Abstract
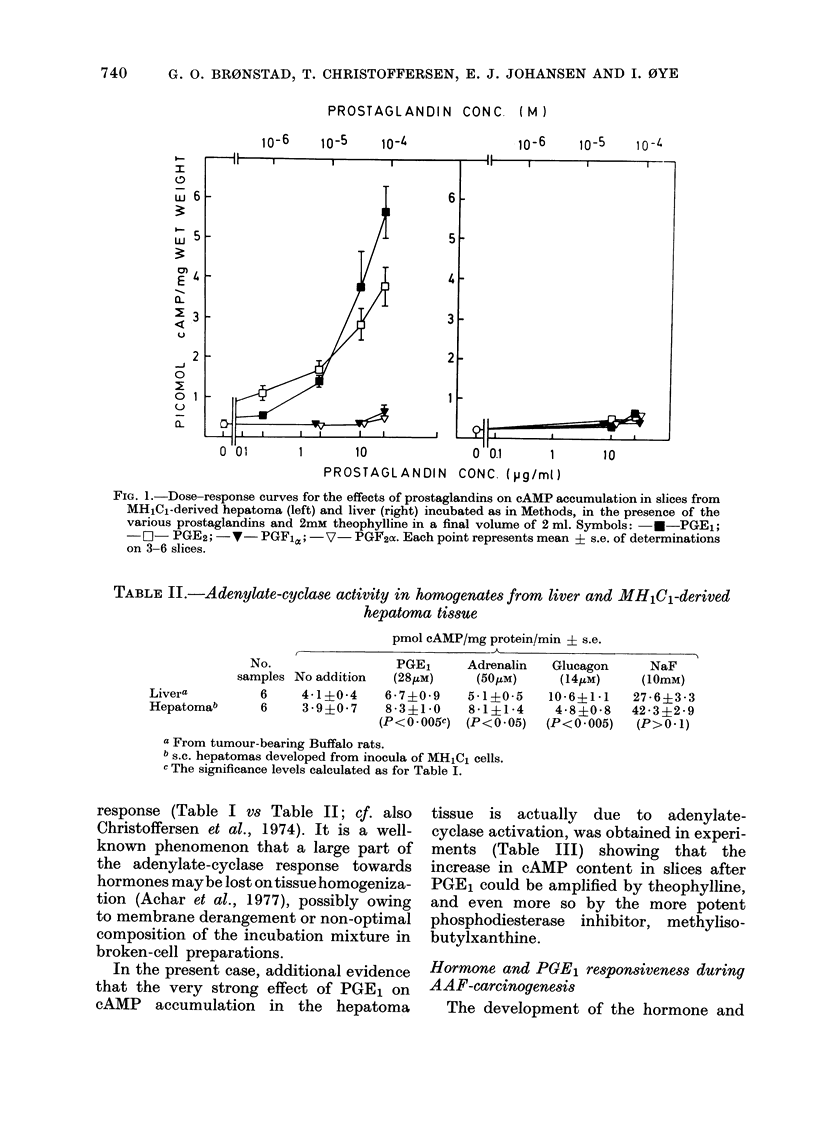
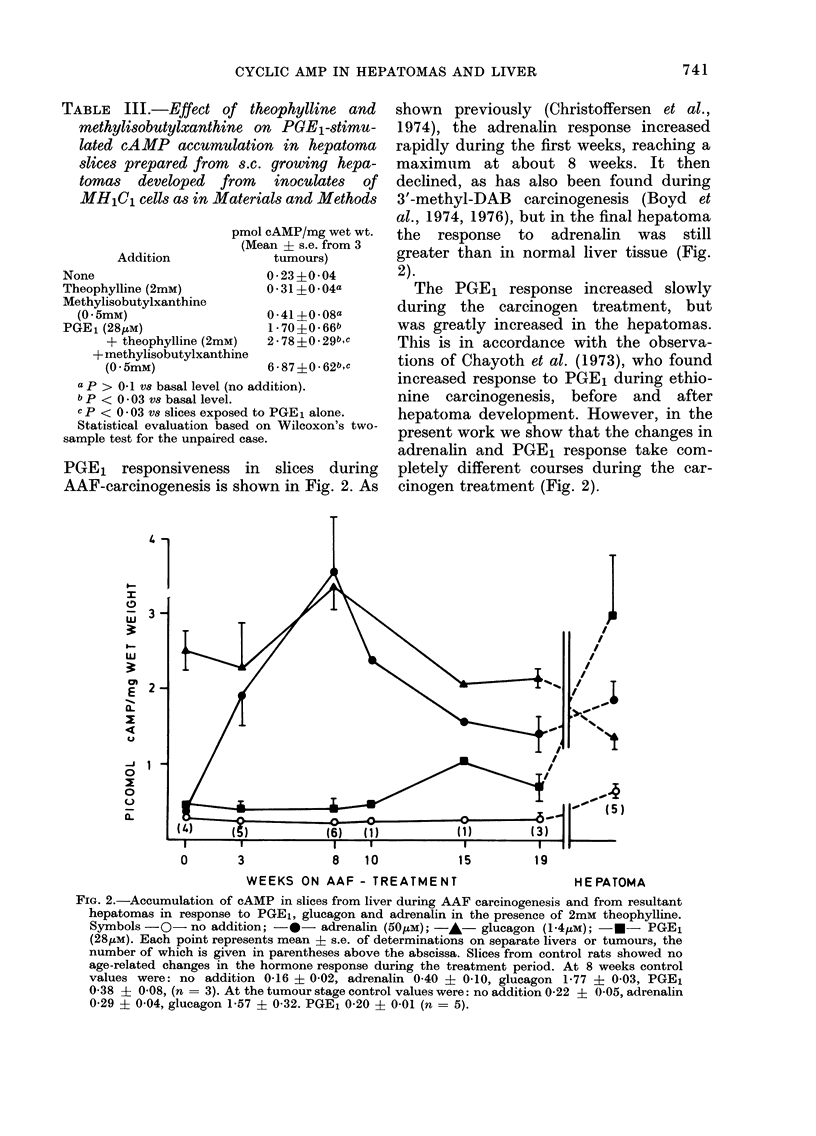
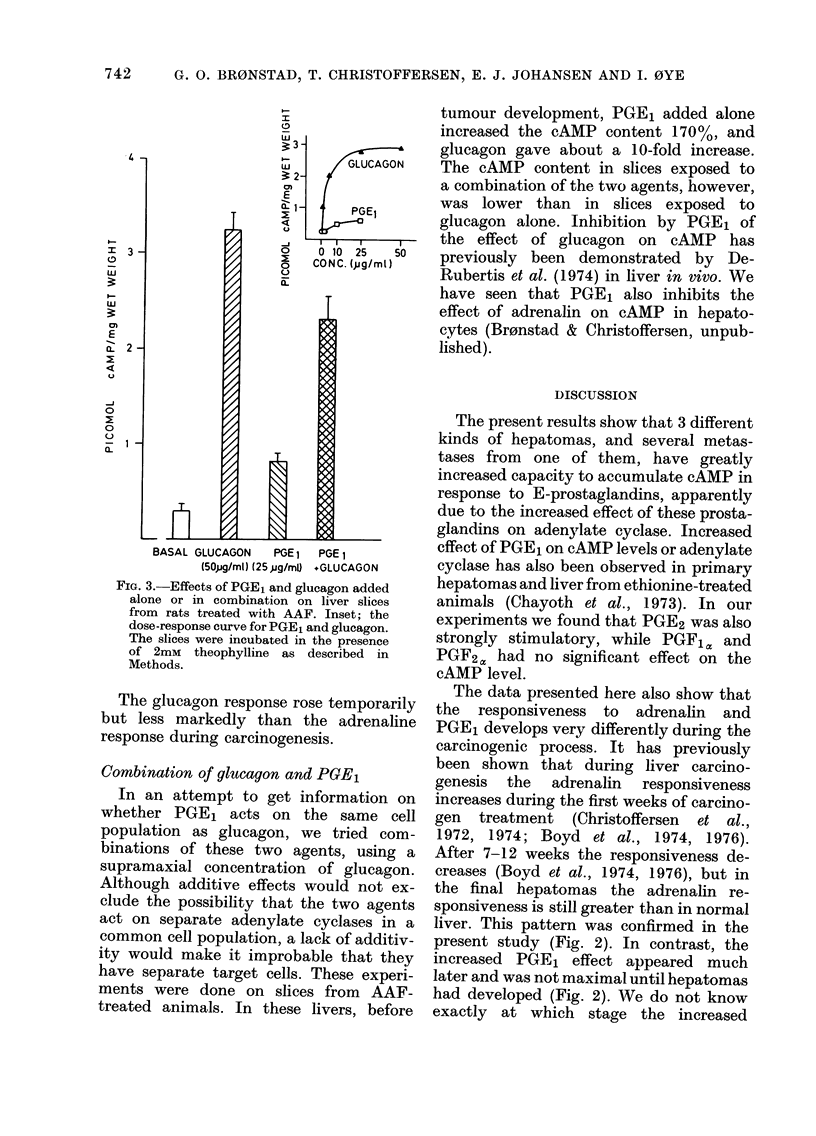
The formation of cyclic AMP was studied in normal liver, subcutaneous hepatomas derived from MH1C1 cells, and premalignant liver and primary hepatomas induced by the carcinogens 2-acetylaminofluorene (AAF) and 4-dimethylamino-azobenzene (DAB). While only very slight effects of prostaglandins (PG) were seen in slices of normal liver, all the hepatomas responded strongly to PGE1 and PGE2. The hepatomas also had increase PGE1-sensitive adenylate-cyclase activity. PGF1alpha and PGF2alpha did not increase the cAMP level significantly either in the liver or in the hepatomas. During AAF carcinogenesis the response to PGE1 increased slightly during the carcinogen feeding, and was greatly elevated only in the fully developed hepatomas. This is in contrast to the increase in adrenalin response seen during carcinogenesis, which starts much earlier, and reaches a peak value within 8--10 weeks. It is concluded that various hepatomas have elevated responsiveness to PGE1 and PGE2 as well as to adrenalin, but the course of change in the tissues' ability to respond to these agents during carcinogenesis is very different.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Achar S. B., Strada S., Sanders R. B., Pledger W. J., Thompson W. J., Robison G. A. Sonication as a tool for the study of adenylyl cyclase activity. J Cyclic Nucleotide Res. 1977 Jun;3(3):189–198. [PubMed] [Google Scholar]
- Allen D. O., Munshower J., Morris H. P., Weber G. Regulation of adenyl cyclase in hepatomas of different growth rates. Cancer Res. 1971 May;31(5):557–560. [PubMed] [Google Scholar]
- Boyd H., Louis C. J., Martin T. J. Activity and hormone responsiveness of adenyl cyclase during induction of tumors in rat liver with 3'-methyl-4-dimethylaminoazobenzene. Cancer Res. 1974 Jul;34(7):1720–1725. [PubMed] [Google Scholar]
- Boyd H., Martin T. J. Changes in catecholamine- and glucagon-responsive adenylate cyclase activity in preneoplastic rat liver. Mol Pharmacol. 1976 Mar;12(2):195–202. [PubMed] [Google Scholar]
- Brown H. D., Chattopadhyay S. K., Morris H. P., Pennington S. N. Adenyl cyclase activity in Morris hepatomas 7777, 7794A, and 9618A. Cancer Res. 1970 Jan;30(1):123–126. [PubMed] [Google Scholar]
- Chayoth R., Epstein S. M., Field J. B. Glucagon and prostaglandin E1 stimulation of cyclic adenosine 3', 5'-monophosphate levels and adenylate cyclase activity in benign hyperplastic nodules and malignant hepatomas of ethionine-treated rats. Cancer Res. 1973 Aug;33(8):1970–1974. [PubMed] [Google Scholar]
- Chayroth R., Epstein S., Field J. B. Increased cyclic AMP levels in malignant hepatic nodules of ethionine treated rats. Biochem Biophys Res Commun. 1972 Dec 18;49(6):1663–1670. doi: 10.1016/0006-291x(72)90534-7. [DOI] [PubMed] [Google Scholar]
- Christoffersen T. Effect of treatment of rats with some chemical carcinogens on the stimulatory effect of adrenaline on cyclic AMP accumulation in liver slices. Acta Pharmacol Toxicol (Copenh) 1975 Sep;37(3):233–236. doi: 10.1111/j.1600-0773.1975.tb00839.x. [DOI] [PubMed] [Google Scholar]
- Christoffersen T., Morland J., Osnes J. B., Elgjo K. Hepatic adenyl cyclase: alterations in hormone response during treatment with a chemical carcinogen. Biochim Biophys Acta. 1972 Sep 15;279(2):363–366. doi: 10.1016/0304-4165(72)90153-5. [DOI] [PubMed] [Google Scholar]
- Christoffersen T., Morland J., Osnes J. B., Oye I. Development of cyclic AMP metabolism in rat liver. A correlative study of tissue levels of cyclic AMP, accumulation of cyclic AMP in slices, adenylate cyclase activity and cyclic nucleotide phosphodiesterase activity. Biochim Biophys Acta. 1973 Jul 28;313(2):338–349. doi: 10.1016/0304-4165(73)90033-0. [DOI] [PubMed] [Google Scholar]
- Criss W. E., Morris H. P. Regulation of the adenylate cyclase system in transplantable hepatomas. Cancer Res. 1976 May;36(5):1740–1743. [PubMed] [Google Scholar]
- De Asua L. J., Clingan D., Rudland P. S. Initiation of cell proliferation in cultured mouse fibroblasts by prostaglandin F2alpha. Proc Natl Acad Sci U S A. 1975 Jul;72(7):2724–2728. doi: 10.1073/pnas.72.7.2724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmelot P., Bos C. J. Studies on plasma membranes. XIV. Adenyl cyclase in plasma membranes isolated from rat and mouse livers and hepatomas, and its hormone sensitivity. Biochim Biophys Acta. 1971 Oct 12;249(1):285–292. doi: 10.1016/0005-2736(71)90106-4. [DOI] [PubMed] [Google Scholar]
- Fehér I., Gidáli J. Prostaglandin E2 as stimulator of haemopoietic stem cell proliferation. Nature. 1974 Feb 22;247(5442):550–551. doi: 10.1038/247550a0. [DOI] [PubMed] [Google Scholar]
- Friedman D. L. Role of cyclic nucleotides in cell growth and differentiation. Physiol Rev. 1976 Oct;56(4):652–708. doi: 10.1152/physrev.1976.56.4.652. [DOI] [PubMed] [Google Scholar]
- Gaudernack G., Rugstad H. E., Hegna I., Prydz H. Synthesis of serum proteins by a clonal strain of rat hepatoma cells. Exp Cell Res. 1973 Mar 15;77(1):25–30. doi: 10.1016/0014-4827(73)90548-x. [DOI] [PubMed] [Google Scholar]
- Gilman A. G. A protein binding assay for adenosine 3':5'-cyclic monophosphate. Proc Natl Acad Sci U S A. 1970 Sep;67(1):305–312. doi: 10.1073/pnas.67.1.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hial V., Horakova Z., Shaff F. E., Beaven M. A. Alteration of tumor growth by aspirin and indomethacin: studies with two transplantable tumors in mouse. Eur J Pharmacol. 1976 Jun;37(2):367–376. doi: 10.1016/0014-2999(76)90044-3. [DOI] [PubMed] [Google Scholar]
- Hickie R. A., Jan S. H., Datta A. Comparative adenylate cyclase activities in homogenate and plasma membrane fractions of Morris hepatoma 5123tc (h). Cancer Res. 1975 Mar;35(3):596–600. [PubMed] [Google Scholar]
- Hickie R. A., Thompson W. J., Strada S. J., Couture-Murillo B., Morris H. P., Robison G. A. Comparison of cyclic adenosine 3':5'-monophosphate and cyclic guanosine 3':5'-monophosphate levels, cyclases, and phosphodiesterases in Morris hepatomas and liver. Cancer Res. 1977 Oct;37(10):3599–3606. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Leffert H. L., Koch K. S., Rubalcava B. Present paradoxes in the environmental control of hepatic proliferation. Cancer Res. 1976 Nov;36(11 Pt 2):4250–4255. [PubMed] [Google Scholar]
- Macmanus J. P., Franks D. J., Youdale T., Braceland B. M. Increases in rat liver cyclic AMP concentrations prior to the initiation of DNA synthesis following partial hepatectomy or hormone infusion. Biochem Biophys Res Commun. 1972 Dec 4;49(5):1201–1207. doi: 10.1016/0006-291x(72)90596-7. [DOI] [PubMed] [Google Scholar]
- Pastan I. H., Johnson G. S., Anderson W. B. Role of cyclic nucleotides in growth control. Annu Rev Biochem. 1975;44:491–522. doi: 10.1146/annurev.bi.44.070175.002423. [DOI] [PubMed] [Google Scholar]
- Richardson U. I., Tashjian A. H., Jr, Levine L. Establishment of a clonal strain of hepatoma cells which secrete albumin. J Cell Biol. 1969 Jan;40(1):236–247. doi: 10.1083/jcb.40.1.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salomon Y., Londos C., Rodbell M. A highly sensitive adenylate cyclase assay. Anal Biochem. 1974 Apr;58(2):541–548. doi: 10.1016/0003-2697(74)90222-x. [DOI] [PubMed] [Google Scholar]
- Short J., Tsukada K., Rudert W. A., Lieberman I. Cyclic adenosine 3':5'-monophosphate and the induction of deoxyribonucleic acid synthesis in liver. J Biol Chem. 1975 May 25;250(10):3602–3606. [PubMed] [Google Scholar]
- Thomas E. W., Murad F., Looney W. B., Morris H. P. Adenosine 3',5'-monophosphate and guanosine 3',5'-monophosphate: concentrations in Morris hepatomas of different growth rates. Biochim Biophys Acta. 1973 Feb 28;297(2):564–567. doi: 10.1016/0304-4165(73)90106-2. [DOI] [PubMed] [Google Scholar]
- Tomasi V., Réthy A., Trevisani A. Soluble and membrane-bound adenylate cyclase activity in Yoshida ascites hepatoma. Life Sci II. 1973 Feb 22;12(4):145–150. doi: 10.1016/0024-3205(73)90206-3. [DOI] [PubMed] [Google Scholar]


