Abstract
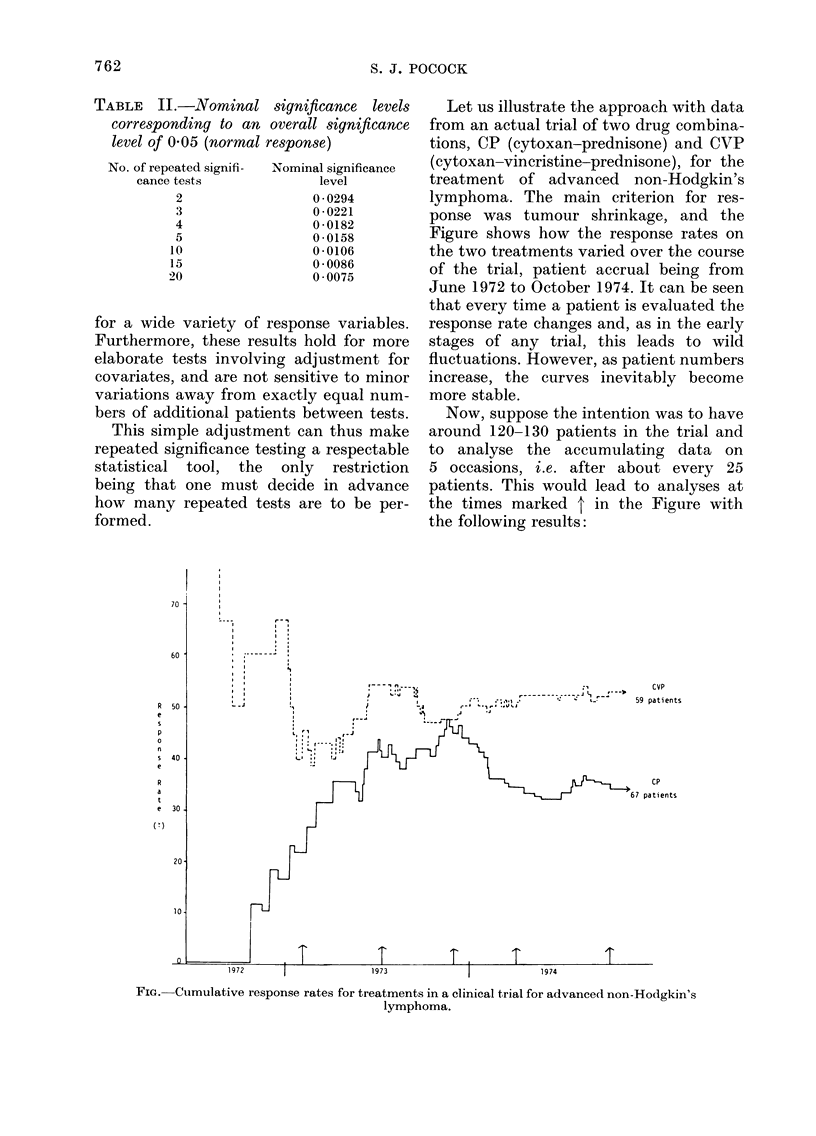
A recent international survey on the size of clinical trials in cancer showed the frequent problem of slow patient accrual, which remains a major hindrance to progress. The survey also revealed that, although the design of most trials specified a fixed number of patients, subsequent experience revealed a much more flexible approach, with analysis of results, say, every 4--6 months. Conventional sequential methods are hardly ever used and unfortunately most trials proceed without any predetermined stopping rules. Some trial organizers use repeated significance tests on accumulating data as a guide to the detection of treatment differences, an approach that can be adapted to a more rigorous statistical framework as a "group sequential design". The major statistical principle involved is that the more often one analyses the data the greater is the probability of achieving a statistically significant result, even when the two treatments are equally effective. Group sequential designs require the adoption of a more stringent significance level to allow for repeated testing. If one intends up to 10 repeated analyses of the data, only a treatment difference significant at the 1% level would merit a decision to stop the trial. For any trial to implement a stopping rule successfully there must also be prompt feedback and processing of response and survival data ready for up-to-date analysis. Such efficiency is often lacking. The repeated presentation of interim results of a trial to participating investigators can seriously affect their future reaction, especially if there are interesting but non-significant differences. Thus, some secrecy about ongoing results is advisable if trials are to achieve an unbiased conclusion.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- McPherson K. Statistics: the problem of examining accumulating data more than once. N Engl J Med. 1974 Feb 28;290(9):501–502. doi: 10.1056/NEJM197402282900907. [DOI] [PubMed] [Google Scholar]


