functional expression of four isoforms of ATPase II, the putative aminophospholipid translocase. Effect of isoform variation on the ATPase activity and phospholipid specificity. J Biol Chem. 2000;275:23378–23386. doi: 10.1074/jbc.M910319199. [DOI] [PubMed] [Google Scholar]
- 21.Hamon Y, et al. ABC1 promotes engulfment of apoptotic cells and transbilayer redistribution of phosphatidylserine. Nat Cell Biol. 2000;2:399–406. doi: 10.1038/35017029. [DOI] [PubMed] [Google Scholar]
- 22.Marguet D, Luciani MF, Moynault A, Williamson P, Chimini G. Engulfment of apoptotic cells involves the redistribution of membrane phosphatidylserine on phagocyte and prey. Nat Cell Biol. 1999;1:454–456. doi: 10.1038/15690. [DOI] [PubMed] [Google Scholar]
- 23.Callahan MK, Williamson P, Schegel RA. Surface expression of phosphatidylserine on macrophages is required for phagocytosis of apoptotic thymocytes. Cell Death Differ. 2000;7:645–653. doi: 10.1038/sj.cdd.4400690. [DOI] [PubMed] [Google Scholar]
- 24.Luciani MF, Chimini G. The ATP binding cassette transporter ABC1, is required for the engulfment of corpses generated by apoptotic cell death. EMBO J. 1996;15:226–235. [PMC free article] [PubMed] [Google Scholar]
- 25.Wu YC, Horvitz HR. The C. elegans cell corpse engulfment gene ced-7 encodes a protein similar to ABC transporters. Cell. 1998;93:951–960. doi: 10.1016/s0092-8674(00)81201-5. [DOI] [PubMed] [Google Scholar]
- 26.Fadok VA, et al. A receptor for phosphatidylserine-specific clearance of apoptotic cells. Nature. 2000;405:85–90. doi: 10.1038/35011084. [DOI] [PubMed] [Google Scholar]
- 27.Casciola-Rosen L, Rosen A, Petri M, Schlissel M. Surface blebs on apoptotic cells are sites of enhanced procoagulant activity: implications for coagulation events and antigenic spread in systemic lupus erythematosus. Proc Natl Acad Sci USA. 1996;93:1624–1629. doi: 10.1073/pnas.93.4.1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gregory CD. CD14-dependent clearance of apoptotic cells: relevance to the immune system. Curr Opin Immunol. 2000;12:27–34. doi: 10.1016/s0952-7915(99)00047-3. [DOI] [PubMed] [Google Scholar]
- 29.Herrmann M, et al. Impaired phagocytosis of apoptotic cell material by monocyte-derived macrophages from patients with systemic lupus erythematosus. Arthritis Rheum. 1998;41:1241–1250. doi: 10.1002/1529-0131(199807)41:7<1241::AID-ART15>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 30.Dini L, Autuori F, Lentini A, Oliverio S, Piacentini M. The clearance of apoptotic cells in the liver is mediated by the asialoglycoprotein receptor. FEBS Lett. 1992;296:174–178. doi: 10.1016/0014-5793(92)80373-o. [DOI] [PubMed] [Google Scholar]
- 31.Duvall E, Wyllie AH, Morris RG. Macrophage recognition of cells undergoing programmed cell death (apoptosis) Immunology. 1985;56:351–358. [PMC free article] [PubMed] [Google Scholar]
- 32.Taylor PR, et al. A hierarchical role for classical pathway complement proteins in the clearance of apoptotic cells in vivo. J Exp Med. 2000;192:359–366. doi: 10.1084/jem.192.3.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ogden CA, et al. C1q and mannose binding lectin (MBL) engagement of cell surface calreticulin and CD91 to initiates macropinocytosis and uptake of apoptotic cells. J Exp Med. 2001;194:781–795. doi: 10.1084/jem.194.6.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schagat TL, Wofford JA, Wright JR. Surfactant protein A enhances alveolar macrophage phagocytosis of apoptotic neutrophils. J Immunol. 2001;166:2727–2733. doi: 10.4049/jimmunol.166.4.2727. [DOI] [PubMed] [Google Scholar]
- 35.Mevorach D, Mascarenhas JO, Gershov D, Elkon KB. Complement-dependent clearance of apoptotic cells by human macrophages. J Exp Med. 1998;188:2313–2320. doi: 10.1084/jem.188.12.2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chang MK, et al. Monoclonal antibodies against oxidized low-density lipoprotein bind to apoptotic cells and inhibit their phagocytosis by elicited macrophages: evidence that oxidation-specific epitopes mediate macrophage recognition. Proc Natl Acad Sci USA. 1999;96:6353–6358. doi: 10.1073/pnas.96.11.6353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pradhan D, Krahling S, Williamson P, Schlegel RA. Multiple systems for recognition of apoptotic lymphocytes by macrophages. Mol Biol Cell. 1997;8:767–778. doi: 10.1091/mbc.8.5.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ii M, Kurata H, Itoh N, Yamashima I, Kawasaki T. Molecular cloning and sequence analysis of cDNA encoding the macrophage lectin specific for galactose and N-acetylgalactosamine. J Biol Chem. 1990;265:11295–11298. [PubMed] [Google Scholar]
- 39.Beppu M. Mechanism of removal of aged cells, oxidized cells and apoptotic cells through carbohydrate chains. Seikagaku. 2001;73:196–200. [PubMed] [Google Scholar]
- 40.Franc NC, Heitzler P, Ezekowitz RA, White K. Requirement for croquemort in phagocytosis of apoptotic cells in Drosophila. Science. 1999;284:1991–1994. doi: 10.1126/science.284.5422.1991. [DOI] [PubMed] [Google Scholar]
- 41.Zhou Z, Hartwieg E, Hrovitz HR. CED-1 is a transmembrane receptor that mediates cell corpse engulfment in C. elegans. Cell. 2001;104:43–56. doi: 10.1016/s0092-8674(01)00190-8. [DOI] [PubMed] [Google Scholar]
- 42.Pearson AM. Scavenger receptors in innate immunity. Curr Opin Immunol. 1996;8:20–28. doi: 10.1016/s0952-7915(96)80100-2. [DOI] [PubMed] [Google Scholar]
- 43.Albert ML, et al. Immature dendritic cells phagocytose apoptotic cells via alphavbeta5 and CD36, and cross-present antigens to cytotoxic T lymphocytes. J Exp Med. 1998;188:1359–1368. doi: 10.1084/jem.188.7.1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Albert ML, Kim JI, Birge RB. alphavbeta5 integrin recruits the CrkII-Dock180-rac1 complex for phagocytosis of apoptotic cells. Nat Cell Biol. 2000;2:899–905. doi: 10.1038/35046549. [DOI] [PubMed] [Google Scholar]
- 45.Balasubramanian K, Chandra J, Schroit AJ. Immune clearance of phosphatidylserine-expressing cells by phagocytes. The role of beta2-glycoprotein I in macrophage recognition. J Biol Chem. 1997;272:31113–31117. doi: 10.1074/jbc.272.49.31113. [DOI] [PubMed] [Google Scholar]
- 46.Ishimoto Y, Ohashi K, Mizuno K, Nakano T. Promotion of the uptake of PS liposomes and apoptotic cells by a product of growth arrest-specific gene, gas6. J Biochem (Tokyo) 2000;127:411–417. doi: 10.1093/oxfordjournals.jbchem.a022622. [DOI] [PubMed] [Google Scholar]
- 47.Fadok VA, et al. Exposure of phosphatidylserine on the surface of apoptotic lymphocytes triggers specific recognition and removal by macrophages. J Immunol. 1992;148:2207–2216. [PubMed] [Google Scholar]
- 48.Clissold PM, Ponting CP. JmjC: cupin metalloenzyme-like domains in jumonji, hairless, and phospholipase A2beta. Trends Biochem Sci. 2001;26:7–9. doi: 10.1016/s0968-0004(00)01700-x. [DOI] [PubMed] [Google Scholar]
- 49.Scott RS, et al. Phagocytosis and clearance of apoptotic cells is mediated by MER. Nature. 2001;411:207–211. doi: 10.1038/35075603. [DOI] [PubMed] [Google Scholar]
- 50.Botto M, et al. Homozygous C1q deficiency causes glomerulonephritis associated with multiple apoptotic bodies. Nat Genet. 1998;19:56–59. doi: 10.1038/ng0598-56. [DOI] [PubMed] [Google Scholar]
- 51.Nakano T, et al. Cell adhesion to phosphatidylserine mediated by a product of growth arrest-specific gene 6. J Biol Chem. 1997;272:29411–29414. doi: 10.1074/jbc.272.47.29411. [DOI] [PubMed] [Google Scholar]
- 52.Herz J, Strickland DK. LRP: a multifunctional scavenger and signaling receptor. J Clin Invest. 2001;108:779–784. doi: 10.1172/JCI13992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Korb LC, Ahearn JM. C1q binds directly and specifically to surface blebs of apoptotic human keratinocytes: complement deficiency and systemic lupus erythematosus revisited. J Immunol. 1997;158:4525–4528. [PubMed] [Google Scholar]
- 54.Ellis RE, Jacobson DM, Horvitz HR. Genes required for the engulfment of cell corpses during programmed cell death in Caenorhabditis elegans. Genetics. 1991;129:79–94. doi: 10.1093/genetics/129.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Reddien PW, Horvitz HR. CED-2/CrkII and CED-10/Rac control phagocytosis and cell migration in Caenorhabditis elegans. Nat Cell Biol. 2000;2:131–136. doi: 10.1038/35004000. [DOI] [PubMed] [Google Scholar]
- 56.Wu YC, Horvitz HR. C. elegans phagocytosis and cell-migration protein CED-5 is similar to human DOCK180. Nature. 1998;392:501–504. doi: 10.1038/33163. [DOI] [PubMed] [Google Scholar]
- 57.Tosello-Trampont A-C, Brugnera E, Ravichandran KS. Evidence for a conserved role for CrKII and Rac in engulfment of apoptotic cells. J Biol Chem. 2001;276:13797–13802. doi: 10.1074/jbc.M011238200. [DOI] [PubMed] [Google Scholar]
- 58.Liu QA, Hengartner MO. Candidate adaptor protein CED-6 promotes the engulfment of apoptotic cells in C. elegans. Cell. 1998;93:961–972. doi: 10.1016/s0092-8674(00)81202-7. [DOI] [PubMed] [Google Scholar]
- 59.Liu QA, Hengartner MO. Human CED-6 encodes a functional homologue of the Caenorhabditis elegans engulfment protein CED-6. Curr Biol. 1999;9:1347–1350. doi: 10.1016/s0960-9822(00)80061-5. [DOI] [PubMed] [Google Scholar]
- 60.Smits E, Van Criekinge W, Plaetinck G, Bogaert T. The human homologue of Caenorhabditis elegans CED-6 specifically promotes phagocytosis of apoptotic cells. Curr Biol. 1999;9:1351–1354. doi: 10.1016/s0960-9822(00)80062-7. [DOI] [PubMed] [Google Scholar]
- 61.Su HP, et al. Identification and characterization of a dimerization domain in CED-6, an adapter protein involved in engulfment of apoptotic cells. J Biol Chem. 2000;275:9542–9549. doi: 10.1074/jbc.275.13.9542. [DOI] [PubMed] [Google Scholar]
- 62.Voll RE, et al. Immunosuppressive effects of apoptotic cells. Nature. 1997;390:350–351. doi: 10.1038/37022. [DOI] [PubMed] [Google Scholar]
- 63.Niederkorn JY. Anterior chamber-associated immune deviation. Chem Immunol. 1999;73:59–71. doi: 10.1159/000058740. [DOI] [PubMed] [Google Scholar]
- 64.Tomimori Y, Ikawa Y, Oyaizu N. Ultraviolet-irradiated apoptotic lymphocytes produce interleukin-10 by themselves. Immunol Lett. 2000;71:49–54. doi: 10.1016/s0165-2478(99)00163-7. [DOI] [PubMed] [Google Scholar]
- 65.Rovere P, et al. Delayed clearance of apoptotic lymphoma cells allows cross-presentation of intracellular antigens by mature dendritic cells. J Leukoc Biol. 1999;66:345–349. doi: 10.1002/jlb.66.2.345. [DOI] [PubMed] [Google Scholar]
- 66.Steinman RM, Turley S, Mellman I, Inaba K. The induction of tolerance by dendritic cells that have captured apoptotic cells. J Exp Med. 2000;191:411–416. doi: 10.1084/jem.191.3.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Binder RJ, Han DK, Srivastava PK. CD91: a receptor for heat shock protein gp96. Nat Immunol. 2000;1:151–155. doi: 10.1038/77835. [DOI] [PubMed] [Google Scholar]
- 68.Green DR, Beere HM. Apoptosis. Gone but not forgotten. Nature. 2000;405:85–90. doi: 10.1038/35011175. [DOI] [PubMed] [Google Scholar]
- 69.Freire-de-Lima CG, et al. Uptake of apoptotic cells drives the growth of a pathogenic trypanosome in macrophages. Nature. 2000;403:199–203. doi: 10.1038/35003208. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.