Abstract
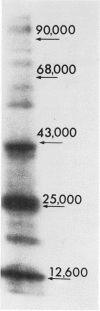
In the present study the tube LAI assay was used to monitor the isolation of the TSA of 4 different types of human cancers. Each tumour antigen was found to be specific for tumours arising in the organ from which the TSA was initially derived and which were histopathologically similar. Immunochemical studies revealed that these molecules co-isolate with normal human HLA antigens and are associated with beta2m. On Sephadex G-150, the majority of the papain-solubilized tumour antigen eluted in the mol. wt range 70,000-150,000. Analysis of this material by SDS-PAGE and 6M guanidine-HC1 column chromatography indicated that the material is composed of smaller subunits with prominent peaks at approximately 40,000, 25,000 and 12,000 mol. wt. Immunoadsorbent affinity chromatography of the solubilized tumour-membrane constituents on AH-Sepharose-linked horse anti-human-beta2m indicated that the tumour antigens, like HLA molecules, contain a beta2m subunit. The specificity of binding of TSA to the immunoadsorbent columns and the immunologically specific abrogation of LAI reactivity were clearly shown. The present study, therefore, indicates that by the isolation of beta2m, human tumour antigens can also be isolated, since human tumour antigens are associated with beta2m. Whether human TSAs may perhaps be modified histocompatibility antigens remains to be answered. Although the change upon malignant transformation in the pattern of the cell-surface proteins expressing the TSA determinant remains obscure, it would appear that for tumours arising within a given organ, a consistent alteration of cell-surface proteins occurs.
Full text
PDF






















Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Artzt K., Bennett D. Analogies between embryonic (T/t) antigens and adult major histocompatibility (H-2) antigens. Nature. 1975 Aug 14;256(5518):545–547. doi: 10.1038/256545a0. [DOI] [PubMed] [Google Scholar]
- Baldwin R. W., Barker C. R. Demonstration of tumour-specific humoral antibody against aminoazo dye-induced rat hepatomata. Br J Cancer. 1967 Dec;21(4):793–800. doi: 10.1038/bjc.1967.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin R. W., Glaves D. Solubilization of tumour-specific antigen from plasma membrane of an aminoazo-dye-induced rat hepatoma. Clin Exp Immunol. 1972 May;11(1):51–56. [PMC free article] [PubMed] [Google Scholar]
- Bevan M. J. The major histocompatibility complex determines susceptibility to cytotoxic T cells directed against minor histocompatibility antigens. J Exp Med. 1975 Dec 1;142(6):1349–1364. doi: 10.1084/jem.142.6.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blank K. J., Lilly F. Evidence for an H-2/viral protein complex on the cell surface as the basis for the H-2 restriction of cytotoxicity. Nature. 1977 Oct 27;269(5631):808–809. doi: 10.1038/269808a0. [DOI] [PubMed] [Google Scholar]
- Bowen J. G., Baldwin R. W. Tumour-specific antigen related to rat histocompatibility antigens. Nature. 1975 Nov 6;258(5530):75–76. doi: 10.1038/258075a0. [DOI] [PubMed] [Google Scholar]
- Comoglio P. M., Bertini M., Forni G. Evidence for a membrane carrier molecule common to embryonal and tumour-specific antigenic determinants expressed by a mouse transplantable tumour. Immunology. 1975 Aug;29(2):353–364. [PMC free article] [PubMed] [Google Scholar]
- Cresswell P., Ayres J. L. HLA antigens: rabbit antisera reacting with all A series or all B series specificities. Eur J Immunol. 1976 Feb;6(2):82–88. doi: 10.1002/eji.1830060203. [DOI] [PubMed] [Google Scholar]
- Doherty P. C., Blanden R. V., Zinkernagel R. M. Specificity of virus-immune effector T cells for H-2K or H-2D compatible interactions: implications for H-antigen diversity. Transplant Rev. 1976;29:89–124. doi: 10.1111/j.1600-065x.1976.tb00198.x. [DOI] [PubMed] [Google Scholar]
- Flores M., Marti J. H., Grosser N., MacFarlane J. K., Thomson D. M. An overview: antitumor immunity in breast cancer assayed by tube leukocyte adherence inhibition. Cancer. 1977 Feb;39(2):494–505. doi: 10.1002/1097-0142(197702)39:2<494::aid-cncr2820390218>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Forman J., Vitetta E. S. Absence of H-2 antigens capable of reacting with cytotoxic T cells on a teratoma line expressing a T/t locus antigen. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3661–3665. doi: 10.1073/pnas.72.9.3661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujisawa T., Waldman S. R., Yonemoto R. H. Leukocyte adherence inhibition by soluble tumor antigens in breast cancer patients. Cancer. 1977 Feb;39(2):506–513. doi: 10.1002/1097-0142(197702)39:2<506::aid-cncr2820390219>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- GREENWOOD F. C., HUNTER W. M., GLOVER J. S. THE PREPARATION OF I-131-LABELLED HUMAN GROWTH HORMONE OF HIGH SPECIFIC RADIOACTIVITY. Biochem J. 1963 Oct;89:114–123. doi: 10.1042/bj0890114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gooding L. R., Edidin M. Cell surface antigens of a mouse testicular teratoma. Identification of an antigen physically associated with H-2 antigens on tumor cells. J Exp Med. 1974 Jul 1;140(1):61–78. doi: 10.1084/jem.140.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon R. D., Simpson E., Samelson L. E. In vitro cell-mediated immune responses to the male specific(H-Y) antigen in mice. J Exp Med. 1975 Nov 1;142(5):1108–1120. doi: 10.1084/jem.142.5.1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graff R. J., Nathenson S. G. Immunogenic properties of papain-solubilized alloantigen. Transplant Proc. 1971 Mar;3(1):249–252. [PubMed] [Google Scholar]
- Grosser N., Marti J. H., Proctor J. W., Thomson D. M. Tube leukocyte adherence inhibition assay for the detection of anti-tumor immunity. I. Monocyte is the reactive cell. Int J Cancer. 1976 Jul 15;18(1):39–47. doi: 10.1002/ijc.2910180107. [DOI] [PubMed] [Google Scholar]
- Grosser N., Thompson D. M. Tube leukocyte (monocyte) adherence inhibition assay for the detection of anti-tumour immunity. III. "Blockade" of monocyte reactivity by excess free antigen and immune complexes in advanced cancer patients. Int J Cancer. 1976 Jul 15;18(1):58–66. doi: 10.1002/ijc.2910180109. [DOI] [PubMed] [Google Scholar]
- Halliday W. J., Maluish A. E., Little J. H., Davis N. C. Leukocyte adherence inhibition and specific immunoreactivity in malignant melanoma. Int J Cancer. 1975 Oct 15;16(4):645–658. doi: 10.1002/ijc.2910160415. [DOI] [PubMed] [Google Scholar]
- Halliday W. J., Miller S. Leukocyte adherence inhibition: a simple test for cell-mediated tumour immunity and serum blocking factors. Int J Cancer. 1972 May 15;9(3):477–483. doi: 10.1002/ijc.2910090304. [DOI] [PubMed] [Google Scholar]
- Holmes E. C., Kahan B. D., Morton D. L. Soluble tumor-specific transplantation antigens from methylcholanthrene-induced guinea pig sarcomas. Cancer. 1970 Feb;25(2):373–379. doi: 10.1002/1097-0142(197002)25:2<373::aid-cncr2820250215>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- Holán V., Hasek M., Bubeník J., Chutná J. Antigen-mediated macrophage adherence inhibition. Cell Immunol. 1974 Jul;13(1):107–116. doi: 10.1016/0008-8749(74)90231-7. [DOI] [PubMed] [Google Scholar]
- Invernizzi G., Parmiani G. Tumour-associated transplantation antigens of chemically induced sarcomata cross reacting with allogeneic histocompatibility antigens. Nature. 1975 Apr 24;254(5502):713–714. doi: 10.1038/254713a0. [DOI] [PubMed] [Google Scholar]
- Kessler S. W. Rapid isolation of antigens from cells with a staphylococcal protein A-antibody adsorbent: parameters of the interaction of antibody-antigen complexes with protein A. J Immunol. 1975 Dec;115(6):1617–1624. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leveson S. H., Howell J. H., Holyoke E. D., Goldrosen M. H. Leukocyte adherence inhibition: an automated microassay demonstrating specific antigen recognition and blocking activity in two murine tumor systems. J Immunol Methods. 1977;17(1-2):153–162. doi: 10.1016/0022-1759(77)90086-2. [DOI] [PubMed] [Google Scholar]
- Lopez M. J., Thomson D. M. Isolation of breast cancer tumour antigen from serum and urine. Int J Cancer. 1977 Dec 15;20(6):834–848. doi: 10.1002/ijc.2910200604. [DOI] [PubMed] [Google Scholar]
- Marti J. H., Grosser N., Thomson D. M. Tube leukocyte adherence inhibition assay for the detection of anti-tumour immunity. II. Monocyte reacts with tumour antigen via cytophilic anti-tumour antibody. Int J Cancer. 1976 Jul 15;18(1):48–57. doi: 10.1002/ijc.2910180108. [DOI] [PubMed] [Google Scholar]
- Marti J. H., Thomson D. M. Anti-tumour immunity in malignant melanoma assay by tube leucocyte adherence inhibition. Br J Cancer. 1976 Aug;34(2):116–133. doi: 10.1038/bjc.1976.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaelson J., Flaherty L., Vitetta E., Poulik M. D. Molecular similarities between the Qa-2 alloantigen and other gene products of the 17th chromosome of the mouse. J Exp Med. 1977 Apr 1;145(4):1066–1070. doi: 10.1084/jem.145.4.1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamuro K., Tanigaki N., Pressman D. Common antigenic structures of HLA antigens. VII. Selective combination binding of beta2-microglobulin with HLA large component in cultured human cell lines. Immunology. 1977 Feb;32(2):139–150. [PMC free article] [PubMed] [Google Scholar]
- OLD L. J., BOYSE E. A. IMMUNOLOGY OF EXPERIMENTAL TUMORS. Annu Rev Med. 1964;15:167–186. doi: 10.1146/annurev.me.15.020164.001123. [DOI] [PubMed] [Google Scholar]
- Ohno S. The original function of MHC antigens as the general plasma membrane anchorage site of organogenesis-directing proteins. Immunol Rev. 1977 Jan;33:59–69. [PubMed] [Google Scholar]
- Ostberg L., Rask L., Wigzell H., Peterson P. A. Thymus leukaemia antigen contains beta2-microglobulin. Nature. 1975 Feb 27;253(5494):735–737. doi: 10.1038/253735a0. [DOI] [PubMed] [Google Scholar]
- Peterson P. A., Rask L., Lindblom J. B. Highly purified papain-solubilized HL-A antigens contain beta2-microglobulin. Proc Natl Acad Sci U S A. 1974 Jan;71(1):35–39. doi: 10.1073/pnas.71.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell A. E., Sloss A. M., Smith R. N., Makley J. T., Hubay C. A. Specific responsiveness of leukocytes to soluble extracts of human tumors. Int J Cancer. 1975 Dec 15;16(6):905–913. doi: 10.1002/ijc.2910160604. [DOI] [PubMed] [Google Scholar]
- Robb R. J., Strominger J. L. Rapid purification of detergent-solubilized HLA antigen by affinity chromatography employing anti-beta2-microglobulin serum. J Biol Chem. 1976 Sep 10;251(17):5427–5428. [PubMed] [Google Scholar]
- Rutherford J. C., Walters B. A., Cavaye G., Halliday W. J. A modified leukocyte adherence inhibition test in the laboratory investigation of gastrointestinal cancer. Int J Cancer. 1977 Jan;19(1):43–48. doi: 10.1002/ijc.2910190107. [DOI] [PubMed] [Google Scholar]
- Sanderson A. R. HLA "help" for human B2-microglobulin across species barriers. Nature. 1977 Sep 29;269(5627):414–417. doi: 10.1038/269414a0. [DOI] [PubMed] [Google Scholar]
- Schrader J. W., Cunningham B. A., Edelman G. M. Functional interactions of viral and histocompatibility antigens at tumor cell surfaces. Proc Natl Acad Sci U S A. 1975 Dec;72(12):5066–5070. doi: 10.1073/pnas.72.12.5066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrader J. W., Edelman G. M. Participation of the H-2 antigens of tumor cells in their lysis by syngeneic T cells. J Exp Med. 1976 Mar 1;143(3):601–614. doi: 10.1084/jem.143.3.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith M., Gold P., Freedman S. O., Shuster J. Studies of the linkage relationship of beta-2-microglobulin in man-mouse somatic cell hybrids. Ann Hum Genet. 1975 Jul;39(1):21–31. doi: 10.1111/j.1469-1809.1975.tb00104.x. [DOI] [PubMed] [Google Scholar]
- Thomson D. M., Alexander P. A cross-reacting embryonic antigen in the membrane of rat sarcoma cells which is immunogenic in the syngeneic host. Br J Cancer. 1973 Jan;27(1):35–47. doi: 10.1038/bjc.1973.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson D. M., Gold P., Freedman S. O., Shuster J. The isolation and characterization of tumor-specific antigens of rodent and human tumors. Cancer Res. 1976 Sep;36(9 Pt 2):3518–3525. [PubMed] [Google Scholar]
- Thomson D. M., Sellens V., Eccles S., Alexander P. Radioimmunoassay of tumour specific transplantation antigen of a chemically induced rat sarcoma: circulating soluble tumour antigen in tumour bearers. Br J Cancer. 1973 Nov;28(5):377–388. doi: 10.1038/bjc.1973.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Zinkernagel R. M., Doherty P. C. Immunological surveillance against altered self components by sensitised T lymphocytes in lymphocytic choriomeningitis. Nature. 1974 Oct 11;251(5475):547–548. doi: 10.1038/251547a0. [DOI] [PubMed] [Google Scholar]
- Zoller M., Matzku S. Antigen and antibody purification by immunoadsorption: elimination of non-biospecifically bound proteins. J Immunol Methods. 1976;11(3-4):287–295. doi: 10.1016/0022-1759(76)90122-8. [DOI] [PubMed] [Google Scholar]