Abstract
Background
Annexin II heavy chain (also called p36, calpactin I) is lost in prostate cancers and in a majority of prostate intraepithelial neoplasia (PIN). Loss of annexin II heavy chain appears to be specific for prostate cancer since overexpression of annexin II is observed in a majority of human cancers, including pancreatic cancer, breast cancer and brain tumors. Annexin II exists as a heterotetramer in complex with a protein ligand p11 (S100A10), and as a monomer. Diverse cellular functions are proposed for the two forms of annexin II. The monomer is involved in DNA synthesis. A leucine-rich nuclear export signal (NES) in the N-terminus of annexin II regulates its nuclear export by the CRM1-mediated nuclear export pathway. Mutation of the NES sequence results in nuclear retention of annexin II.
Results
Annexin II localized in the nucleus is phosphorylated, and the appearance of nuclear phosphorylated annexin II is cell cycle dependent, indicating that phosphorylation may play a role in nuclear entry, retention or export of annexin II. By exogenous expression of annexin II in the annexin II-null LNCaP cells, we show that wild-type annexin II is excluded from the nucleus, whereas the NES mutant annexin II localizes in both the nucleus and cytoplasm. Nuclear retention of annexin II results in reduced cell proliferation and increased doubling time of cells. Expression of annexin II, both wild type and NES mutant, causes morphological changes of the cells. By site-specific substitution of glutamic acid in the place of serines 11 and 25 in the N-terminus, we show that simultaneous phosphorylation of both serines 11 and 25, but not either one alone, prevents nuclear localization of annexin II.
Conclusion
Our data show that nuclear annexin II is phosphorylated in a cell cycle-dependent manner and that substitution of serines 11 and 25 inhibit nuclear entry of annexin II. Aberrant accumulation of nuclear annexin II retards proliferation of LNCaP cells.
Background
Annexins are a family of proteins that have been isolated from a variety of cells and tissues and involved in diverse physiological activities. The amino acid sequence of annexins consists of a variable amino terminal "tail" domain followed by four or eight conserved repeats. The common characteristic of the annexins is that they bind to biological membranes and anionic phospholipids in a Ca2+-dependent manner through their conserved four or eight repeats [1]. The unique amino terminal tail of each annexin confers its functional and regulatory specificity. Annexin II exists as two forms in the cells, as a heterotetramer and as a 36 kD monomer. In the heterotetramer, 2 molecules of annexin II bind to 2 molecules S100A10. The annexin II tetramer exists in the sub-plasmalemmal cytoskeleton network in different cells types [2,3], and is implicated in a number of membrane-related events, including the Ca2+-dependent regulation of exocytosis in chromaffin cells and endocytotic pathway [4-6]. It was shown that the binding of p11 to annexin II could increase the affinity of annexin II for Ca2+ and account for exocytosis in adrenergic cells [7]. As a monomer, annexin II is found in both the cytoplasm and nucleus, but predominantly in the cytoplasm. Given the difference between annexin II and p11 expression levels in different cell types, the heterotetramer and the monomer may have different functions [8,9]. The function of the annexin II monomer in the nucleus was suggested by its purification as part of a primer recognition protein complex that enhances DNA polymerase α activity in vitro [10,11]. The role of annexin II in DNA synthesis and cell proliferation was demonstrated by immunodepletion of annexin II from Xenopus egg extracts which resulted in the loss of DNA replication in these extracts [12], and antisense oligonucleotides to annexin II reduced DNA synthesis in HeLa cells and retarded progression of cells through the cell cycle [13,14]. Like many other members of annexin family, annexin II can be phosphorylated in its N-terminus. Serine 25 was reported to be phosphorylated by protein kinase C (PKC) both in vivo and in vitro, while serine 11 can only be phosphorylated in vitro [15,16]. In addition to serine 11 and 25, tyrosine 23 is the phosphorylation site of protein tyrosine kinase (PTK) pp60src, suggesting a function in cell growth [17]. The effects of Tyr or Ser phosphorylation on annexin II function are largely unknown, although previous studies indicate that phosphorylation affects the lipid binding characteristics of the protein. For example, Tyr phosphorylation of annexin II by pp60src decreases the binding of the protein to phospholipid vesicles at low Ca2+ concentrations [18], whereas Ser phosphorylation of the protein by protein kinase C inhibits its ability to aggregate phospholipid vesicles [19].
Annexin II expression is lost in prostate cancers and in a majority of prostatic intraepithelial neoplasia (PIN) [20]. Loss of annexin II appears to be specific for prostate cancer because over expression of annexin II is observed in other human cancers, including lung cancer, pancreatic cancer, breast cancer and brain tumors [21-39]. We have previously reported that annexin II is present at high levels in human and hamster pancreatic cancer cells and tissues [38,39], and its expression was localized to invasive areas of the cancer and in metastatic foci.
Recently, a nuclear export signal was identified in the N-terminal of annexin II, which overlaps the p11 binding site and is close to the phosphorylation sites of a 60 kD phosphoprotein encoded by the src oncogene (pp60src) and protein kinase C [40]. The role of phosphorylation of annexin II is not understood, and because of the proximity of the potential phosphorylation sites to the nuclear export signal, we propose that phosphorylation of these sites may modulate the nucleo-cytoplasmic distribution of annexin II. Our data show that phosphorylated annexin II localizes in the cell nucleus, and that annexin II phosphorylation and the nuclear retention is cell cycle dependent. Annexin II is exported from the nucleus in the annexin II-null LNCaP cells transfected with wild-type annexin II either transiently or stably, and this nuclear export is mediated by CRM1 pathway. Ectopic expression of annexin II results in changes in cell morphology. Accumulation of annexin II in the nucleus of NES mutant significantly reduces cell proliferation. Site directed changes of serines 11 and 25 to glutamic acid prevents nuclear entry of annexin II.
Results
Cell cycle-dependent phosphorylation and nuclear retention of annexin II in cells
The annexin II N-terminus is distinct from the N-termini of other annexins and contains sites for p11 binding and phosphorylation by PKC and pp60src. Thus, modifications in the N-terminus of annexin II may regulate its cellular function. Recently, a nuclear export signal (NES) was identified in the N-terminus of annexin II [40]. The NES overlaps the p11 binding site and one of the PKC phosphorylation sites, and it is also in proximity to the PKC and pp60src phosphorylation sites. Phosphorylation regulates nuclear export of other NES-containing proteins [41,42]. Cytosolic and nuclear extracts from unsynchronized human K562 cells that express endogenous annexin II were subjected to SDS-PAGE and immunoblot analysis to determine whether annexin II in the cell nucleus is phosphorylated. The results shown in Figure 1 demonstrate that nuclear annexin II has a slower mobility in the SDS-PAGE than the cytosolic annexin II. The slower mobility form is sensitive to calf intestinal alkaline phosphatase (CIAP) treatment, which results in a change in gel mobility. These data suggest that nuclear annexin II is phosphorylated, and this phosphorylation could regulate the nuclear retention of annexin II. Additionally, we observed that treatment of cytosolic extract with CIAP resulted in a protein with faster mobility, suggesting annexin II exists in multiple phosphorylation states. Previous reports have described the mono- and diphosphorylated state of annexin II [43], and our data are consistent with existence of annexin II in these different phosphorylation states.
Figure 1.
Annexin II is phosphorylated in nuclear extract of K562 cells. Human K562 cells were fractionated into nuclear extract (NE) and cytosolic extract (CE). One aliquot of NE and CE was treated with CIAP prior to SDS-PAGE. Untreated CE and NE were subjected to incubation in the phosphatase buffer without CIAP. 20 μg protein from each extract were subjected to SDS-PAGE and immunoblotting with anti-annexin II antisera. Positions of the phosphorylated (upper two) and dephosphorylated annexin II are indicated by arrows.
The activities of many cell cycle regulatory proteins are controlled both by expression levels and phosphorylation status. Annexin II expression is cell cycle regulated [44,45]. We examined if phosphorylation of nuclear annexin II is cell cycle-dependent. K562 cells were subjected to centrifugal elutriation, a method that isolates cells according to their different sizes in different cell cycle phases. The cells in different cell cycle phases were collected, and cell cycle distribution in each of the fractions from centrifugal elutriation was confirmed by flow cytometry. Nuclear and cytosolic extracts prepared from cells in different cell cycle phases were subjected to SDS-PAGE and western blot analysis. Figure 2 shows the cell cycle dependent phosphorylation and nuclear retention of annexin II. Phosphorylated annexin II is seen in nuclear extracts of G1, G1/S and S/G2 cells, but no phosphorylated annexin II is observed when most of the cells are in S and G2/M phase of the cell cycle. The appearance of phosphorylated annexin II in the nucleus mimics the annexin II RNA levels during the HeLa cell cycle previously reported [44]. Curiously, when the cells were in the S/G2 phase of the cell cycle, we found reappearance of phosphorylated annexin II. Since Figure 1 shows that the anti-annexin II antibody can recognize both faster-mobility cytosolic annexin II and slower-mobility nuclear annexin II, the absence of annexin II in certain phases of cell cycle cannot be explained by the modification of annexin II that blocks the antibody recognition.
Figure 2.
Phosphorylation of nuclear annexin II and cell cycle distribution. Human K562 cells were subjected to centrifugal elutriation followed by flow cytometry to determine the cell cycle distribution of phosphorylated annexin II. Cells enriched in each indicated phase were fractionated to cytosolic extract (CE) and nuclear extract (NE). Twenty μg protein from each sample were subjected to SDS-PAGE followed by immunoblotting with anti-annexin II antibody. Immunoblot of PGK was used as an internal control. The flow cytometric profile of each fraction is presented in the upper panels and the percentage of cells in each cell cycle phase is indicated below the immunoblot under each fraction.
Annexin II-null LNCaP cells for the investigation of the functions of annexin II
Annexin II is over-expressed in most cancers and cell lines derived from such cancer tissues. However, annexin II expression is lost in prostate cancers. Hence, we examined a panel of established human prostate cancer cell lines to find an annexin II-null cell line that would serve as a useful model for studying the physiological role of annexin II. Figure 3 shows a western blot analysis using whole cell lysates from a panel of prostate cancer cell lines, including both androgen-responsive (LNCaP) and androgen-unresponsive (PC-3 and DU-145) cell lines, and cell lines used as positive controls (NIH 3T3 and SkBr-3). High levels of annexin II is expressed in PC-3, DU-145, NIH 3T3 and SkBr-3 cells, but no annexin II is detected in LNCaP cells of different passage numbers tested. LNCaP cells that are androgen-responsive progress to androgen-unresponsive upon continuous passages under normal growth conditions [46]. We analyzed if annexin II expression is related to androgen responsiveness. Annexin II expression was not observed in androgen-responsive and androgen-unresponsive LNCaP cells, either in the presence or absence of dihydrotestosterone (DHT) in the growth medium (data not shown). Northern blot (Figure 4) analysis was performed to determine if the loss of annexin II expression occurs at the transcriptional or at the translational level. Figure 4 shows that annexin II message is expressed at a high level in PC-3 and DU-145 cells. The density of cells growing in monolayer can also influence the expression of genes [47], so the LNCaP cells were seeded in either high or low density and the expression of annexin II gene was observed. No annexin II expression was detected in LNCaP cells plated at either high or low density with or without treatment with DHT. Our data indicate that annexin II expression is lost at the transcriptional level in LNCaP cells.
Figure 3.
Immunoblot analysis of annexin II in prostate cancer cells. Cell lysates of NIH3T3 (lane 1), SkBr-3 (lane 2), DU-145 (lane 3), PC-3 (lane 4) and LNCaP (passage 181, lane 5; passage 142, lane 6; passage 92, lane 7; passage 34, lane 8) were subjected to western blot analysis with mouse anti-human monoclonal anti-annexin II antibody as described in materials and methods. Lane 9 is the protein molecular markers used for SDS-PAGE. The position of annexin II band is indicated on the right side of the panel.
Figure 4.
Northern blot analysis of annexin II in prostate cancer cells. Prior to RNA extraction, LNCaP cells of both low level and high level of confluence were treated with 10 nM dihydrotestosterone (DHT) for 2 days. 20 μg RNA were extracted from PC-3 cells (lane 1), DU-145 cells (lane 2) and LNCaP cells treated with (lanes 3, 6) or without (lanes 4, 5) DHT. [32P]-labeled annexin II cDNA was used as probe (Panel A). The location of full length annexin II cDNA is indicated by an arrow on the left side of the panel. The membrane from panel A was probed with [32P]-labeled GAPDH cDNA for normalization of the annexin II level (Panel B).
Thus, LNCaP cells, which are annexin II-null, serve as useful recipient cells to investigate the function of exogenously expressed annexin II in the absence of background from endogenous annexin II.
Export of annexin II from the nucleus involves the CRM1 pathway
To confirm whether the annexin II-null LNCaP cells regulate NES containing proteins by the CRM1 mediated nuclear export pathway, we transfected LNCaP cells with pEGFP-C1 vector, pEGFP-C1-AnxII or pEGFP-C1-AnxII/L10AL12A, which encode GFP alone, GFP fused to N-terminus of wild-type annexin II (GFP-WT), and GFP fused to N-terminus of NES mutant annexin II (GFP-NES), respectively. Confocal microscopy images of transiently transfected LNCaP cells are shown in Figure 5. The presence of GFP was detected by auto-fluorescence of GFP (green). Immunostaining of annexin II was performed with secondary antibody conjugated to rhodamine (red). Colocalization of GFP and annexin II results in the appearance of yellow fluorescence. Figure 5 shows that both GFP and GFP-fused NES mutant annexin II distribute equally throughout the cell, whereas the GFP fused wild-type annexin II is observed only in the cytoplasm, indicating that the NES of wild type annexin II is still functional. The cellular distribution of wild-type annexin II without GFP is identical to the GFP fused wild-type annexin II (data not shown) indicating no effect of GFP fusion on the cellular distribution of annexin II. Mutation of the conserved leucines (L10 and L12) of the annexin II NES results in retention of annexin II in the nucleus.
Figure 5.
Localization of wild type and NES mutant annexin II in transiently transfected LNCaP cells. Transiently transfected LNCaP cells expressing GFP alone, GFP-wild type (GFP-WT) annexin II or GFP-NES mutant (GFP-NES) annexin II were fixed and subjected to immunocytochemistry with anti-annexin II antibody as described in materials and methods. The images were scanned using laser scanning confocal microscope (LSCM). The green color represents auto-fluorescence of GFP, the red color represents the rhodamine staining of annexin II antigen-antibody complex, and the yellow color is the overlay of GFP and annexin II images. Bar: 25 μm.
We established stable clones of LNCaP cells expressing GFP alone, wild-type annexin II or NES mutant annexin II. In these stable clones, we examined the distribution of annexin II by fractionating the cells and preparing cytosolic and nuclear extracts. These extracts were subjected to western blot analysis for annexin II. Figure 6 shows the distribution of GFP, GFP-WT and GFP-NES in LNCaP cells. As expected, there is no annexin II expressed in the LNCaP cell transfected with vector alone, and also there is no endogenous annexin II expressed in LNCaP cells (Figure 6A, lanes 1 and 2). However, in GFP-WT, cytosolic fraction contains a majority of annexin II (Figure 6A, lanes 3 and 4). GFP-NES was found to distribute equally between the cytoplasmic and nuclear extracts (Figure 6, panel A, lanes 5 and 6). In the nuclear extract, the unphosphorylated and phosphorylated annexin II are observed. These results are in agreement with data from K562 cells (Figure 2), as well as previously published literature [48]. We determined that the slower mobility band in the nuclear extract is phosphorylated by subjecting the nuclear extract to dephosphorylation with potato acid phosphatase. Figure 6 (panel C) shows that upon treatment with the phosphatase, the slower mobility band is shifted to the position corresponding to annexin II in the cytosolic extracts. These results are consistent with our observations in K562 cells (Figure 1). We performed immunoblot analysis of PGK to ensure that nuclear extracts were devoid of cytosolic contamination (Figure 6, panel B).
Figure 6.
Distribution of GFP and GFP-fused wild type or NES mutant annexin II in LNCaP cells. LNCaP cells expressing GFP, GFP-wild type annexin II (GFP-WT), and GFP-NES mutant annexin II (GFP-NES) were fractionated into cytosolic extract (CE) and nuclear extract (NE). Each extract was subjected to SDS-PAGE and immunoblot with anti-GFP (Panel A) and anti-PGK antibody (panel B). Cytosolic and nuclear extracts from the GFP-NES cells were subjected to potato acid phosphatase (PACP) treatment as described in materials and methods, and an immunoblot analysis was performed for annexin II (Panel C).
Stable expression of NES-Annexin II retards LNCaP cell proliferation
Because nuclear retention of annexin II is cell cycle dependent, this protein may play a role in regulating cell cycle progression and cell proliferation. We examined the effect of exogenous expression of wild type and NES mutant annexin II on the growth of LNCaP cells. Reexpression of annexin II results in significant morphological changes to the cells (Figure 7). The vector-alone transfection does not result in any morphological changes. The vector-alone transfection does not result in any morphological changes. However, expression of wild-type annexin II or NES mutant results in the cells taking on a mesenchymal phenotype. To eliminate clonal variation, which may occur in stably transfected cancer cells, and the possibility that NES mutant itself is affecting proliferation other than nuclear localization, we examined different clones or NES-transfected LNCaP cells. Data from two such clones (NES-1 and NES-4) are shown in Figure 8. The vector transfected cells and wild type annexin II transfected cells underwent cell doubling with a doubling time of approximately 34 hours as compared to 30 hours for the untransfected LNCaP cells, whereas the NES-mutant cells had a doubling time of 48 hours. The inhibition of cell proliferation by NES mutant annexin II suggests that abnormal accumulation of annexin II in the nucleus may lead to deregulation or inhibition of DNA replication. We compared the cell cycle distribution of logarithmically growing LNCaP cells and the three transfectants using flow cytometry, and observed no changes in the parental and transfected cells in the distribution of cells in different cell cycle phases (data not shown). This observation indicated that nuclear retention of NES-mutant annexin II does not inhibit the growth of LNCaP cells by blocking the cells at any certain phase of the cell cycle. These observations are similar to the finding that the expression of human Cdc6 mutant, HsCdc6E1E2E3, which is exclusively nuclear, inhibits initiation of DNA replication [49].
Figure 7.
Morphology of LNCaP cells transfected with wild type annexin II and NES-annexin II. Untransfected LNCaP and stable clones of vector-alone, GFP-WT and GFP-NES cells were cultured as described in materials and methods. Light microscopy images of each of the cultures are shown.
Figure 8.
Accumulation of annexin II in the nucleus retards cell proliferation. Parental LNCaP cells and stable clones of vector-alone (GFP), GFP-WT or GFP-NES mutant annexin II were cultured as described in materials and methods for up to 8 days. Two different GFP-NES clones (NES-1 and NES-4) were included. The cell numbers on the indicated days were obtained from a standard curve (data not shown) according to the OD570–690 values. The experiment was done in triplicate and the data were analyzed using GraphPad Prism 3.0. Cell doubling time was determined for each culture, and the values were: LNCaP (30.32 ± 2.1 hours), GFP (33.8 ± 4.6 hours), GFP-WT (34.5 ± 5.1 hours) and GFP-NES (48.1 ± 8.3 hours).
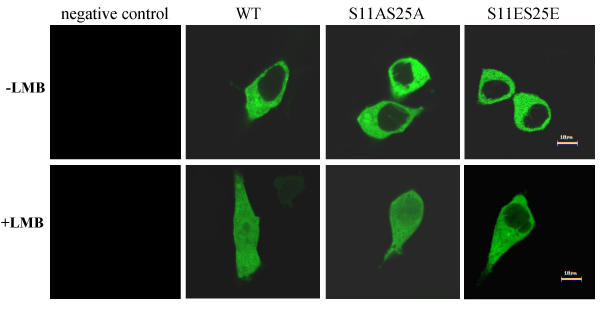
Site-directed mutagenesis of serines 11 and 25 to glutamic acid prevents nuclear entry of annexin II
We examined the role of phosphorylation in the nuclear entry and export of annexin II. Initial experiments with exposure of cells to staurosporine, a protein kinase inhibitor, in combination with LMB showed no effect of staurosporine on nuclear accumulation of annexin II (data not shown), indicating that phosphorylation is not required for annexin II to enter the cell nucleus. To more specifically address the role of phosphorylation in nuclear entry and export of annexin II, we examined the effect of site-directed changes to known phosphoamino acids. Because serines 11 and 25 are known to be phosphorylated by protein kinase C [50,51], these residues were mutated to glutamic acid to mimic the negative charge of a phosphate group, or to alanine to mimic an unphosphorylated state. The mutants were constructed as GFP-fusion proteins and included single mutants (S11A, S11E, S25A, S25E) or in combination (S11AS25A, S11AS25E, S11ES25A, S11ES25E). Transfection of LNCaP cells with these mutants showed no effect on nuclear entry or CRM1-mediated inhibition of nuclear export in all of the mutants except the S11ES25E double mutant. Cells harboring this double mutation displayed inhibition of annexin II entry to the nucleus (Figure 9), indicating the important regulatory role of the serines at 11 and 25 in the cellular distribution of annexin II. We find annexin II in the nucleus as a phosphoprotein in a cell cycle-dependent manner (Figures 1 and 2), so we conclude that serine 11 and 25 are not the phosphorylation sites involved in nuclear retention of annexin II, and phosphorylation of other residues may be important in nuclear entry or export of annexin II. The contribution of other phosphoamino acid residues in annexin II to this regulatory function needs further investigation.
Figure 9.
Site-directed change of ser11 and ser25 to glutamic acid prevents nuclear entry of annexin II. GFP-fused annexin II containing Ser to Ala or Ser to Glu either singly or in combination were generated as described in materials and methods. Transient transfection of LNCaP cells was performed followed by treatment with or without LMB. Confocal microscopic observations were made and representative images are shown. Bar: 10 μm.
Discussion
Phosphorylation of annexin II can regulate its multiple functions in the cell. In the N-terminus of annexin II there are multiple phosphorylation sites suggesting that phosphorylation of these residues may regulate annexin II physiological functions. When primary cultured chromaffin cells are stimulated by nicotine, annexin II is phosphorylated by protein kinase C at Ser-25 and phosphorylation at this residue appears to be a prerequisite for the stimulation of Ca2+-dependent secretion by the annexin II-p11 complex observed in permeabilized chromaffin cells [52], indicating that phosphorylation is involved in regulating the exocytotic function of annexin II. In resting chromaffin cells, annexin II is in monomeric form and predominantly exists in cytosol. However, when the cells are stimulated by nicotine, annexin II forms heterotetramer with p11 and restrict its location to the membrane-associated cytoskeleton [53]. The regulatory role of phosphorylation at tyrosine 23 is less well understood. It was shown that Tyr-23 can be phosphorylated by pp60src [17] and the insulin receptor [54], suggesting its role in growth regulation. Although there is no evidence shown for the function of Tyr-23 phosphorylation in exocytosis, we predict that it may not account for the membrane related processes since Tyr-23 phosphorylation inhibits the formation of annexin II heterotetramer with p11 [18]. Despite the studies mentioned above regarding the role of annexin II phosphorylation on its membrane associated functions, there is no information on the role of phosphorylation on the nuclear function of annexin II. Annexin II was identified to be in a multiprotein complex with DNA polymerase α-primase [11]. We have previously demonstrated that annexin II plays a role in DNA synthesis and cell proliferation. Antisense oligonucleotides to annexin II reduce DNA synthesis in HeLa cells and retard progression of cells through the cell cycle [13]. Immunodepletion of annexin II from Xenopus egg extract results in loss of DNA replication [12]. Replication extracts made from cells expressing antisense-annexin II in a regulatable vector do not support SV40 replication in vitro [14]. Annexin II expression is regulated in the mammalian cell cycle [44], and its levels are enhanced in many cancers [38].
DNA replication is regulated at the level of initiation [55]. The initiation of DNA replication is mediated by a common set of protein factors and some of these factors are activated by phosphorylation catalyzed by cyclin-dependent kinase (CDKs) and the Cdc 7 family of protein kinases [55]. Although the specific substrates of phosphorylation have not been identified with certainty, the phosphorylation pattern is definitely cell cycle dependent. Another important mechanism that regulates cell cycle transition is nuclear protein export [56-58]. For example, CDC6 protein, which is essential for initiation of DNA replication, contains a NES involved in exporting phosphorylated forms of CDC6 in a cell cycle-dependent manner [49,59]. Cyclin B is exported from the nucleus and degraded when cells exit mitosis. The N-terminal peptide of annexin II contains both Ser/Thr phosphorylation sites and a Tyr phosphorylation site, and these sites are in proximity to the nuclear export signal (NES). Thus, the activity of annexin II in the nucleus may be regulated not only by its expression levels but also by phosphorylation and nuclear export. In this study, we have demonstrated that nuclear annexin II is phosphorylated. Interestingly, after CIAP treatment, nuclear annexin II still migrates slower than cytosolic annexin II. This observation suggests that nuclear annexin II may be phosphorylated differently from that in the cytosol, and nuclear and cytosolic annexin II may be regulated through different pathways.
Our data also show that the phosphorylation and nuclear retention of annexin II is cell cycle-dependent. In G1 phase of the cell cycle, annexin II is phosphorylated and accumulates in the nucleus, whereas in S phase annexin II is not detected in the nucleus. These findings are consistent with previous reports [44,48] and similar to the observations that the human CDC6 homolog, HsCDC6, accumulates in the nucleus in G1 phase, but in S phase it is phosphorylated and exported from the nucleus [49]. This similarity indicates that phosphorylation may play a role in the regulation of nuclear entry and export of annexin II. However, another possibility is that annexin II may interact with one or more partner proteins and get transported into the nucleus in a cell-cycle dependent manner, and the phosphorylation may be just a requirement for its function in the nucleus. Unlike HsCDC6, which contains both NLS and NES, annexin II contains no NLS that can mediate its entry into the nucleus, so it is possible that its nuclear entry is through interaction with a partner protein containing NLS. Our data shows that annexin II NES mutant is found distributed equally between the cytosol and the nucleus. Thus, the nuclear export pathway for annexin II appears to be more active than the nuclear entry. We have shown that annexin II is exported out of the nucleus by the CRM1 dependent export pathway.
Ectopic expression of NES mutant annexin II results in a drastic reduction in the proliferation of LNCaP cells with an associated increase in cell doubling time. This reduction in cell proliferation may be due to abnormal presence of annexin II during the S phase of the cell cycle where it is usually not present (Figure 2). On the other hand, it is possible that LNCaP cells lack a binding partner for annexin II in the cell nucleus and this results in annexin II becoming a negative regulator of cell proliferation in the prostate cells. LNCaP cells expressing either the wild-type or the NES mutant change to a more flattened and elongated morphology, indicating that annexin II may cause stabilization of the cytoskeleton. Several lines of evidence suggest that annexin II may be involved in cytoskeleton regulation. First, annexin II has been shown to be associated with the cytoskeleton [60]. Second, it has been suggested that intracellular annexin II heterotetramer acts as a link between the cytoskeleton and the plasma membrane, although the physiological role of this proposed interaction are unknown [61]. Third, both annexin II monomer and tetramer can bind F-actin in vitro, and annexin II tetramer can bundle F-actin [62]. It is possible that these annexin II molecules may form complexes with p11 underneath plasma membrane and bind to cytoskeletal molecules and the annexin II heterotetramer then stabilize cytoskeleton, which results in the morphology change of LNCaP cells. The stabilization of cytoskeleton also accounts for inhibition of cell migration. Our results are also consistent with recent finding showing that re-expression of annexin II inhibits prostate cancer cell migration [63]. Epithelial mesenchymal transition (EMT) has been estimated to occur in as much as 18% of breast tumors in vivo and the EMT generally depicts a more aggressive behavior of the tumor cells [64]. The functional consequence of mesenchymal appearance of annexin II-transfected cells need to be investigated further.
The annexin II NES overlaps the interaction site for p11. Binding of p11 targets annexin II to the cell membrane, and p11 is not localized to the nucleus. Thus, the heterotetramer formed by annexin II and p11 is exclusively cytosolic and has no role in the nucleus. Previous studies of other proteins containing NES and NLS motifs show that the proteins are shuttling between cytoplasm and nucleus until NES and/or NLS is masked either by phosphorylation, association with other proteins, or other modification [65,66]. Phosphorylation of annexin II may confer a conformational change resulting in lack of recognition by the CRM1-mediated nuclear export pathway. Alternatively, phosphorylated annexin II can interact with additional protein factors in the nucleus, and this interaction masks annexin II NES preventing its nuclear export. The three known phosphorylation sites in the N-terminus of annexin II, Ser11, Ser25, and Tyr23, were identified from studies of the heterotetrameric form of annexin II. The exact phosphorylation sites of the nuclear monomer of annexin II are not known, and it is possible that other potential sites are phosphorylated in the nuclear annexin II. In this study, we have shown that phosphorylation of both Ser11 and Ser 25 prevents annexin II entry to the nucleus. Annexin II phosphorylated in its Ser-11 and 25 cannot form a heterotetramer with p11 [16]. However, diphosphorylated form of annexin II exists as homodimer instead of as monomer [16]. The formation of annexin II homodimer devoid of p11 suggests that the phosphorylation of the heavy chains could induce a head-to-head association [16]. This kind of dimerization has also been observed on a monomeric annexin I in placenta [67] and brain [68]. Thus, this head-to-head dimerization may account for the inhibition of nuclear entry of the diphosphorylated form of annexin II. From these results, we also conclude that ser11 and ser25 residues are not involved in the regulation of nuclear retention of annexin II.
Conclusions
Data from our studies show that annexin II localized in the cell nucleus is phosphorylated in a cell cycle-dependent manner. The entry of annexin II into the nucleus is prevented by phosphorylation of serines 11 and 25. Stable expression of the NES mutant annexin II results in reduced cell proliferation of LNCaP cells. These results indicate an important role for nuclear annexin II in cell proliferation and the regulation of nucleo-cytoplasmic shuttling of annexin II.
Methods
Cell lines and culture conditions
LNCaP cells were cultured in RPMI 1640 containing 7% fetal bovine serum (FBS) (Gibco-BRL, Rockville, MD), 100 μg/ml penicillin, and 100 μg/ml streptomycin at 37° in a 5% CO2 cell culture incubator. Plasmids that express green fluorescence protein (GFP) alone (pEGFP-C1), GFP-fused wild type annexin II (pEGFP-C1-wild type annexin II), or GFP-fused NES mutant annexin II with lysine 10 and 12 mutated to alanine (pEGFP-C1-AnxII L10A/L12A) were transfected into LNCaP cells using Lipofectamine Plus (Gibco-BRL). Stably transfected cell populations were generated by culturing cells in the presence of 0.2 mg/ml G418 and subsequent subcloning. K562 cells were grown in suspension in RPMI 1640 containing 10% FBS, 2 mM L-glutamine, 100 μg/ml penicillin, and 100 μg/ml streptomycin.
Cell fractionation
K562 cell pellets were rinsed twice with Hank's balanced salt solution (HBSS) and once with hypotonic buffer (10 mM Tris-HCl, pH 7.5, 10 mM NaCl and 1 mM MgCl2). Cells were resuspended and held in hypotonic buffer for 2 hours at 4°C. Cells were disrupted with a Dounce homogenizer, and disruption was monitored by light microscopy. No intact cells were observed by microscopy after homogenization. The homogenate was subjected to centrifugation in a Sorvall SS-34 rotor at 2,000 revs/min for 30 minutes at 4°C. The supernatant was designated as cytoplasmic extract (CE). The nuclear pellet was further rinsed three times with the hypotonic buffer and the supernatant from each wash was added to the cytoplasmic extract. The nuclei were resuspended in 0.4 M potassium phosphate, pH 7.2, 1 mM EDTA, 1 mM dithiothreitol (DTT) and 10% glycerol and extracted for 1 hour at 4°C. Following centrifugation in a Sorvall SS-34 rotor at 10,000 g for 30 minutes at 4°C, the supernatant was collected as nuclear extract (NE). The cytoplasmic and nuclear extracts were dialyzed against 50 mM potassium phosphate, pH 7.2, 1 mM DTT, 1 mM EDTA and 10% glycerol, and stored at minus 80°C until further use. LNCaP cell transfectants grown in 80% confluency were harvested, and the cytosolic extract (CE) and nuclear extract (NE) were obtained using NE-PER Nuclear and Cytoplasmic Extraction Reagents (PIERCE, Rockford, IL).
Electrophoresis and Immunoblot analyses
Protein extracts were prepared from indicated cells. The total amount of protein extracts was quantitated using Bio-Rad BCA protein assay (Pierce). Indicated amount of protein from each extract was separated by 12% SDS-PAGE. After electrophoresis, protein was transferred to polyvinylidene fluoride (PVDF) membranes (Bio-Rad Hercules CA). The membranes were blocked in 1X Tris Buffered Saline with 0.01% Tween 20 (1XTTBS) with 7% powdered milk for one hour at room temperature then probed with monoclonal anti-human annexin II at 1:5000 dilution (BD Transduction Laboratories) and polyclonal anti-human PGK at 1:2000 dilution overnight at 4°C. Appropriate secondary antibodies conjugated to horseradish peroxidase (HRP) were used to detect antigen-antibody complexes. Membranes were developed using ECL plus (Amersham Pharmacia Biotech, UK) and exposed to films for appropriate amounts of time.
Phosphatase treatment of cytosolic and nuclear extract of LNCaP cells expressing GFP-NES mutant annexin II
LNCaP cells expressing GFP-NES were fractionated into cytosolic and nuclear extracts using NE-PER nuclear and cytosolic extraction reagents (PIERCE, Rockford IL). The extracts were concentrated with Centricon-10 centrifugal concentrator (Millipore Co.), and 10 μg protein was treated with 0.5 units of potato acid phosphatase (Sigma Chemical Co.) for 30 minutes at 37°C in 50 mM PIPES pH 6.0 buffer. The reaction was terminated by adding SDS-PAGE sample buffer and both the untreated and treated extracts were subjected to SDS-PAGE.
Northern blot
Total RNA was extracted using TRIzol standard protocols. A total of 20 μg RNA was used in northern blot experiments. Annexin II probe was prepared from the vector pGAF5-1 by the random primer method of labeling with 32P greater than 1 × 108 cpm/μg. Hybridization of the probe to the membrane was carried out at 42°C overnight in a water bath. The membrane was stripped and re-probed with glyceraldehyde-3-phosphate dehydrogenase (GAPDH) probe and its expression was used as a control to normalize data for annexin II levels.
Immunocytochemistry
LNCaP cells seeded on the glass coverslips were grown to 80% confluency then fixed for 30 minutes in 4% paraformaldehyde in phosphate buffered saline (PBS) and permeabilized in methanol for 10 minutes. After several rinses with PBS, cells were pretreated with 10% bovine serum albumin (BSA) in PBS for 3 hours at room temperature to reduce nonspecific staining. Cells were incubated with monoclonal anti-human annexin II primary antibody (BD Transduction Laboratories) at 1:5000 dilution in PBS containing 3% BSA for 2 hours at room temperature, washed, and subsequently incubated for 2 hours with biotinylated secondary antibody (Vector Laboratories, Inc. Burlingame, CA) diluted 1:500 followed by staining with rhodamine-avidin D (Vector Laboratories Inc. Burlingame, CA) diluted 1:2000 for 30 minutes. The coverslips were washed extensively with PBS and mounted in VECTASHIELD mounting medium for fluorescence (Vector Laboratories Inc. Burlingame, CA). Cells were visualized on a Zeiss confocal microscope.
Leptomycin B (LMB) treatment of LNCaP cells
LNCaP cells expressing GFP or GFP-wild type annexin II were grown to 80% confluence on the surface of round glass coverslips placed in 12-well plates. The cells were then treated with 20 nM LMB for 2 hours. The cells were fixed with 4% paraformaldehyde in 1X PBS and observed under confocal microscopy.
Cell proliferation assay
Cell proliferation was measured using 3-(4,5-cimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) cell proliferation assay. MTT was dissolved in serum-free and phenol red-free RPMI 1640 at a concentration of 1 mg/ml. A total of 30,000 cells were seeded into 6-well plates. On days 2, 4, 6 and 8, cells were incubated with MTT solution for 3 hours in a CO2 incubator at 37°C. The MTT reagent was removed and placed in 2.0 ml microcentrifuge tubes and centrifuged at 14,000 × g for 5 minutes to pellet and collect any detached cells. The formazan dye was dissolved by adding 2.0 ml of 99% isopropanol to each sample, and the absorbance was read at 570–690 nm using spectrophotometer. For the standard curve, 20,000 to 140,000 cells were seeded in 6-well plates, and after 24 hours, the cells were treated with MTT and processed as described above. Data were plotted using Prizm 3.0 (GraphPad Software, San Diego, CA).
Construction of GFP-fused site directed mutants of annexin II
GFP fusion proteins with annexin II containing mutations in the Ser11 and Ser25 were constructed by inserting mutant annexin II cDNA into the pEGFP-C1 vector. Ser to Ala and Ser to Glu mutants were generated as previously reported [43]. Annexin II cDNA was generated by PCR with primers containing appropriate restriction sites and inserted into the corresponding restriction sites of the pEGFP-C1 vector. GFP-fusion constructs of single mutants (S11A, S25A, S11E, S25E) and double mutants (S11AS25A, S11AS25E, S11ES25A, S11ES25E) were generated for use in our experiments.
Authors' contributions
JL carried out the phosphorylation experiments, constructed the GFP-annexin II phosphorylation mutants, conducted the proliferation experiments and drafted the manuscript. CAR performed the transfections. JAS constructed the annexin II phosphorylation mutants. JKV conceived of the study, and participated in its design and coordination. All authors read and approved the final manuscript.
Acknowledgments
Acknowledgements
We thank Dr. David A. Eberhard (University of Virginia Health Science Center) for the gift of wild type and NES mutant annexin II vectors and Dr. Minoru Yoshida (University of Tokyo) for the gift of Leptomycin B. We thank the Confocal Microscopy Facility for assistance in our work. This study was supported in part by grants from the Gustavus and Louise Pfeiffer Foundation, Phillip Morris Incorporated and Nebraska Cancer and Smoking Disease Research Program (2003–30).
Contributor Information
Jie Liu, Email: jieliu@unmc.edu.
Christy A Rothermund, Email: crothermun@unmc.edu.
Jesus Ayala-Sanmartin, Email: joyala@chusa.jussieu.fr.
Jamboor K Vishwanatha, Email: jvishwan@unmc.edu.
References
- Geisow MJ, Walker JH, Boustead C, Taylor W. Annexins--new family of Ca2+-regulated-phospholipid binding protein. Biosci Rep. 1987;7:289–298. doi: 10.1007/BF01121450. [DOI] [PubMed] [Google Scholar]
- Gerke V, Weber K. Identity of p36K phosphorylated upon Rous sarcoma virus transformation with a protein purified from brush borders; calcium-dependent binding to non-erythroid spectrin and F-actin. EMBO J. 1984;3:227–233. doi: 10.1002/j.1460-2075.1984.tb01789.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiel C, Osborn M, Gerke V. The tight association of the tyrosine kinase substrate annexin II with the submembranous cytoskeleton depends on intact p11- and Ca(2+)-binding sites. J Cell Sci. 1992;103 ( Pt 3):733–742. doi: 10.1242/jcs.103.3.733. [DOI] [PubMed] [Google Scholar]
- Ali SM, Geisow MJ, Burgoyne RD. A role for calpactin in calcium-dependent exocytosis in adrenal chromaffin cells. Nature. 1989;340:313–315. doi: 10.1038/340313a0. [DOI] [PubMed] [Google Scholar]
- Creutz CE. The annexins and exocytosis. Science. 1992;258:924–931. doi: 10.1126/science.1439804. [DOI] [PubMed] [Google Scholar]
- Sarafian T, Pradel LA, Henry JP, Aunis D, Bader MF. The participation of annexin II (calpactin I) in calcium-evoked exocytosis requires protein kinase C. J Cell Biol. 1991;114:1135–1147. doi: 10.1083/jcb.114.6.1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chasserot-Golaz S, Vitale N, Sagot I, Delouche B, Dirrig S, Pradel LA, Henry JP, Aunis D, Bader MF. Annexin II in exocytosis: catecholamine secretion requires the translocation of p36 to the subplasmalemmal region in chromaffin cells. J Cell Biol. 1996;133:1217–1236. doi: 10.1083/jcb.133.6.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munz B, Gerke V, Gillitzer R, Werner S. Differential expression of the calpactin I subunits annexin II and p11 in cultured keratinocytes and during wound repair. J Invest Dermatol. 1997;108:307–312. doi: 10.1111/1523-1747.ep12286470. [DOI] [PubMed] [Google Scholar]
- Zokas L, Glenney-JR Jr. The calpactin light chain is tightly linked to the cytoskeletal form of calpactin I: studies using monoclonal antibodies to calpactin subunits. J Cell Biol. 1987;105:2111–2121. doi: 10.1083/jcb.105.5.2111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jindal HK, Vishwanatha JK. Purification and characterization of primer recogniton proteins from HeLa cells. Biochemistry. 1990;29:4767–4773. doi: 10.1021/bi00472a004. [DOI] [PubMed] [Google Scholar]
- Jindal HK, Chaney WG, Anderson CW, Davis RG, Vishwanatha JK. The protein-tyrosine kinase substrate, calpactin I heavy chain (p36), is part of the primer recognition protein complex that interacts with DNA polymerase a. Journal of Biological Chemistry. 1991;266:5169–5176. [PubMed] [Google Scholar]
- Vishwanatha JK, Kumble S. Involvement of annexin II in DNA replication: evidence from cell-free extracts of Xenopus eggs. Journal of Cell Science. 1993;105:533–540. doi: 10.1242/jcs.105.2.533. [DOI] [PubMed] [Google Scholar]
- Kumble KD, Iversen PL, Vishwanatha JK. The role of primer recognition proteins in DNA replication: Inhibition of cellular proliferation by antisense oligodeoxyribonucleotides. Journal of Cell Science. 1992;101:35–41. doi: 10.1242/jcs.101.1.35. [DOI] [PubMed] [Google Scholar]
- Chiang Y, Rizzino A, Sibenaller ZA, Wold MS, Vishwanatha JK. Specific down-regulation of annexin II expression in human cells interferes with cell proliferation. Mol Cell Biochem. 1999;199:139–147. doi: 10.1023/A:1006942128672. [DOI] [PubMed] [Google Scholar]
- Johnsson N, Nguyen Van P., Soling HD, Weber K. Functionally distinct serine phosphorylation sites of p36, the cellular substrate of retroviral protein kinase; differential inhibition of reassociation with p11. EMBO J. 1986;5:3455–3460. doi: 10.1002/j.1460-2075.1986.tb04669.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regnouf F, Sagot I, Delouche B, Devilliers G, Cartaud J, Henry JP, Pradel LA. "In vitro " phosphorylation of annexin 2 heterotetramer by protein kinase C - Comparative properties of the unphosphorylated and phosphorylated annexin 2 on the aggregation and fusion of chromaffin granule membranes. Journal of Biological Chemistry. 1995;270:27143–27150. doi: 10.1074/jbc.270.45.27143. [DOI] [PubMed] [Google Scholar]
- Glenney J.R.,Jr., Tack BF. Amino-terminal sequence of p36 and associated p10: identification of the site of tyrosine phosphorylation and homology with S-100. Proc Natl Acad Sci U S A. 1985;82:7884–7888. doi: 10.1073/pnas.82.23.7884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell MA, Glenney JR. Regulation of calpactin I phospholiped binding by calpactin I light-chain binding and phosphorylation by p60v-src. Biochemistry Journal. 1987;247:321–328. doi: 10.1042/bj2470321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnstone SA, Hubaishy I, Waisman DM. Phosphorylation of annexin II tetramer by protein kinase C inhibits aggregation of lipid vesicles by the protein. Journal of Biological Chemistry. 1992;267:25976–25981. [PubMed] [Google Scholar]
- Chetcuti A, Margan SH, Russell P, Mann S, Millar DS, Clark SJ, Rogers J, Handelsman DJ, Dong Q. Loss of annexin ii heavy and light chains in prostate cancer and its precursors. Cancer Res. 2001;61:6331–6334. [PubMed] [Google Scholar]
- Andronicos NM, Ranson M. The topology of plasminogen binding and activation on the surface of human breast cancer cells. Br J Cancer. 2001;85:909–916. doi: 10.1054/bjoc.2001.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brichory FM, Misek DE, Yim AM, Krause MC, Giordano TJ, Beer DG, Hanash SM. An immune response manifested by the common occurrence of annexins I and II autoantibodies and high circulating levels of IL-6 in lung cancer. Proc Natl Acad Sci U S A. 2001;98:9824–9829. doi: 10.1073/pnas.171320598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emoto K, Yamada Y, Sawada H, Fujimoto H, Ueno M, Takayama T, Kamada K, Naito A, Hirao S, Nakajima Y. Annexin II overexpression correlates with stromal tenascin-C overexpression: a prognostic marker in colorectal carcinoma. Cancer. 2001;92:1419–1426. doi: 10.1002/1097-0142(20010915)92:6<1419::AID-CNCR1465>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Choi S, Kobayashi M, Wang J, Habelhah H, Okada F, Hamada J, Moriuchi T, Totsuka Y, Hosokawa M. Activated leukocyte cell adhesion molecule (ALCAM) and annexin II are involved in the metastatic progression of tumor cells after chemotherapy with Adriamycin. Clin Exp Metastasis. 2000;18:45–50. doi: 10.1023/A:1026507713080. [DOI] [PubMed] [Google Scholar]
- Kaczan-Bourgois D, Salles JP, Chap H. Expression of annexin II and associated p11 protein by differentiated choriocarcinoma Jar cells. Am J Obstet Gynecol. 1999;181:1273. doi: 10.1016/s0002-9378(99)70128-6. [DOI] [PubMed] [Google Scholar]
- Menell JS, Cesarman GM, Jacovina AT, McLaughlin MA, Lev EA, Hajjar KA. Annexin II and bleeding in acute promyelocytic leukemia. N Engl J Med. 1999;340:994–1004. doi: 10.1056/NEJM199904013401303. [DOI] [PubMed] [Google Scholar]
- Balch C, Dedman JR. Annexins II and V inhibit cell migration. Exp Cell Res. 1997;237:259–263. doi: 10.1006/excr.1997.3817. [DOI] [PubMed] [Google Scholar]
- Blanchard S, Barwise JL, Gerke V, Goodall A, Vaughan PF, Walker JH. Annexins in the human neuroblastoma SH-SY5Y: demonstration of relocation of annexins II and V to membranes in response to elevation of intracellular calcium by membrane depolarisation and by the calcium ionophore A23187. J Neurochem. 1996;67:805–813. doi: 10.1046/j.1471-4159.1996.67020805.x. [DOI] [PubMed] [Google Scholar]
- Mohiti J, Walker JH, Caswell AM. Studies on annexins in primary cultures of human osteoblasts and in the human osteosarcoma cell line MG-63. Biochem Soc Trans. 1995;23:36S. doi: 10.1042/bst023036s. [DOI] [PubMed] [Google Scholar]
- Roseman BJ, Bollen A, Hsu J, Lamborn K, Israel MA. Annexin II marks astrocytic brain tumors of high histologic grade. Oncol Res. 1994;6:561–567. [PubMed] [Google Scholar]
- Tressler RJ, Updyke TV, Yeatman T, Nicolson GL. Extracellular annexin II is associated with divalent cation- dependent tumor cell-endothelial cell adhesion of metastatic RAW117 large-cell lymphoma cells. Journal of Cellular Biochemistry. 1993;53:265–276. doi: 10.1002/jcb.240530311. [DOI] [PubMed] [Google Scholar]
- Ozaki T, Sakiyama S. Molecular cloning of rat calpactin I heavy-chain cDNA whose expression is induced in v-src-transformed rat culture cell lines. Oncogene. 1993;8:1707–1710. [PubMed] [Google Scholar]
- Fox MT, Prentice DA, Hughes JP. Increases in p11 and annexin II proteins correlate with differentiation in the PC12 pheochromocytoma. Biochemical and Biophysical Research Communications. 1991;177:1188–1193. doi: 10.1016/0006-291x(91)90666-u. [DOI] [PubMed] [Google Scholar]
- Gress TM, Wallrapp C, Frohme M, Muller Pillasch F., Lacher U, Friess H, Buchler M, Adler G, Hoheisel JD. Identification of genes with specific expression in pancreatic cancer by cDNA representational difference analysis. Genes Chromosomes Cancer. 1997;19:97–103. doi: 10.1002/(SICI)1098-2264(199706)19:2<97::AID-GCC5>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- Manda R, Kohno T, Matsuno Y, Takenoshita S, Kuwano H, Yokota J. Identification of genes (SPON2 and C20orf2) differentially expressed between cancerous and noncancerous lung cells by mRNA differential display. Genomics. 1999;61:5–14. doi: 10.1006/geno.1999.5939. [DOI] [PubMed] [Google Scholar]
- Nygaard SJ, Haugland HK, Kristoffersen EK, Lund Johansen M., Laerum OD, Tysnes OB. Expression of annexin II in glioma cell lines and in brain tumor biopsies. J Neurooncol. 1998;38:11–18. doi: 10.1023/A:1005953000523. [DOI] [PubMed] [Google Scholar]
- Cole SPC, Pinkoski MJ, Bhardwaj G, Deeley RG. Elevated expression of Annexin II (Lipocortin II, p36) in a multidrug resistant small cell lung cancer cell line. British Journal of Cancer. 1992;65:498–502. doi: 10.1038/bjc.1992.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumble KD, Hirota M, Pour PM, Vishwanatha JK. Enhanced levels of annexins in pancreatic carcinoma cells of Syrian hamsters and their intrapancreatic allografts. Cancer Research. 1992;52:163–167. [PubMed] [Google Scholar]
- Vishwanatha JK, Chiang Y, Kumble KD, Hollingsworth MA, Pour PM. Enhanced expression of annexin II in human pancreatic carcinoma cells and primary pancreatic cancers. Carcinogenesis. 1993;14:2575–2579. doi: 10.1093/carcin/14.12.2575. [DOI] [PubMed] [Google Scholar]
- Eberhard DA, Karns LR, VandenBerg SR, Creutz CE. Control of the nuclear-cytoplasmic partitioning of annexin II by a nuclear export signal and by p11 binding. J Cell Sci. 2001;114:3155–3166. doi: 10.1242/jcs.114.17.3155. [DOI] [PubMed] [Google Scholar]
- Engel K, Kotlyarov A, Gaestel M. Leptomycin B-sensitive nuclear export of MAPKAP kinase 2 is regulated by phosphorylation. EMBO J. 1998;17:3363–3371. doi: 10.1093/emboj/17.12.3363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Xiong Y. A p53 amino-terminal nuclear export signal inhibited by DNA damage-induced phosphorylation. Science. 2001;292:1910–1915. doi: 10.1126/science.1058637. [DOI] [PubMed] [Google Scholar]
- Ayala-Sanmartin J, Gouache P, Henry JP. N-Terminal domain of annexin 2 regulates Ca(2+)-dependent membrane aggregation by the core domain: a site directed mutagenesis study. Biochemistry. 2000;39:15190–15198. doi: 10.1021/bi000764r. [DOI] [PubMed] [Google Scholar]
- Chiang Y, Schneiderman MH, Vishwanatha JK. Annexin II expression is regulated during mammalian cell cycle. Cancer Research. 1993;53:6017–6021. [PubMed] [Google Scholar]
- Keutzer JC, Hirschhorn RR. The growth-regulated gene 1B6 is identified as the heavy chain of calpactin I. Exp Cell Res. 1990;188:153–159. doi: 10.1016/0014-4827(90)90291-h. [DOI] [PubMed] [Google Scholar]
- Rothermund CA, Kondrikov D, Lin MF, Vishwanatha JK. Regulation of Bcl-2 during androgen-unresponsive progression of prostate cancer. Prostate Cancer Prostatic Dis. 2002;5:236–245. doi: 10.1038/sj.pcan.4500582. [DOI] [PubMed] [Google Scholar]
- Frisa PS, Jacobberger JW. Cell density related gene expression: SV40 large T antigen levels in immortalized astrocyte lines. BMC Cell Biol. 2002;3:10. doi: 10.1186/1471-2121-3-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang Y, Davis RG, Vishwanatha JK. Altered expression of annexin II in human B-cell lymphoma cell lines. Biochim Biophys Acta. 1996;1313:295–301. doi: 10.1016/0167-4889(96)00103-6. [DOI] [PubMed] [Google Scholar]
- Saha P, Chen J, Thome KC, Lawlis SJ, Hou ZH, Hendricks M, Parvin JD, Dutta A. Human CDC6/Cdc18 associates with Orc1 and cyclin-cdk and is selectively eliminated from the nucleus at the onset of S phase. Mol Cell Biol. 1998;18:2758–2767. doi: 10.1128/mcb.18.5.2758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould KL, Woodgett JR, Isacke CM, Hunter T. The protein-tyrosine kinase substrate p36 is also a substrate for protein kinase C in vitro and in vivo. Mol Cell Biol. 1986;6:2738–2744. doi: 10.1128/mcb.6.7.2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jost M, Gerke V. Mapping of a regulatory important site for protein kinase C phosphorylation in the N-terminal domain of annexin II. Biochim Biophys Acta. 1996;1313:283–289. doi: 10.1016/0167-4889(96)00101-2. [DOI] [PubMed] [Google Scholar]
- Sarafian T, Pradel L-A, Henry J-P, Aunis D, Bader M-F. The participation of annexin II (calpactin I) in calcium- evoked exocytosis requires protein kinase C. Journal of Cell Biology. 1991;114:1135–1147. doi: 10.1083/jcb.114.6.1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagot I, Regnouf F, Henry JP, Pradel LA. Translocation of cytosolic annexin 2 to a Triton-insoluble membrane subdomain upon nicotine stimulation of chromaffin cultured cells. FEBS Lett. 1997;410:229–234. doi: 10.1016/S0014-5793(97)00594-2. [DOI] [PubMed] [Google Scholar]
- Karasik A, Pepinsky RB, Shoelson SE, Kahn CR. Lipocortins 1 and 2 as substrates for the insulin receptor kinase in rat liver. J Biol Chem. 1988;263:11862–11867. [PubMed] [Google Scholar]
- Kelly TJ, Brown GW. Regulation of chromosome replication. Annu Rev Biochem. 2000;69:829–880. doi: 10.1146/annurev.biochem.69.1.829. [DOI] [PubMed] [Google Scholar]
- Dupont S, Sharova N, DeHoratius C, Virbasius CM, Zhu X, Bukrinskaya AG, Stevenson M, Green MR. A novel nuclear export activity in HIV-1 matrix protein required for viral replication. Nature. 1999;402:681–685. doi: 10.1038/45272. [DOI] [PubMed] [Google Scholar]
- Kudo N, Khochbin S, Nishi K, Kitano K, Yanagida M, Yoshida M, Horinouchi S. Molecular cloning and cell cycle-dependent expression of mammalian CRM1, a protein involved in nuclear export of proteins. J Biol Chem. 1997;272:29742–29751. doi: 10.1074/jbc.272.47.29742. [DOI] [PubMed] [Google Scholar]
- Wen W, Meinkoth JL, Tsien RY, Taylor SS. Identification of a signal for rapid export of proteins from the nucleus. Cell. 1995;82:463–473. doi: 10.1016/0092-8674(95)90435-2. [DOI] [PubMed] [Google Scholar]
- Delmolino LM, Saha P, Dutta A. Multiple mechanisms regulate subcellular localization of human CDC6. J Biol Chem. 2001;276:26947–26954. doi: 10.1074/jbc.M101870200. [DOI] [PubMed] [Google Scholar]
- Pol A, Ortega D, Enrich C. Identification of cytoskeleton-associated proteins in isolated rat liver endosomes. Biochem J. 1997;327 ( Pt 3):741–746. doi: 10.1042/bj3270741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerke V, Moss SE. Annexins and membrane dynamics. Biochim Biophys Acta. 1997;1357:129–154. doi: 10.1016/S0167-4889(97)00038-4. [DOI] [PubMed] [Google Scholar]
- Ikebuchi NW, Waisman DM. Calcium-dependent regulation of actin filament bundling by lipocortin-85. J Biol Chem. 1990;265:3392–3400. [PubMed] [Google Scholar]
- Liu JW, Shen JJ, Tanzillo-Swarts A, Bhatia B, Maldonado CM, Person MD, Lau SS, Tang DG. Annexin II expression is reduced or lost in prostate cancer cells and its re-expression inhibits prostate cancer cell migration. Oncogene. 2003;22:1475–1485. doi: 10.1038/sj.onc.1206196. [DOI] [PubMed] [Google Scholar]
- Petersen OW, Nielsen HL, Gudjonsson T, Villadsen R, Rank F, Niebuhr E, Bissell MJ, Ronnov-Jessen L. Epithelial to mesenchymal transition in human breast cancer can provide a nonmalignant stroma. Am J Pathol. 2003;162:391–402. doi: 10.1016/S0002-9440(10)63834-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komeili A, O'Shea EK. Roles of phosphorylation sites in regulating activity of the transcription factor Pho4. Science. 1999;284:977–980. doi: 10.1126/science.284.5416.977. [DOI] [PubMed] [Google Scholar]
- Zhu J, Shibasaki F, Price R, Guillemot JC, Yano T, Dotsch V, Wagner G, Ferrara P, McKeon F. Intramolecular masking of nuclear import signal on NF-AT4 by casein kinase I and MEKK1. Cell. 1998;93:851–861. doi: 10.1016/s0092-8674(00)81445-2. [DOI] [PubMed] [Google Scholar]
- Pepinsky RB, Sinclair LK, Chow EP, O'Brine-Greco B. A dimeric form of lipocortin-1 in human placenta. Biochem J. 1989;263:97–103. doi: 10.1042/bj2630097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pradel LA, Rendon A. Annexin 1 is present in different molecular forms in rat cerebral cortex. FEBS Letters. 1993;327:41–44. doi: 10.1016/0014-5793(93)81035-X. [DOI] [PubMed] [Google Scholar]