Abstract
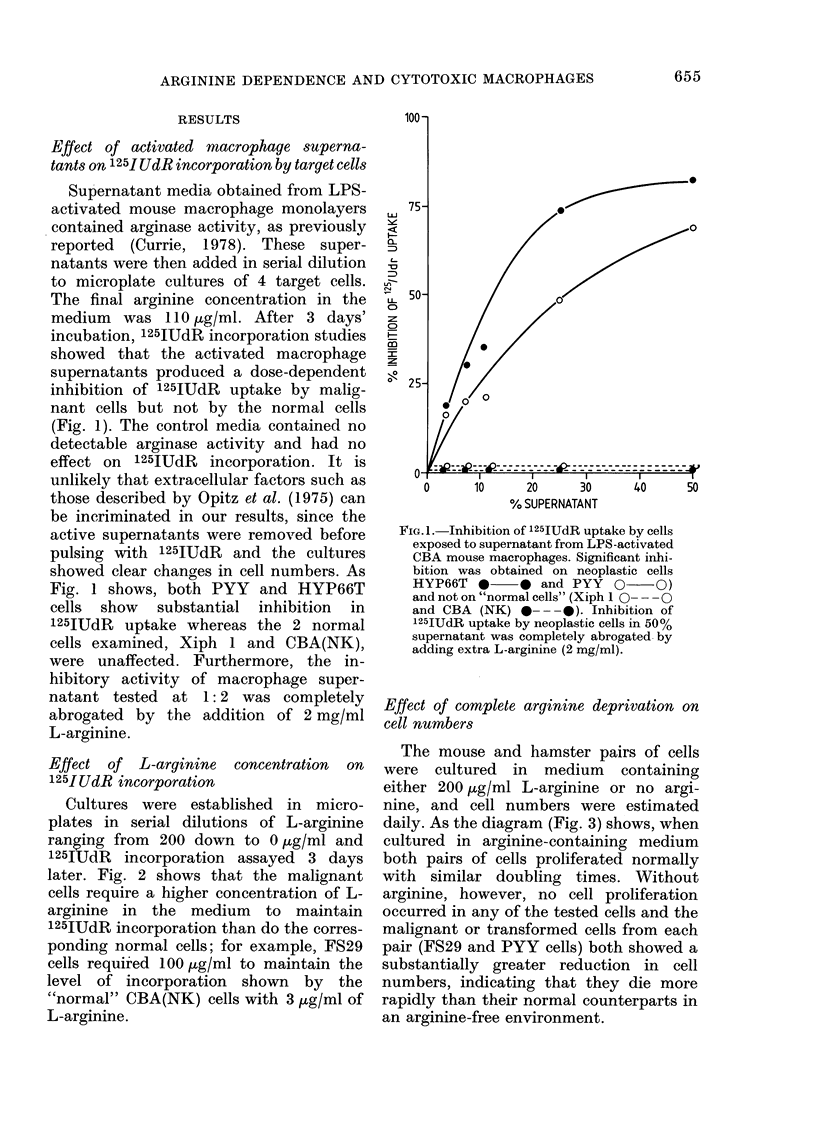
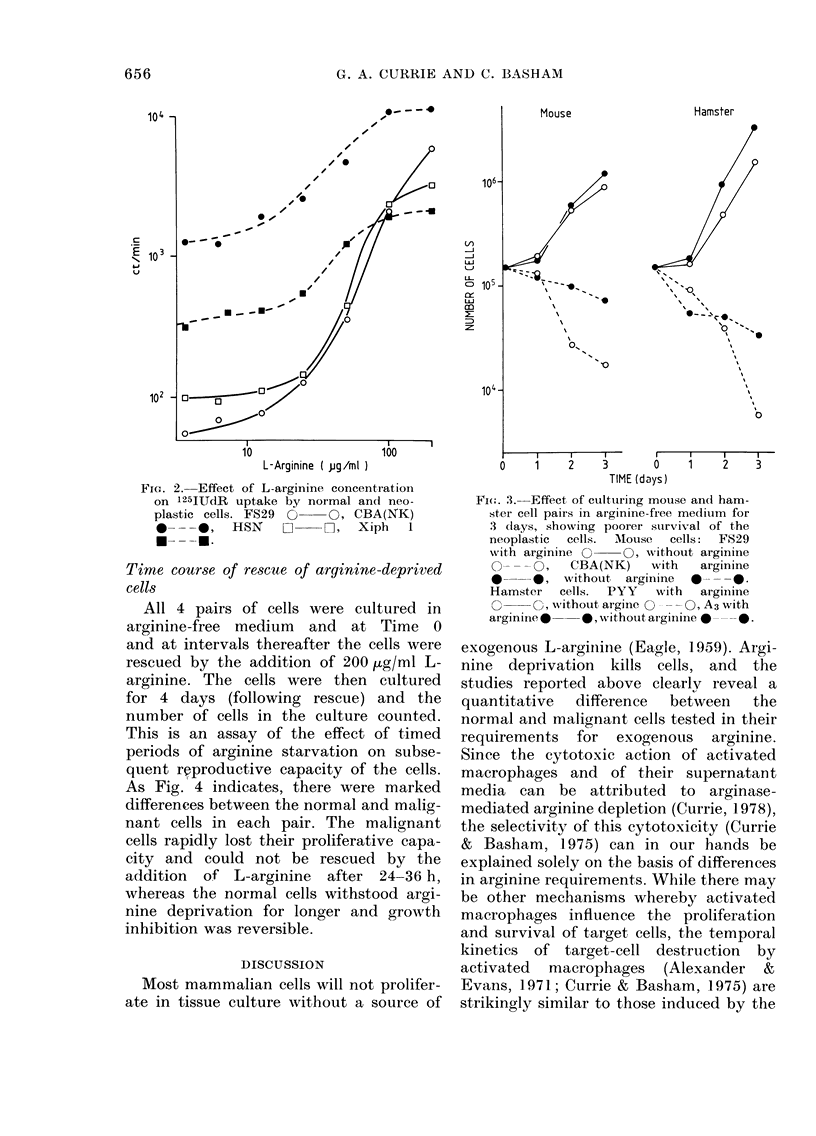
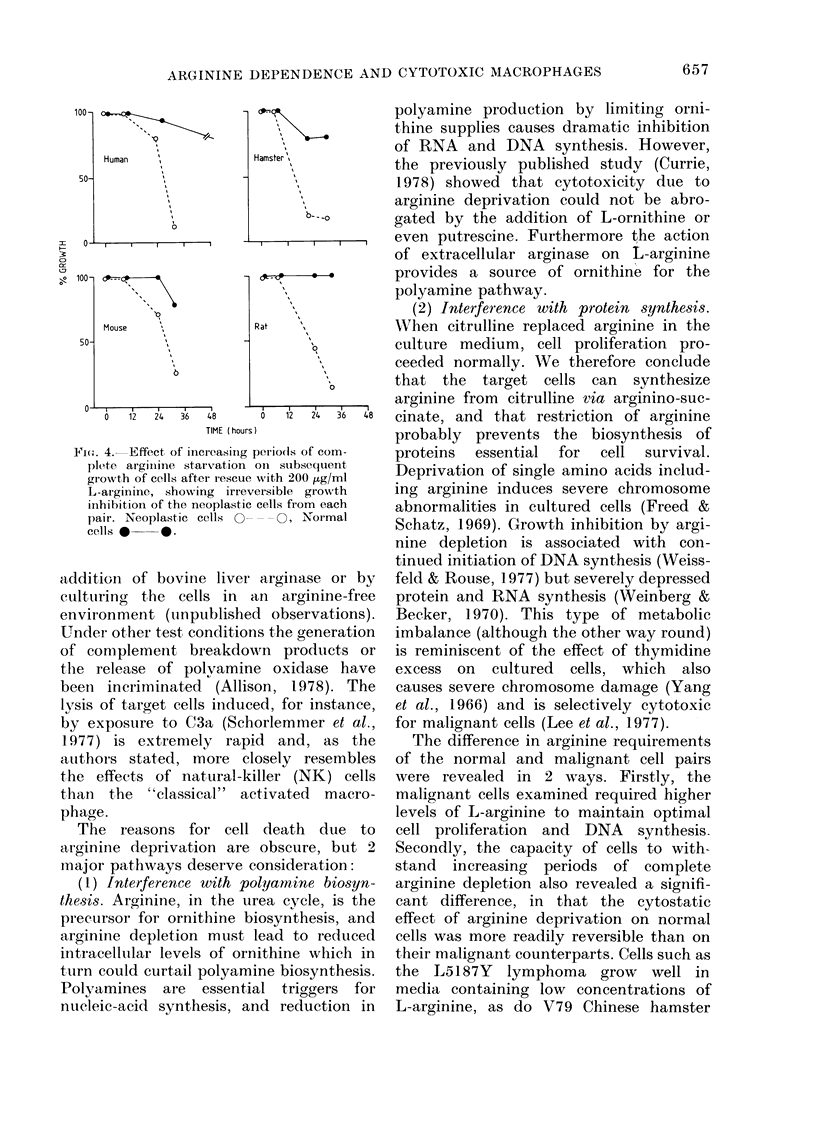
Normal and neoplastic cells from 4 species (man, rat, mouse and hamster) were examined for their dependence on exogenous L-arginine in tissue culture. The malignant cells required a higher concentration of L-arginine in the medium than their normal counterparts (with similar doubling times) to maintain optimal proliferation. Complete arginine deprivation resulted in equal growth inhibition of normal and malignant cells, but more rapid cytolysis of the malignant cell. Deprivation of L-arginine, followed 24 h later by rescue with L-arginine, allowed normal cells to proliferate, but the reproductive capacity of the malignant cells was irreversibly impaired. Since the cytotoxic activity of LPS-activated macrophages was associated with the release of arginase and was abrogated by excess L-arginine, it is suggested that the biological basis for the selective effects of such macrophages may reside in the L-arginine dependence of the target cells.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allison A. C. Mechanisms by which activated macrophages inhibit lymphocyte responses. Immunol Rev. 1978;40:3–27. doi: 10.1111/j.1600-065x.1978.tb00399.x. [DOI] [PubMed] [Google Scholar]
- Currie G. A. Activated macrophages kill tumour cells by releasing arginase. Nature. 1978 Jun 29;273(5665):758–759. doi: 10.1038/273758a0. [DOI] [PubMed] [Google Scholar]
- Currie G. A., Basham C. Activated macrophages release a factor which lyses malignant cells but not normal cells. J Exp Med. 1975 Dec 1;142(6):1600–1605. doi: 10.1084/jem.142.6.1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzfeld A., Raper S. M. The heterogeneity of arginases in rat tissues. Biochem J. 1976 Feb 1;153(2):469–478. doi: 10.1042/bj1530469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kung J. T., Brooks S. B., Jakway J. P., Leonard L. L., Talmage D. W. Suppression of in vitro cytotoxic response by macrophages due to induced arginase. J Exp Med. 1977 Sep 1;146(3):665–672. doi: 10.1084/jem.146.3.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S. S., Giovanella B. C., Stehlin J. S., Jr Selective lethal effect of thymidine on human and mouse tumor cells. J Cell Physiol. 1977 Sep;92(3):401–405. doi: 10.1002/jcp.1040920308. [DOI] [PubMed] [Google Scholar]
- Opitz H. G., Niethammer D., Lemke H., Flad H. D., Huget R. Inhibition of 3H-thymidine incorporation of lymphocytes by a soluble factor from macrophages. Cell Immunol. 1975 Apr;16(2):379–388. doi: 10.1016/0008-8749(75)90126-4. [DOI] [PubMed] [Google Scholar]
- Piessens W. F., Churchill W. H., Jr, David Macrophages activated in vitro with lymphocyte mediators kill neoplastic but not normal cells. J Immunol. 1975 Jan;114(1 Pt 2):293–299. [PubMed] [Google Scholar]
- Simberkoff M. S., Thorbecke G. J., Thomas L. Studies of PPLO infection. V. Inhibition of lymphocyte mitosis and antibody formation by mycoplasmal extracts. J Exp Med. 1969 Jun 1;129(6):1163–1181. doi: 10.1084/jem.129.6.1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg A., Becker Y. Effect of arginine deprivation on macromolecular processes in Burkitt's lymphoblasts. Exp Cell Res. 1970 Jun;60(3):470–474. doi: 10.1016/0014-4827(70)90548-3. [DOI] [PubMed] [Google Scholar]
- Weissfeld A. S., Rouse H. Continued initiation of DNA synthesis in arginine-deprived Chinese hamster ovary cells. J Cell Biol. 1977 Apr;73(1):200–205. doi: 10.1083/jcb.73.1.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S. J., Hahn G. M., Bagshaw M. A. Chromosome aberrations induced by thymidine. Exp Cell Res. 1966 Apr;42(1):130–135. doi: 10.1016/0014-4827(66)90326-0. [DOI] [PubMed] [Google Scholar]


