Abstract
To determine whether a functional type II receptor of transforming growth factor β (TGF-β) is required to mediate the growth inhibitory effect of TGF-β on the skin in vivo, we have generated transgenic mice that overexpress a dominant negative-type II TGF-β receptor (ΔβRII) in the epidermis. The ΔβRII mice exhibited a thickened and wrinkled skin, and histologically the epidermis was markedly hyperplastic and hyperkeratotic. In vivo labeling with BrdUrd showed a 2.5-fold increase in the labeling index over controls, with labeled nuclei occurring in both basal and suprabasal cells of transgenic epidermis. In heterozygotes, this skin phenotype gradually diminished, and by 10–14 days after birth the transgenic mice were indistinguishable from their normal siblings. However, when F1 mice were mated to homozygosity, perinatal lethality occurred due to the severe hyperkeratotic phenotype, which restricted movement. Cultured primary keratinocytes from ΔβRII mice also exhibited an increased rate of growth in comparison with nontransgenic controls, and were resistant to TGF-β-induced growth inhibition. These data document the role of the type II TGF-β receptor in mediating TGF-β-induced growth inhibition of the epidermis in vivo and in maintenance of epidermal homeostasis.
Keywords: keratinocytes, hyperplasia/hyperkeratosis, serine/threonine-kinase
The transforming growth factor β (TGF-β) family regulates cell proliferation and differentiation, tissue remodeling, and repair (for reviews, see refs. 1 and 2). Within a given organ, such as the skin, TGF-β often has opposite effects on different cell types. For example, TGF-β is a potent growth inhibitor of the epidermis, playing an important role in maintenance of tissue homeostasis (3, 4), whereas in the dermis, TGF-β acts as a positive growth factor, inducing the synthesis of extracellular matrix proteins during wound healing (2, 5). TGF-β signals through a heteromeric complex of the type I and II TGF-β receptors, which have serine/threonine kinase activities in their cytoplasmic domains (6, 7); however, little is known about how these receptors mediate different biological responses.
One of the initial approaches to address this question used a truncated form of the type II receptor (ΔβRII) which acts as a dominant-negative mutant due to absence of the cytoplasmic serine/threonine kinase domain (8, 9). Expression of ΔβRII in stably transfected MvlLu cells blocked TGF-β-mediated growth inhibition, but failed to suppress the induction of extracellular matrix proteins such as fibronectin and plasminogen activator inhibitor I (8), suggesting that the type I and type II receptors might signal separate responses. Subsequent studies, using a similar approach resulted in a block of both responses (9, 10), thus arguing against independent signaling. More recent data suggest that different TGF-β responses require different levels of signaling and that differential inhibition may depend on the expression level of the dominant-negative receptor (10).
To determine whether the dominant-negative strategy could be used to block TGF-β-mediated growth inhibition in vivo, we have targeted overexpression of ΔβRII to the epidermis of transgenic mice. Mice expressing ΔβRII exhibit a thickened and wrinkled skin, which in homozygotes restricts mobility and leads to perinatal lethality. This study documents the role of the type II TGF-β receptor in mediating growth inhibition of the epidermis in vivo, and further emphasizes the importance of the TGF-β signaling pathway in maintenance of epidermal homeostasis.
MATERIALS AND METHODS
Generation and Identification of ML.ΔβRII Transgenic Mice.
The ΔβRII construct with a downstream human c-myc epitope (GEQKLISEEDLN) was generated as described (8, 11). After introducing ClaI restriction sites to both ends of this construct, ΔβRII was ligated into the unique ClaI site of the mouse loricrin (ML) vector (see Fig. 1A). The ML.ΔβRII transgene was sequenced to confirm that the PCR generated product was correct and then released from the pGem 7Z-pSL1180 fusion plasmid by digestion with BamHI. The ML.ΔβII transgene was purified and then microinjected into mouse embryos obtained from mating ICR females to strain FVB males. A total of 15–20 embryos were transferred to the oviduct of each ICR pseudopregnant foster mother (12). After birth, transgenic mice were confirmed by PCR analysis of their tail DNA using ΔβRII-specific oligonucleotides 1 (5′-ccatcgattggtctgccatgggtcggg-3′) and 2 (5′-gtatcgattctaattcaggtcctcctcg-3′; see Fig. 1A), under conditions previously described (13).
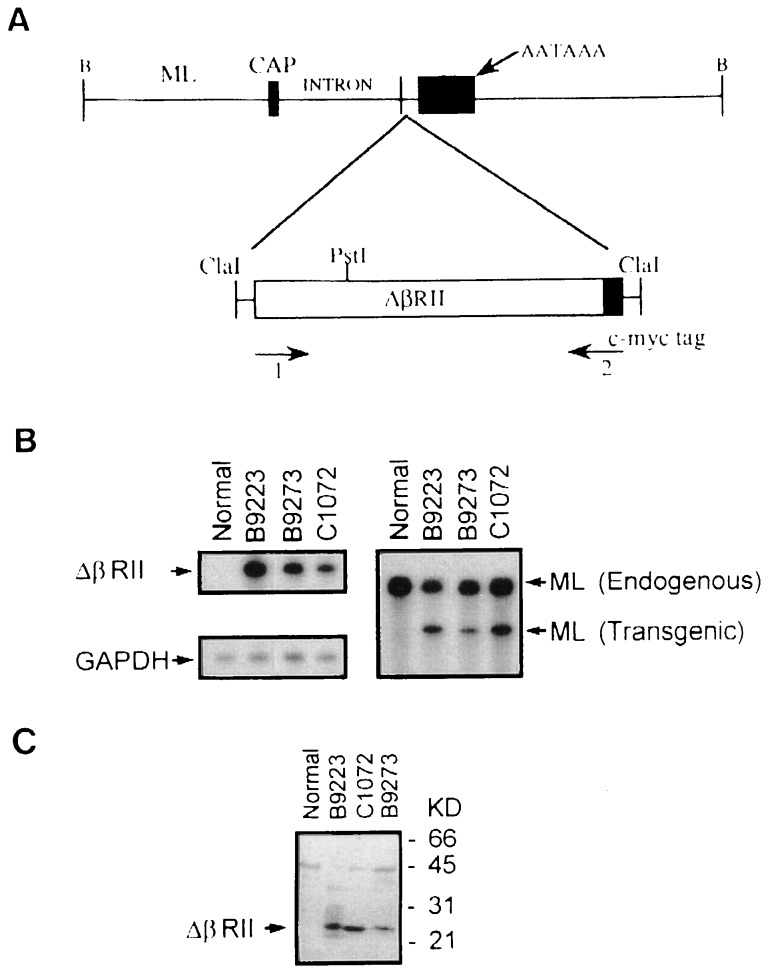
Figure 1.
Transgene construct and expression. (A) Schematic showing structure of the ML.ΔβRII transgene. Construction and characterization of the ML targeting vector have been described (19). The ΔβRII was produced by PCR, which retained the entire extracellular domain and the transmembrane domain, but deletes most of the cytoplasmic domain (8). A sequence tag, encoding the human c-myc epitope (11), was introduced to the 3′ end of the transgene. This transgene was then inserted into the ClaI site of the ML vector. Primers 1 and 2, specific for the TGF-β type II receptor and human c-myc tag, respectively, were used for PCR to screen transgenic mice. (B) RNase protection assays to identify ML.ΔβRII transgene expression. Note that the transgene expression detected by the ΔβRII probe was exclusively in transgenic epidermis (Left), and the ratio of the signal intensity between ΔβRII and GAPDH was 4:1 for RNA from line B9273, and 5:1 for RNAs from transgenic lines B9223 and C1072. The ML probe was used to detect both the endogenous loricrin and transgene expression (Right). The intensity of ΔβRII expression versus endogenous loricrin was 0.4:1 from transgenic line B9273 and 0.6:1 from lines B9223 and C1072. (C) Western blotting showing ΔβRII protein expression. A 23-kDa band is detected in transgenic epidermis from all lines, but not in the normal control, and this represents the size predicated for the truncated ΔβRII protein. Epidermal extracts from lines C1072 and B9223 exhibited a higher intensity than line B9273, which was consistent with RNA expression levels (see B). Nonspecific bands, resulting from the rabbit anti-mouse secondary antibody, were seen in a similar pattern in all of the lanes. The B9223 extract gave a slightly higher background due to partial degradation.
Preparation and Analysis of RNA.
Total RNA was isolated from neonatal epidermis with RNAzol B (Tel-Test, Friendswood, TX) as described (13). To determine expression levels of the ML.ΔβRII transgene, RNase protection assays were performed using a RPA II kit (Ambion, Austin, TX) and a 32P-labeled riboprobe specific for ΔβRII. To normalize each RNA sample for differences in loading, a 32P-labeled glyceraldehyde-3-phosphate dehydrogenase (GAPDH) riboprobe was included in each analysis. To compare ML transgene expression levels with that of the endogenous ML gene, a 32P-labeled riboprobe corresponding to 200 bp spanning both 3′ coding and noncoding sequences of the native ML gene was synthesized. The entire 200-bp probe annealed to native ML transcripts, but only 150 bp of the probe annealed to transcripts of the ML transgene. The intensity of protected bands was determined by densitometeric scanning of x-ray films.
Western Blot Analysis.
The epidermis from transgenic or control littermates was separated from the dermis (13), and total epidermal protein was extracted as described (14). An equal amount of protein from each sample was separated by electrophoresis on a 12.5% SDS/PAGE gel and transferred onto a nitrocellulose membrane. Western blot analysis was performed as described (15) by using 10 μg/ml of a mouse mAb against the human c-myc epitope (Oncogene Science) and an ECL Western blotting detection system (Amersham).
Tissue Histology.
Pieces of skin were fixed in half strength Karnovsky’s at 4°C, postfixed in OsO4, and embedded in EMbed 812 (Electron Microscopy Sciences, Fort Washington, PA) (16). Sections were cut at 1 micron and stained with Richardson’s stain (16).
BrdUrd Uptake and Staining.
Newborn transgenic and control littermates were injected (i.p.) with BrdUrd (Sigma) at 250 mg/kg in 0.9% NaCl and sacrificed after 2 hr. Pieces of skin were fixed and processed as described (13). Sections were incubated with fluorescein isothiocyanate-conjugated mAb to BrdUrd (Becton Dickenson) and a guinea-pig antiserum to mouse keratin 14 (K14). K14 was visualized by biotinylated anti-guinea-pig IgG (Vector Laboratories) and Streptavidin-Texas Red (GIBCO). Three skin samples were used in each group, and three sections from each skin were stained with an anti-BrdUrd antibody. Each epidermal section was measured by a micrometer, and the mean of BrdUrd-positive cells per mm ± SD from three skin samples represents the labeling index.
Immunofluorescence.
Frozen sections from transgenic and control skins were incubated with a guinea pig antibody against K14 and rabbit antibodies against keratins K1 and K6, loricrin, and filaggrin. The reactions were visualized with biotinylated goat anti-guinea pig IgG and Streptavidin-Texas Red, and fluorescein isothiocyanate-labeled anti-rabbit IgG (Dakopatts, Glostrup, Denmark) (see ref.13 for details).
Cell Culture and [3H]Thymidine (TdR) Incorporation.
Primary keratinocytes were prepared from newborn transgenic and control littermates as described (17) and grown in 50% fibroblast conditioned medium supplemented with 0.05 mM Ca2+ (18). To determine the growth rate, keratinocytes from normal and ML.ΔβRII mice were each plated at 5 × 105 cells per 60-mm dish. To confirm that the ΔβRII did in fact block the inhibitory effect of TGF-β1 on keratinocyte growth, [3H]TdR labeling of keratinocytes was performed after addition of exogenous TGF-β1. Keratinocytes from nontransgenic and ML.ΔβRII mice were cultured to confluency in 24-well dishes, then human TGF-β1 (from 0.015 ng/ml to 1 ng/ml; Oncogene Science) was added to the cultures. Twenty-two hours later, cultures were pulse-labeled for 2 hr with 1 μCi/ml (1 Ci = 37 GBq) of [3H]TdR (Amersham). Control cultures of nontransgenic and ML.ΔβRII keratinocytes received no TGF-β1 before [3H]TdR labeling. Incorporated [3H]TdR was precipitated with 5% trichloroacetic acid and counted in a scintillation counter. [3H]TdR incorporation in TGF-β1-treated cultures was expressed as percent of control of [3H]TdR incorporation in control cultures.
RESULTS
Expression of the ML.ΔβRII Transgene in the Epidermis.
To target expression of the ΔβRII (8) to the epidermis, we used a truncated ML promoter, which expresses transgenes in both basal and suprabasal layers of the epidermis. The construction and characterization of this vector have been described (19). Fig. 1A is a schematic of this vector containing the ΔβRII mutant (ML.ΔβRII). Three transgenic lines, B9223, B9273, and C1072, were confirmed as transgene expressors by RNase protection analysis. The ML.ΔβRII transgene was expressed at a very high level—i.e., 4- to 5-fold over that of the GAPDH gene in transgenic epidermis (Fig. 1B Left). To compare expression levels of ML.ΔβRII with that of the endogenous ML gene, a probe that recognizes both the endogenous (200 bp) and transgene (150 bp) transcripts was used. Note that transgene expression levels are about 50% of that of the endogenous loricrin gene (Fig. 1B Right). Western blot analysis, using an antibody against the human c-myc tag, detected a predicated ΔβRII molecule of 23 kDa in extracts of transgenic, but not control, epidermis (Fig. 1C). Expression of the ML.ΔβRII transgene, as determined at both RNA and protein levels, correlated with the phenotypic severity of each line (see below).
ML.ΔβRII Transgenic Mice Exhibit a Hyperplastic/Hyperkeratotic Skin Phenotype.
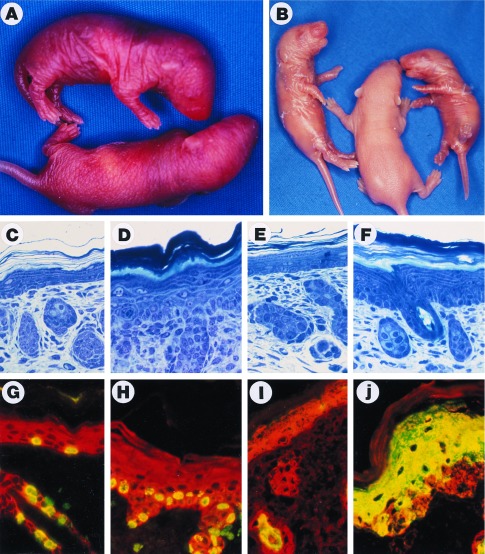
All three of the ML.ΔβRII transgenic lines exhibited a similar skin phenotype, although there were differences in phenotypic severity. Each of the founders was fertile and able to transmit the phenotype to progeny. B9223, the highest expressor, exhibited the most severe skin phenotype. A markedly thickened and wrinkled skin was evident at birth (Fig. 2A), and this progressed to a peeling skin phenotype at 3 days of age (Fig. 2B). Histological analysis of F1 progeny from this founder revealed a marked hyperplasia and hyperkeratosis of the epidermis at birth, and the presence of hypertrophic keratinocytes (Fig. 2D), which were absent in control littermates (Fig. 2C). At 3 days of age, the hyperplastic/hyperkeratotic phenotype was still apparent (Fig. 2F) as compared with nontransgenic controls (Fig. 2E). In addition, the stratum corneum appeared to be flaky, and more loosely attached (Fig. 2F), consistent with the gross phenotype of peeling skin (Fig. 2B). The morphology of hair follicles (Fig. 2 D and F), as well as the onset of hair growth appeared normal in ML.ΔβRII transgenic mice. The hyperplastic/hyperkeratotic phenotype gradually diminished as founder mice developed into adulthood. However, when F1 mice from line B9223 were mated to homozygosity, perinatal lethality occurred due to the severe hyperkeratosis that restricted mobility.
Figure 2.
ML.ΔβRII gross phenotype, histopathology, BrdUrd labeling, and K6 expression. (A) A transgenic pup (Upper) from line B9223 shows a thickened and wrinkled skin compared with the nontransgenic sibling (Lower) 24 hr after birth. (B) Transgenic mice (Left and Right) from line B9223 exhibited a peeling skin phenotype 3 days after birth. (C–F) Richardson’s staining of 1 μm sections. (×20.) (C) Nontransgenic skin 24 hr after birth. (D) ML.ΔβRII transgenic skin from line B9223 shows a 3-fold increase in thickness of the epidermis, hyperkeratosis, and hypertrophic keratinocytes compared with the control (C). (E) Nontransgenic skin 3 days after birth. (F) ML.ΔβRII transgenic skin from line B9223 shows a moderate hyperplasia at 3 days, and a more loosely attached stratum corneum compared with the transgenic skin at 24 hr (D). (G–J) Double-label immunofluorescence. (×20.) (Red represents K14.) (G) BrdUrd labeling (green or yellow) in a nontransgenic epidermis. (H) ML.ΔβRII transgenic epidermis exhibits about a 3-fold increase in BrdUrd labeling. (I) K6 is only expressed in the hair follicles of the normal skin (green or yellow). (J) K6 overexpression in ML.ΔβRII transgenic epidermis.
Increased Labeling Index.
To determine the rate of proliferation of ML.ΔβRII epidermis relative to controls, F1 littermates were labeled with BrdUrd at 24 hr after birth. In normal epidermis, basal cells were labeled with BrdUrd at a rate of 36 ± 8.3 nuclei/mm (Fig. 2G), whereas in ML.ΔβRII epidermis BrdUrd labeling was increased to 92 ± 2.1 nuclei/mm (Fig. 2H). Although most of the labeling occurred in basal cells of ML.ΔβRII epidermis, some suprabasal cells were also labeled (Fig. 2H). Thus, these data confirm both an increased rate of proliferation and an expansion of the proliferative compartment in ML.ΔβRII epidermis.
Aberrant Differentiation Marker Expression.
To assess potential changes in markers of epidermal differentiation and proliferation, frozen sections from normal and transgenic skin samples were analyzed by double-label immunofluorescence with antibodies to keratins K1, K6, K13, and K14, to loricrin, and to filaggrin. K14 is detectable throughout most of the epidermis, as well as in hair follicles. K1 is normally expressed at an early stage during differentiation of the epidermis, routinely detected in the first suprabasal layer and occasionally in basal cells that have just withdrawn from the cell cycle (20). The expression of K1 was delayed in ML.ΔβRII epidermis as compared with controls (data not shown), presumably due to expansion of the proliferative compartment. K6 is normally expressed in the outer root sheath of the hair follicles but not in the interfollicular epidermis (ref. 21; Fig. 2I). We have previously shown that the induction of K6 can occur in the epidermis of transgenic mice that has been pathologically altered by expression of a variety of transgenes (3, 13, 22–24), including those that induce hyperplasia (13, 22, 23). Therefore, it was not surprising to find that K6 was expressed in the epidermis of ML.ΔβRII mice (Fig. 2J). Loricrin and filaggrin, which are markers for late stages of terminal differentiation and normally expressed in the granular layer of the epidermis (25, 26), did not appear to be altered in the ML.ΔβRII epidermis (data not shown).
Cultured ML.ΔβRII Keratinocytes Exhibit Accelerated Growth and Resistance to TGF-β.
Because it was not possible to obtain direct in vivo evidence that the hyperplastic phenotype was due to resistance to TGF-β, we decided to confirm this using primary cultures of ML.ΔβRII keratinocytes. When cultures of primary keratinocytes are grown in low Ca2+ medium, they exhibit a basal cell phenotype—i.e., they proliferate but do not stratify or express differentiation markers (14). Initially, we observed that primary ML.ΔβRII keratinocytes exhibited an accelerated rate of growth, reaching confluency by day 4, when 5 × 105 cells were plated in a 60-mm culture dish (Fig. 3B). In contrast, normal control keratinocytes exhibited a much slower rate of growth when plated at the same density (Fig. 3A) and did not reach confluency before the cells started to slough from the dish at day 9. When exogenous TGF-β1 was added to primary cultures grown in low Ca2+ medium, an immediate inhibitory effect on DNA synthesis was observed in nontransgenic keratinocytes, with inhibition ranging from 50% with 0.015 ng/ml TGF-β1 to nearly 100% with 1 ng/ml TGF-β1 (Fig. 3C). In contrast, ML.ΔβRII keratinocytes require 30 times more TGF-β1 (0.5 ng/ml) to show a similar (50%) decrease in [3H]TdR labeling, and at 1 ng/ml, which totally inhibited nontransgenic keratinocytes, ML.ΔβRII keratinocytes still retained 40% of DNA synthesis (Fig. 3C). These data demonstrate that basal keratinocytes from transgenic ML.ΔβRII epidermis have an increased rate of growth due to loss of sensitivity to TGF-β-mediated growth inhibition.
Figure 3.
Analysis of ML.ΔβRII keratinocytes in culture. (A) Nontransgenic keratinocytes were subconfluent at day 4 of culture. (B) Keratinocytes from ML.ΔβRII epidermis grew faster and were confluent by day 4. (C) [3H]TdR labeling of keratinocytes 22 hr after addition of TGF-β1. [3H]TdR labeling for cultures receiving different concentrations of TGF-β1 was expressed as percent of control. Each point is the average of results determined in triplicate. Error bars represent SD. Note that nontransgenic keratinocytes showed a marked decrease in [3H]TdR labeling at the lowest concentration of TGF-β1, while ML.ΔβRII keratinocytes were resistant to TGF-β1 until the concentration reached 0.5 ng/ml.
DISCUSSION
We have previously used a transgenic approach to demonstrate that TGF-β is a potent negative growth regulator of the epidermis in vivo (3). Overexpression of a mutant form of TGF-β1 (mutations of Cys-223 → Ser and Cys-225 → Ser, TGF-β1S223/225), which is constitutively active (27), results in an inhibition of normal skin development due to almost complete suppression of epidermal cell proliferation. Arresting growth of the epidermis at a stage of development when the remainder of the embryo continued to grow rapidly produced phenotypic pups with a very tautly stretched skin that restricted movement and breathing, and resulted in death within 24 hr. In this study, we have targeted overexpression of a dominant-negative mutant form of the type II TGF-β receptor and created just the opposite effect–i.e., by blocking signaling via wild-type II TGF-β receptors, we have disrupted the normal mechanism regulating epidermal homeostasis. In the case of homozygotes, the accelerated rate of proliferation leads to lethality due to the marked increase in thickness of the epidermis (hyperkeratosis), which severely restricts movement. The phenotypes observed in both of these transgenic studies correlate well with the TGF-β signaling pathway functioning primarily as a regulator of epidermal growth, rather than an inducer of differentiation. In our initial study, overexpression of TGF-β1 resulted in growth arrest of the epidermis without altering the normal pattern of terminal differentiation. In the present study, delayed expression of K1, an early differentiation marker, was observed. However, delayed expression of K1 has been previously observed in other transgenic lines exhibiting a hyperproliferative phenotype (22, 23), and most likely occurs as a result of expansion of the proliferative compartment.
The more severe phenotype exhibited by homozygotes suggests a dosage effect from expression of ΔβRII. We have previously observed an increase in phenotypic severity when other lines expressing epidermally targeted transgenes were mated to homozygosity (22–24). The gradual reduction in the hyperplastic/hyperkeratotic phenotype during the development of heterozygous mice into adulthood has also been observed previously. This is most obvious in the case of mice expressing transgenes that increase the rate of proliferation of the epidermis, such as rasHa (22) and TGF-α (23, 28). It is well established that the rate of proliferation of newborn mouse epidermis is higher than adult epidermis (29). Therefore, the mechanisms that control the gradual reduction in the rate of proliferation during this transition may exert a normalizing or suppressive effect as observed in this and previous transgenic studies. However, it is more likely that this represents an adaptive response by the mouse to restore tissue homeostasis. This backup regulatory system is successful in the case of heterozygotes but not for homozygotes derived from founders with high expression levels. Whatever the mechanism, in the case of rasHa or TGF-α expressing lines, it can only maintain tissue homeostasis if the epidermis remains unchallenged. If the skin of heterozygous rasHa or TGF-α mice is exposed to the tumor promoter, 12-O-tetradecanylphorbol-13-acetate, or wounded, the backup system fails and benign tumors appear (30, 31).
It remains to be seen how the ML.ΔβRII mice will respond to such challenges; however, we predict that these mice will prove to be a valuable resource to address several unanswered questions concerning TGF-β and its potential pathogenic role in several diseases. For example, as discussed above, TGF-β is a potent growth inhibitor of the epidermis and other epithelia, yet it is overexpressed in many epithelial cancers. It has been postulated that secretion of TGF-β at a late stage of carcinogenesis may be a critical factor for invasion and metastasis via stromal remodeling (32). If TGF-β does play a promoting role in epithelial carcinogenesis, it must occur at a stage after epithelial cells have lost responsiveness to its potent growth inhibitory effects. In support of this hypothesis, mutations in the type II TGF-β receptor have recently been detected in TGF-β-resistant cell lines of colon (33–35), gastric (34), and head and neck (36) cancers, and even more importantly, similar mutations have also recently been reported in primary tumors of the colon (34). These data suggest that inactivation of the TGF-β type II receptor might be one mechanism by which epithelial tumor cells escape from TGF-β-induced growth inhibition and progress to malignancy, and our ML.ΔβRII transgenic mice will provide a useful experimental model to assess the effect of blocking TGF-β-mediated growth inhibition during various stages of carcinogenesis. Other unanswered questions center on TGF-β and diseases of connective tissue. Although TGF-β is thought to play a critical role in normal wound healing by regulating connective tissue deposition, its inappropriate expression has been implicated in the pathogenesis of fibrotic diseases such as hypertrophic scarring, superficial types of scleroderma, and keloids (37). Our initial attempt to make a transgenic animal model to determine whether expression of TGF-β in the epithelial compartment of the skin might contribute to the development of fibrosis in the superficial dermis resulted in neonatal lethality due to growth arrest of the epidermis (3). Because we have succeeded in blocking the growth inhibitory effect of TGF-β on the epidermis via expression of ΔβRII, it should now be feasible to introduce another transgene into the ML.ΔβRII germ line that controls expression of TGF-β1S223/225 in an inducible fashion. This would allow overproduction of TGF-β1S223/225 in the epidermis, without deleterious effects, and assessment of the phenotypic consequences of its secretion into the dermis. Such transgenic lines are currently being developed.
Acknowledgments
We thank Matti Brooks and Michael Peterson for excellent technical assistance and Ms. Janelle Laminack for invaluable secretarial assistance. This work was supported in part by National Institutes of Health Grant CA52607 (D.R.R.) and a Max Planck Research Award from the Alexander von Humboldt Foundation (T.K. and D.R.R.).
ABBREVIATIONS
- TGF-β
transforming growth factor β
- ΔβRII
dominant negative type II TGF-β receptor
- ML
mouse loricrin
- TdR
thymidine
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
References
- 1.Roberts A B, Flanders K C, Kondaiah P, Thompson N L, Obberghen-Schilling E V, Wakefield L, Rossi P, Crombrugghe B D, Heine U, Sporn M B. Recent Prog Horm Res. 1988;44:157–193. doi: 10.1016/b978-0-12-571144-9.50010-7. [DOI] [PubMed] [Google Scholar]
- 2.Pittelkow M R, Coffey R J, Moses H L. Ann NY Acad Sci. 1988;548:211–224. doi: 10.1111/j.1749-6632.1988.tb18809.x. [DOI] [PubMed] [Google Scholar]
- 3.Sellheyer K, Bickenbach J R, Rothnagel J A, Bundman D S, Longley M A, Krieg T, Roche N S, Roberts A B, Roop D R. Proc Natl Acad Sci USA. 1993;90:5237–5241. doi: 10.1073/pnas.90.11.5237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cui W, Fowlis D J, Cousins F M, Duffie E, Bryson S, Balmain A, Akhurst R J. Genes Dev. 1995;9:945–955. doi: 10.1101/gad.9.8.945. [DOI] [PubMed] [Google Scholar]
- 5.Roberts A B, Sporn M B, Assoian R K, Smith J M, Roche N S, Wakefield L M, Heine U I, Liotta L A, Falanga V, Kehrl J H, Fauci A S. Proc Natl Acad Sci USA. 1986;83:4167–4171. doi: 10.1073/pnas.83.12.4167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Derynck R. Trends Biol Sci. 1994;19:548–553. doi: 10.1016/0968-0004(94)90059-0. [DOI] [PubMed] [Google Scholar]
- 7.Massague J, Attisano L, Wrana J L. Trends Cell Biol. 1994;4:172–178. doi: 10.1016/0962-8924(94)90202-x. [DOI] [PubMed] [Google Scholar]
- 8.Chen R-H, Ebner R, Derynck R. Science. 1993;260:1335–1338. doi: 10.1126/science.8388126. [DOI] [PubMed] [Google Scholar]
- 9.Wieser R, Attisano L, Wrana J L, Massague J. Mol Cell Biol. 1993;13:7239–7247. doi: 10.1128/mcb.13.12.7239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Feng X-H, Filvaroff E H, Derynck R. J Biol Chem. 1995;270:24237–24245. doi: 10.1074/jbc.270.41.24237. [DOI] [PubMed] [Google Scholar]
- 11.Chen R-H, Derynck R. J Biol Chem. 1994;269:22868–22874. [PubMed] [Google Scholar]
- 12.Hogan B, Constantini F, Lacey E. Manipulating the Mouse Embryo: A Laboratory Manual. Plainview, NY: Cold Spring Horbor Lab. Press; 1986. [Google Scholar]
- 13.Wang X-J, Greenhalgh D A, Lu X, Bickenbach J R, Roop D R. Oncogene. 1995;10:279–289. [PubMed] [Google Scholar]
- 14.Yuspa S H, Kilkenny A E, Steinert P M, Roop D R. J Cell Biol. 1989;109:1207–1217. doi: 10.1083/jcb.109.3.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Greenhalgh D A, Rothnagel J A, Wang X-J, Quintanill M I, Orengo C C, Gagne T A, Bundman D S, Longley M A, Fisher C, Roop D R. Oncogene. 1993;8:2145–2157. [PubMed] [Google Scholar]
- 16.Hayat M A. Basic Electron Microscopy Techniques. New York: Van Nostrand Reinhold; 1972. [Google Scholar]
- 17.Yuspa, S. H., Hawley-Nelson, P., Stanley, J. R. & Hennings, H. (1980) Transplant. Proc. 12 (Suppl. 1), 114–122. [PubMed]
- 18.Greenhalgh D A, Welty D J, Strickland J E, Yuspa S H. Mol Carcinog. 1989;2:199–207. doi: 10.1002/mc.2940020406. [DOI] [PubMed] [Google Scholar]
- 19.DiSepio D, Jones A, Longley M A, Bundman D, Rothnagel J A, Roop D R. J Biol Chem. 1995;270:10792–9. doi: 10.1074/jbc.270.18.10792. [DOI] [PubMed] [Google Scholar]
- 20.Roop D R, Huitfeldt H, Kilkenny A, Yuspa S H. Differentiation (Berlin) 1987;35:143–150. doi: 10.1111/j.1432-0436.1987.tb00162.x. [DOI] [PubMed] [Google Scholar]
- 21.Weiss R A, Eichner R, Sun T T. J Cell Biol. 1984;98:1397–1406. doi: 10.1083/jcb.98.4.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Greenhalgh D A, Rothnagel J A, Quintanill M I, Orengo C C, Gagne T A, Bundman D S, Longley M A, Roop D R. Mol Carcinog. 1993;7:99–110. doi: 10.1002/mc.2940070208. [DOI] [PubMed] [Google Scholar]
- 23.Dominey A M, Wang X-J, King L E, Jr, Nanney L B, Gagne T A, Sellheyer K, Bundman D S, Longley M A, Rothnagel J A, Greenhalgh D A, Roop D R. Cell Growth Differ. 1993;4:1071–82. [PubMed] [Google Scholar]
- 24.Imakado S, Bickenbach J R, Bundman D S, Rothnagel J A, Attar P S, Wang X-J, Walczak V R, Wisniewski S, Pote J, Gordon J S, Heyman R A, Evans R M, Roop D R. Genes Dev. 1995;9:317–329. doi: 10.1101/gad.9.3.317. [DOI] [PubMed] [Google Scholar]
- 25.Mehrel T, Hohl D, Rothnagel J A, Longley M A, Bundman D, Cheng C, Licht U, Bisher M E, Steven A C, Steinert P M, Yuspa S H, Roop D R. Cell. 1990;61:1103–1112. doi: 10.1016/0092-8674(90)90073-n. [DOI] [PubMed] [Google Scholar]
- 26.Steven A C, Bisher M E, Roop D R, Steinert P M. J Struct Biol. 1990;104:150–162. doi: 10.1016/1047-8477(90)90071-j. [DOI] [PubMed] [Google Scholar]
- 27.Brunner A M, Marquardt H, Malacko A R, Lioubin M N, Purchio A F. J Biol Chem. 1989;264:13660–13664. [PubMed] [Google Scholar]
- 28.Vassar R, Fuchs E. Genes Dev. 1991;5:714–727. doi: 10.1101/gad.5.5.714. [DOI] [PubMed] [Google Scholar]
- 29.Marks F, Bertsch S. Cell Tissue Kinet. 1982;15:81–87. doi: 10.1111/j.1365-2184.1982.tb01026.x. [DOI] [PubMed] [Google Scholar]
- 30.Greenhalgh D A, Roop D R. Adv Cancer Res. 1994;64:247–296. doi: 10.1016/s0065-230x(08)60840-4. [DOI] [PubMed] [Google Scholar]
- 31.Wang X-J, Greenhalgh D A, Eckhardt J N, Bundman D S, Rothnagel J A, Roop D R. Mol Carcinog. 1994;10:15–22. doi: 10.1002/mc.2940100104. [DOI] [PubMed] [Google Scholar]
- 32.Wright J A, Turley E A, Greenberg A H. Crit Rev Oncog. 1993;4:473–492. [PubMed] [Google Scholar]
- 33.Markowitz S, Wang J, Myeroff L, Parsons R, Sun L Z, Lutterbaugh J, Fan R S, Zborowska E, Kinzler K W, Vogelstein B, Brattain M, Willson J K V. Science. 1995;268:1336–1338. doi: 10.1126/science.7761852. [DOI] [PubMed] [Google Scholar]
- 34.Myeroff L L, Parsons R, Kim S-J, Hedrick L, Cho K R, Orth K, Mathis M, Kinzler K W, Lutterbaugh J, Park K, Bang Y-J, Lee H Y, Park J G, Lynch H T, Roberts A B, Bogelstein B, Markowitz S D. Cancer Res. 1995;55:5545–5547. [PubMed] [Google Scholar]
- 35.Wang J, Sun L, Myeroff L, Wang X, Gentry L E, Yang J, Liang J, Zborowska E, Markowitz S, Willson J K V, Brattain M G. J Biol Chem. 1995;270:22044–22049. doi: 10.1074/jbc.270.37.22044. [DOI] [PubMed] [Google Scholar]
- 36.Garrigue-Antar L, Munoz-Antonia T, Antonia S J, Gesmonde J, Vellucci V P, Reiss M. Cancer Res. 1995;55:3982–3987. [PubMed] [Google Scholar]
- 37.Mauch C, Eckes B, Hunzelmann N, Oono T, Kozlowska E, Krieg T. J Invest Dermatol. 1993;100:92s–96s. doi: 10.1111/1523-1747.ep12356293. [DOI] [PubMed] [Google Scholar]