Abstract
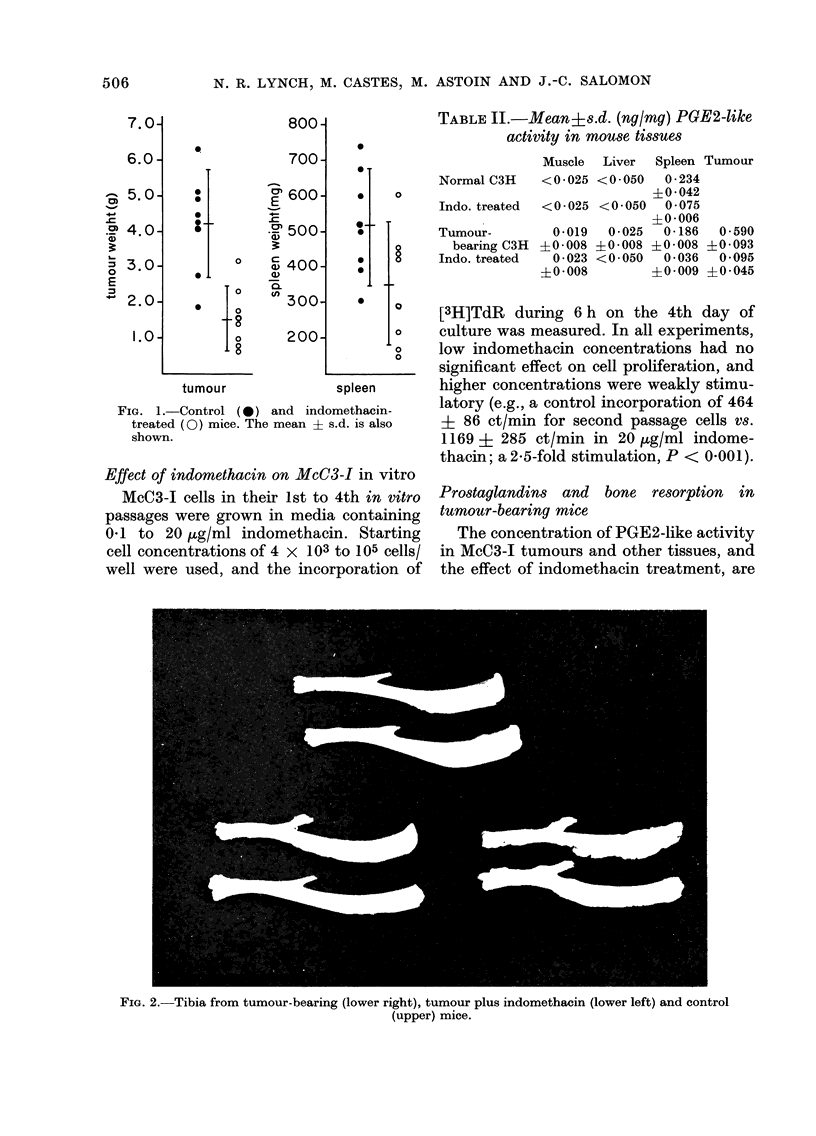
The growth of a 3-methylcholanthrene-induced fibrosarcoma of C3H mice was inhibited by aspirin and indomethacin. While the tumour contained relatively high concentrations of PGE2-like material, that were markedly diminished by indomethacin treatment, our results did not confirm the recently proposed hypothesis that the anti-tumour effect arises from a restoration of depressed immune function. For example, mice that had completely eliminated their tumours under indomethacin administration were not immune to rechallenge. The tumour-bearing animals were not non-specifically immunodepressed, as their splenic PFC responses against SRBC were enhanced. However, while indomethacin augmented the PFC response in normal mice, this adjuvant effect was depressed in tumour-bearing animals. The spleen-cell PHA responses of tumour bearers were severely depressed, and such cells suppressed the PHA response of normal cells. Only after prolonged indomethacin treatment did animals (with comparable tumour burdens) show weak PHA responses and somewhat diminished suppressive activity. Possible alternative mechanisms, such as direct cytotoxicity, or inhibition of inflammation, phosphodiesterase activity, blood coagulation or calcium availability were not implicated (nor definitively excluded) in the anti-tumour effect.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atkins D., Ibbotson K. J., Hillier K., Hunt N. H., Hammonds J. C., Martin T. J. Secretion of prostaglandins as bone-resorbing agents by renal cortical carcinoma in culture. Br J Cancer. 1977 Nov;36(5):601–607. doi: 10.1038/bjc.1977.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett A., Tacca M. D., Stamford I. F., Zebro T. Prostaglandins from tumours of human large bowel. Br J Cancer. 1977 Jun;35(6):881–884. doi: 10.1038/bjc.1977.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulson C. J., Ford R. E., Marshall S., Walker J. L., Wooldridge K. R., Bowden K., Coombs T. J. Interrelationship of cyclic nucleotides and anaphylactic reactions. Nature. 1977 Feb 10;265(5594):545–547. doi: 10.1038/265545a0. [DOI] [PubMed] [Google Scholar]
- Dunn C. J., Willoughby D. A., Giroud J. P., Yamamoto S. An appraisal of the interrelationships between prostaglandins and cyclic nucleotides in inflammation. Biomedicine. 1976;24(4):214–220. [PubMed] [Google Scholar]
- Goodwin J. S., Messner R. P., Bankhurst A. D., Peake G. T., Saiki J. H., Williams R. C., Jr Prostaglandin-producing suppressor cells in Hodgkin's disease. N Engl J Med. 1977 Nov 3;297(18):963–968. doi: 10.1056/NEJM197711032971802. [DOI] [PubMed] [Google Scholar]
- Hial V., De Mello M. C., Horakova Z., Beaven M. A. Antiproliferative activity of anti-inflammatory drugs in two mammalian cell culture lines. J Pharmacol Exp Ther. 1977 Aug;202(2):446–454. [PubMed] [Google Scholar]
- Hial V., Horakova Z., Shaff F. E., Beaven M. A. Alteration of tumor growth by aspirin and indomethacin: studies with two transplantable tumors in mouse. Eur J Pharmacol. 1976 Jun;37(2):367–376. doi: 10.1016/0014-2999(76)90044-3. [DOI] [PubMed] [Google Scholar]
- Lewis G. P. Prostaglandins in inflammation. J Reticuloendothel Soc. 1977 Oct;22(4):389–402. [PubMed] [Google Scholar]
- Lynch N. R., Salomon J. C. Passive local anaphylaxis: demonstration of antitumor activity and complementation of intratumor BCG. J Natl Cancer Inst. 1977 Apr;58(4):1093–1098. doi: 10.1093/jnci/58.4.1093. [DOI] [PubMed] [Google Scholar]
- Lynch N. R., Salomon J. C. Tumour-associated inhibition of immediate hypersensitivity reactions in mice. Immunology. 1977 May;32(5):645–650. [PMC free article] [PubMed] [Google Scholar]
- Manku M. S., Horrobin D. F. Chloroquine, quinine, procaine, quinidine, tricyclic antidepressants, and methylxanthines as prostaglandin agonists and antagonists. Lancet. 1976 Nov 20;2(7995):1115–1117. doi: 10.1016/s0140-6736(76)91090-4. [DOI] [PubMed] [Google Scholar]
- Moroz L. A. Increased blood fibrinolytic activity after aspirin ingestion. N Engl J Med. 1977 Mar 10;296(10):525–529. doi: 10.1056/NEJM197703102961001. [DOI] [PubMed] [Google Scholar]
- Pelus L. M., Strausser H. R. Indomethacin enhancement of spleen-cell responsiveness to mitogen stimulation in tumorous mice. Int J Cancer. 1976 Nov 15;18(5):653–660. doi: 10.1002/ijc.2910180514. [DOI] [PubMed] [Google Scholar]
- Plescia O. J., Smith A. H., Grinwich K. Subversion of immune system by tumor cells and role of prostaglandins. Proc Natl Acad Sci U S A. 1975 May;72(5):1848–1851. doi: 10.1073/pnas.72.5.1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powles T. J., Dowsett M., Easty G. C., Easty D. M., Neville A. M. Breast-cancer osteolysis, bone metastases, and anti-osteolytic effect of aspirin. Lancet. 1976 Mar 20;1(7960):608–610. doi: 10.1016/s0140-6736(76)90416-5. [DOI] [PubMed] [Google Scholar]
- Santoro M. G., Philpott G. W., Jaffe B. M. Inhibition of tumour growth in vivo and in vitro by prostaglandin E. Nature. 1976 Oct 28;263(5580):777–779. doi: 10.1038/263777a0. [DOI] [PubMed] [Google Scholar]
- Schechter B., Feldman M. Hydrocortisone affects tumor growth by eliminating precursors of suppressor cells. J Immunol. 1977 Nov;119(5):1563–1568. [PubMed] [Google Scholar]
- Strausser H. R., Humes J. L. Prostaglandin synthesis inhibition: effect on bone changes and sarcoma tumor induction in balb/c mice. Int J Cancer. 1975 May 15;15(5):724–730. doi: 10.1002/ijc.2910150503. [DOI] [PubMed] [Google Scholar]
- Tashjian A. H., Jr, Voelkel E. F., McDonough J., Levine L. Hydrocortisone inhibits prostaglandin production by mouse fibrosarcoma cells. Nature. 1975 Dec 25;258(5537):739–741. doi: 10.1038/258739a0. [DOI] [PubMed] [Google Scholar]
- Webb D. R., Jr, Jamieson T. Control of mitogen-induced transformation: characterization of a splenic suppressor cell and its mode of action. Cell Immunol. 1976 Jun 1;24(1):45–57. doi: 10.1016/0008-8749(76)90130-1. [DOI] [PubMed] [Google Scholar]
- Webb D. R., Osheroff P. L. Antigen stimulation of prostaglandin synthesis and control of immune responses. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1300–1304. doi: 10.1073/pnas.73.4.1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb D., Braun W., Plescia O. J. Antitumor effects of polynucleotides and theophylline. Cancer Res. 1972 Sep;32(9):1814–1819. [PubMed] [Google Scholar]
- Winchurch R. A., Foschi G. V., Walz D. T. Inhibition of the lymphocyte-mediated cytotoxic reaction by anti-inflammatory drugs. J Reticuloendothel Soc. 1974 Feb;15(2):112–117. [PubMed] [Google Scholar]




