Abstract
Peroxisome proliferator–activated receptor-γ (PPARγ) controls adipogenesis and glucose metabolism. It was reported recently that PPARγ activation by its agonistic ligands modifies lymphocyte function. Since synthetic ligands are known to exert their effect via PPARγ-dependent and -independent pathways, we examined the physiological role of PPARγ in lymphocytes by using heterozygote mutant mice in which one allele of PPARγ is deleted (PPARγ+/–). In contrast to T cells, which did not exhibit a significant difference, B cells from PPARγ+/– showed an enhanced proliferative response to stimulation by either lipopolysaccharide or cross-linking of antigen receptors. Dysregulation of the NF-κB pathway in B cells from PPARγ+/– was indicated by spontaneous NF-κB activation, as well as increased IκBα phosphorylation and gel-shift activity following LPS stimulation. Mice primed with either ovalbumin or methylated BSA also showed enhanced antigen-specific immune response of both T and B cells, an immunological abnormality that exacerbated antigen-induced arthritis. These findings indicate that PPARγ plays a critical role in the control of B cell response and imply a role in diseases in which B cell hyperreactivity is involved, such as arthritis and autoimmunity.
Introduction
Peroxisome proliferator–activated receptor-γ (PPARγ) is one of the isotypes of peroxisome proliferator–activated receptors, which are a group of closely related nuclear receptors. The primary role of PPARγ, which is mainly expressed in adipose tissue, is to promote lipogenesis under anabolic conditions (1–6). PPARγ is expressed in various other tissues, including colon, retina, and spleen, although in lesser amounts (2, 6). PPARγ is also known to regulate inflammatory responses. In monocytes and monocyte-derived macrophages, activation of PPARγ inhibits the expression of such inflammatory cytokines as TNF-α, IL-1α, and IL-6 (7, 8). It was reported recently that PPARγ activation modifies lymphocyte function. Agonistic ligands for PPARγ inhibit proliferation of T helper cell clones (9–11) as well as B cells and B cell lymphoma cells (12, 13). PPARγ ligands are even cytotoxic for B cells by induction of apoptosis. These findings led to the proposal that manipulation of PPARγ has potential as a therapeutic strategy for controlling inflammatory and immunological diseases (6).
Most studies described above employed PPARγ agonists, such as 15-deoxy-Δ12, 14-prostaglandin J2 (15d-PGJ2) and thiazolidinediones (TZDs), both of which have affinity for PPARγ (3, 4, 14, 15). It was demonstrated that the anti-inflammatory and antiproliferative effects of PPARγ activation by these ligands are involved in suppression of NF-κB, which is a key transcription factor for proliferation, apoptosis, and cytokine synthesis of inflammatory cells (7, 8). Straus et al. reported that 15d-PGJ2 inhibits multiple steps in the NF-κB signaling pathway (16). Another study also suggested that 15d-PGJ2 directly regulates NF-κB as well as IKKβ (17). Interestingly, in those studies that used PPARγ agonists on lymphocytes, 15d-PGJ2 always showed more efficient suppression of lymphocytes and induction of apoptosis than other ligands (9–13), indicating that 15d-PGJ2 exhibits its effect not only via PPARγ but also via other pathways. Finally, Chawla et al. reported that both 15d-PGJ2 and TZDs exert anti-inflammatory effect in macrophages derived from mice homozygous for a null mutation in the PPARγ gene, indicating that they can work by a PPARγ-independent mechanism (18). PPARγ expression is dispensable for some function of macrophages including differentiation of the macrophage lineage and lipid metabolism (18). Therefore, it is possible that the role of PPARγ in lymphocytes is overestimated since the ligands are used at concentrations exceeding those required to bind PPARγ (18).
Rheumatoid arthritis (RA) is a representative systemic chronic inflammatory disease in the pathogenesis of which immunological abnormalities, including autoimmune responses, are also involved. Persistent inflammation in hyperplastic synovium and pannus accompanied by massive cell infiltration in joints finally leads to the destruction of cartilage and bone (19). T cells infiltrate massively into the pannus and expand clonally in the synovium and synovial fluid (20, 21). Lymphoid follicles composed mainly of B cells are also formed in the pannus, and rheumatoid factor as well as hypergammaglobulinemia are the prominent serological features of RA (22).
Recently, it was reported that PPARγ agonistic ligands, 15d-PGJ2 and troglitazone, could ameliorate adjuvant-induced arthritis in rats, which is an experimental model of RA (23). The authors addressed that the PPARγ agonistic ligands regulate the inflammation in the synovial macrophages and synovial cells to exhibit their therapeutic effects since apoptosis is induced in synovial cells by PPARγ ligands in vitro and synovial macrophages express PPARγ. We suspect that not only modification of immunological components but also PPARγ-independent effect of the PPARγ agonistic ligands might also contribute to the amelioration, since adjuvant arthritis involves immunological pathogenic mechanisms.
We have generated PPARγ gene–targeted mice (24). Complete elimination of a functional PPARγ gene results in embryonic lethality, whereas PPARγ heterozygote knockout mice (PPARγ+/–) exhibit resistance to high-fat diet–induced obesity and insulin resistance (24, 25–27). This heterozygote shows a 50% reduction in PPARγ (24, 25). This PPARγ mutant permits us to investigate the physiological role of PPARγ in the immune system without employing PPARγ synthetic agonists.
We found that haploinsufficiency of PPARγ affects B cells but not T cells. The B cell proliferative response was enhanced in PPARγ+/–. These results indicate that PPARγ plays a critical role in regulating homeostasis of B cell function. Furthermore, the antigen-specific immune response is enhanced in PPARγ+/–, probably due to B cell hyperreactivity. Finally, when we induced arthritis experimentally, the arthritis in PPARγ+/– was exacerbated compared with wild-type. These findings provide new insight into the physiological role of PPARγ in the immune system and its pathogenic role in arthritis and autoimmune diseases since B cell hyperreactivity is involved in the pathogenesis of these diseases.
Methods
Animals.
The generation of PPARγ gene–targeted mice was described previously (24). Heterozygous PPARγ-deficient mice were crossed to the ICR background for more than eight generations and were maintained in a temperature- and light-controlled environment with free access to food and water under specific-pathogen free (SPF) conditions. Female, age-matched mice were used in all experiments, and the mice were 7–10 weeks old at the start of each experiment. For each experiment littermates were used as a control. ICR mice for mating were obtained from Japan SLC Inc. (Hamamatsu, Shizuoka, Japan).
Materials.
The 15d-PGJ2 was obtained from Cayman Chemical (Ann Arbor, Michigan, USA). Troglitazone, pioglitazone, and rosiglitazone (BRL49653) were kindly donated by Sankyo Co. (Toyko, Japan), Takeda Chemical Industries Ltd. (Osaka, Japan), and GlaxoSmithKline (Greenford, Middlesex, United Kingdom) respectively. Ovalbumin (OVA), methylated-BSA (mBSA), and lipopolysaccharide (LPS) were obtained from Sigma Chemical Co. (St. Louis, Missouri, USA). Anti-mouse IgM F(ab′)2 was obtained from Jackson Immunoresearch Laboratories Inc. (West Grove, Pennsylvania, USA).
Lymphocyte proliferation assay.
Single cell suspension from splenocytes or lymph nodes was cultured at 105 cells/well with various concentrations of OVA and mBSA in RPMI-1640 medium supplemented with 2 mM L-glutamine, 100 U/ml penicillin, 100 μg/ml streptomycin, 10% heat-inactivated FCS, and 5 × 10–5 M 2-mercaptoethanol (2-ME) for 5 days. This was followed by a final 16 hours of culture in the presence of 1 μCi of [3H]thymidine per well. The incorporated radioactivity was counted with a β-scintillation counter. The proliferative response was expressed as the mean cpm of test cultures ± SD.
Naive T cells were purified using a MACS (Miltenyi Biotech GmbH, Bergisch Gladbach, Germany), as described previously (28, 29). They were stimulated with 1 or 10 μg/ml of anti-CD3 Ab in the presence of 1 μg/ml of anti-CD28 Ab for 24 hours. Proliferation was measured by [3H]thymidine incorporation (30).
B220+ spleen cells were isolated using a MACS according to the manufacturer’s instructions. Purified B cells were cultured at 105 cells/well in RPMI-1640 with 10% heat-inactivated FCS. Cells were incubated for 56 hours with 10 μg/ml of LPS or 20 μg/ml of goat anti-mouse IgM F(ab′)2 (31). One μCi of [3H] thymidine was added per well for the final 16 hours. Cells were harvested and counted with a scintillation counter as T cells. The purity of fractionated cells was monitored by an EPIX-system II (Coulter Electronics Ltd., Hialeah, Florida, USA) using anti-CD3 and anti-B220 (RA3-9B2) (PharMingen, San Diego, California, USA).
Quantitation of cytokines.
IFN-γ and IL-2 in the supernatant of mBSA-stimulated T cells from the mice primed with mBSA were quantified using sandwich ELISA kits (Endogen Inc., Woburn, Massachusetts, USA).
Ab assay.
The level of anti-OVA or anti-mBSA Ab in sera was measured by ELISA as described previously (29).
Examination of cell viability.
Viable cells were counted by trypan blue–exclusion method. For propidium iodide (PI) staining, cells were washed with PBS and fixed in 80% ethanol/PBS. After fixation, cells were washed again and then incubated with 500 μg/ml DNase-free RNase, 20 μg/ml PI for 30 minutes at 37°C. Thereafter, sub-G0 peaks, which correspond to dead or dying cells, were determined using a flow cytometer (32, 33).
Electrophoretic mobility shift assay.
Electrophoretic mobility shift assays (EMSAs) were performed as described previously (34). Briefly, 107 cells were washed with cold PBS and resuspended in buffer A (10 mM HEPES, pH 7.9, 1.5 mM MgCl2, 10 mM KCl, and 0.5 mM DTT) with 0.1% Nonidet P-40 and protease inhibitors (leupeptin, aprotinin, and PMSF) at 0°C for 10 minutes, followed by centrifugation and resuspension of the nuclear pellet in buffer C (20 mM HEPES, pH 7.9, 25% glycerol, 0.42 M NaCl, 1.5 mM MgCl2, 0.2 mM EDTA, and 0.5 mM DTT) with protease inhibitors at 0°C for 20 minutes. To remove insoluble debris, nuclear lysates were centrifuged and stored at –80°C. The NF-κB–binding sequence (Promega Corp., Madison, Wisconsin, USA) was labeled using T4 kinase. The nuclear lysates were incubated with a 32P-labeled probe in DNA-binding buffer for 20 minutes at room temperature before electrophoresis in a 4% polyacrylamide gel.
IκB immunoblot.
Cells were lysed in NP-40 lysis buffer containing 1% NP-40, 20 mM Tris-HCl (pH 7.4), 150 mM NaCl, 1 mM EDTA, 1 mM PMSF, 10 mM NaF, 0.5 mM Na3VO4, 10 μg/ml aprotinin, and 10 μg/ml leupeptin. The same amount of protein in each sample was separated by SDS-PAGE on 12.5% gel and transferred to a nitrocellulose membrane (Schleicher & Schnell GmbH, Dassel, Germany) (34). Blots were incubated with either rabbit anti–phospho-IκBα mAb or rabbit anti-IκBα mAb (Cell Signaling Technology, Beverly, Massachusetts, USA). After washing, blots were incubated with anti-rabbit Ig Ab coupled with peroxidase and visualized by the enhanced chemiluminescence (ECL) system (Amersham Pharmacia Biotech, Buckinghamshire, United Kingdom).
Induction of AIA.
Mice were immunized intradermally at the base of the tail with 100 μg of mBSA dissolved in 100 μl of PBS and emulsified with an equal volume of CFA (Difco Laboratories, Detroit, Michigan, USA) (day 0) as described previously (29). Fourteen days later, 20 μg mBSA dissolved in 20 μl of PBS was injected intra-articularly into the left ankle joint. The right ankle joint was injected with 20 μl PBS only as a negative control. The joint thickness was measured with dial-gauge calipers (Mitutoyo, Kawasaki, Kanagawa, Japan). The net increase in joint thickness attributable to the antigenic challenge was calculated by subtracting the increase in thickness of the right ankle from the increase in thickness of the left ankle.
Histology.
Ankles and knees were fixed in 10% phosphate-buffered formalin and decalcified. Tissues were then dehydrated in a gradient of alcohols, paraffin-embedded, sectioned, mounted on glass slides, and stained with hematoxylin and eosin (29).
Statistical analysis.
Statistical evaluation was performed with both paired t test and Mann-Whitney’s U test. In addition to statistical analysis using the whole samples, to decrease the genetic variance due to the ICR background we also compared the data of PPARγ+/– mice with that of their PPARγ+/+ littermates derived from the same litter.
Results
T cell from PPARγ+/– mice appeared to be normal.
The immune system of PPARγ+/– mice developed normally, since the size of the thymus and spleen, the number of T cells in both the thymus and spleen, the ratio of CD4/CD8 T cells, and the number of B cells in the spleen were not different from those of wild-type mice (data not shown).
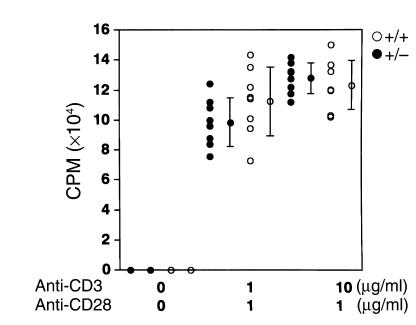
To investigate the influence of the reduced amount of PPARγ in PPARγ+/– mice on the function of lymphocytes, first we examined T cell function, since it has been reported that PPARγ agonists inhibit proliferation of T helper cell clones (9–11). To verify this, we stimulated purified naive T cells with anti-CD3 and anti-CD28 Ab. No significant difference was observed between the proliferation of naive T cells from PPARγ+/– mice and that from wild-type littermate mice (Figure 1). Although the variance between individual mice is rather high, partly due to the heterogeneous genetic background of ICR mice, there was no statistical difference. This result suggests that the reduced amount of PPARγ does not affect the proliferative response of naive T cells.
Figure 1.
Proliferative response of naive T cells from PPARγ+/– mice does not differ from wild-type mice. T cells were purified from the spleen of each naive mouse and stimulated with 1 or 10 μg/ml of anti-CD3 Ab in the presence of 1 μg/ml of anti-CD28 Ab for 24 hours. The proliferation was measured by [3H]thymidine incorporation. Bars show the mean ± SD (n = 8 per group). Data from every littermate group of PPARγ+/– and PPARγ+/+ mice were statistically analyzed. There was no significant difference in the naive T cell response between the PPARγ+/– mice (filled circles, +/–) and the littermate ICR (open circles, +/+) wild-type mice.
B cells from PPARγ+/– mice proliferate vigorously upon stimulation.
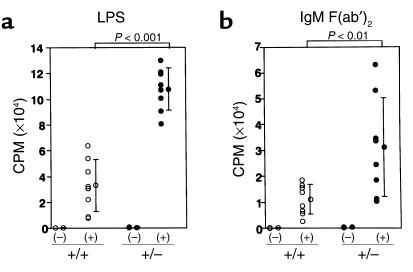
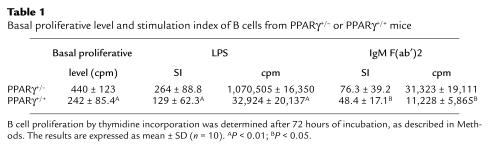
Next, we investigated the B cell proliferative response. Naive B cells purified from both types of unimmunized mice were stimulated with either LPS or anti-mouse IgM F(ab′)2. B cells from PPARγ+/– mice showed a remarkably exaggerated response to each stimulus compared with B cells from wild-type littermates (Figure 2, a and b). To decrease the influence of genetic heterogeneity of ICR mice, we compared PPARγ+/– and PPARγ+/+ littermates from the same litter. A statistical analysis using the littermates as well as the whole sample gave a significant difference (P < 0.01 in the case of LPS stimulation and P < 0.05 in the case of B cell receptor cross-linking). Since the basal proliferative response of B cells from PPARγ+/– mice was significantly higher than PPARγ+/+ mice (242 ± 85.4 cpm in PPARγ+/+ and 440 ± 123 cpm in PPARγ+/–), we analyzed stimulation index (SI) of the B cell response to exclude the possibility that the higher response after stimulation simply reflects the basal proliferative level. As summarized in Table 1, the SI of LPS-stimulated B cells from PPARγ+/– mice was significantly higher in each littermate group (P < 0.05). Although the SI did not differ significantly in B cells stimulated with B cell receptor cross-linking from a littermate group, the SI of B cells stimulated with anti-mouse IgM F(ab′)2 from the other littermate group, as well as the whole sample, showed significant differences (P < 0.05 in the case of a littermate analysis as well as the whole sample). These results suggest that B cells from PPARγ+/– mice are intrinsically abnormal.
Figure 2.
Enhanced proliferative response of B cells from PPARγ+/– mice. Naive B cells (105) purified from the spleen of each mouse were stimulated with either 10 μg/ml of LPS (a) or 20 μg/ml of anti-mouse IgM F(ab′)2 (b) for 72 hours. The proliferation was measured by [3H]thymidine incorporation. Bars show the mean ± SD (n = 8 per group). B cells from PPARγ+/– mice (+/–) demonstrated a significantly stronger response to LPS or anti–mouse IgM F(ab′)2 compared with the cells from wild-type littermates (+/+). Statistical analysis using data from all mice as well as every littermate group gave a significant difference.
Table 1.
Basal proliferative level and stimulation index of B cells from PPARγ+/– or PPARγ+/+ mice
The PPARγ agonists are not cytotoxic for B cells from PPARγ+/– and PPARγ+/+ mice.
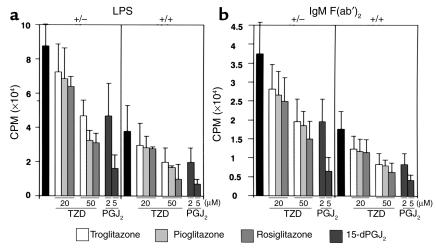
To confirm that this different trait of B cells from PPARγ+/– mice depends on PPARγ haploinsufficiency, we studied whether PPARγ activation is involved in B cell function. The B cell proliferative response induced by either LPS or anti–mouse IgM F(ab′)2 was suppressed by all PPARγ agonists tested, including 15d-PGJ2, troglitazone, pioglitazone, and rosiglitazone (BRL49653) (Figure 3, a and b). The degree of inhibition depended on the dose of the agonist. The enhanced proliferative response of B cells from PPARγ+/– mice was also suppressed by the PPARγ agonists. Interestingly, a higher dose was required to achieve almost the same degree of suppression of B cell proliferation from PPARγ+/– mice as for the wild-type.
Figure 3.
PPARγ agonistic ligands suppress the proliferation of naive B cells from both PPARγ+/– and PPARγ+/+ mice stimulated with LPS or anti–mouse IgM F(ab′)2. B cells (105) purified from spleens were stimulated with 10 μg/ml of LPS (a) or 20 μg/ml of anti–mouse IgM F(ab′)2 (b) for 72 hours in the absence or presence of PPARγ agonistic ligands at various concentrations (TZDs: 20, 50 μM; 15d-PGJ2: 2, 5 μM). Proliferation was measured by [3H]thymidine incorporation. Bars show the mean ± SD (n = 10 per group). All of the tested PPARγ agonistic ligands (15d-PGJ2, troglitasone, pioglitasone, and rosiglitasone) demonstrated suppressive activity. Data from every littermate group were analyzed statistically using paired t test.
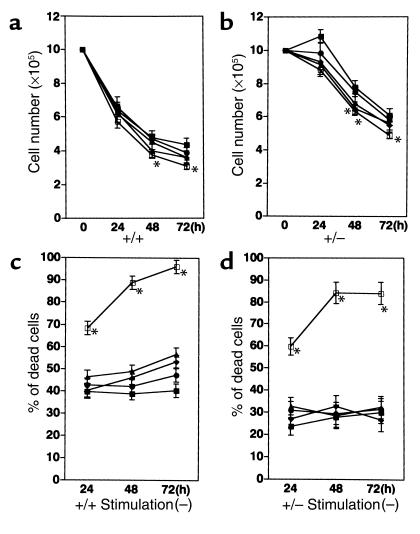
Since Padilla et al. demonstrated that PPARγ agonists induce apoptosis in normal as well as malignant B-lineage cells (13), we examined the cell viability to examine the possibility that suppressive activity of the PPARγ agonists is due to their cytotoxic effect. In both PPARγ+/+ and PPARγ+/– mice the B cell viability was not significantly altered by the presence of either 20 μM or 50 μM of troglitazone or 2 μM of 15d-PGJ2, whereas the viable cell count decreased significantly at 5 μM of 15d-PGJ2 (Figure 4, a and b). The other TZDs (pioglitazone and rosiglitazone) did not alter the cell viability as well (data not shown). Although it seems that the proportion of dead cells assayed by PI staining gradually increased during the culture in the presence of PPARγ agonists (see Figure 4c), a no significant difference was observed in the ratio of dead cells except 5 μM of 15d-PGJ2, which induced massive cell death in B cells from both PPARγ+/+ and PPARγ+/– mice (Figure 4, c and d). These results indicate that at the concentrations we used PPARγ agonists are not cytotoxic except 5 μM of 15d-PGJ2 and that they counteract the proliferative response, although it is true that PPARγ agonists at rather higher concentration can induce cell death. The reason for this partial inconsistency regarding the concentration of PPARγ agonists that induce cell death between our data and the previous report might be that we used B cells from a different strain; we used B cells from ICR mice while Padilla et al. used BDF1 mice.
Figure 4.
The cell viability as well as the proportion of dead/dying cells are different between PPARγ+/+ and PPARγ+/– mice, whereas the addition of PPARγ agonists did not alter those parameters, except 5 μM of 15d-PGJ2. The B cells from PPARγ+/+ (a) or PPARγ+/– (b) are cultured in the absence or presence of PPARγ agonists and their viable cell number were counted by trypan blue exclusion. The percentage of dead or dying cells of B cells from PPARγ+/+ (c) or PPARγ+/– (d) in the culture were counted by PI staining. B cells were cultured without agonist (closed squares), or with troglitazone 20 μM (filled circles), troglitazone 50 μM (filled triangles), 15d-PGJ2 2 μM (filled diamonds), and 15d-PGJ2 5 μM (open squares). *P < 0.05 for the cell number or percentage of dead cells compared to those in the absence of the agonist.
The increased viability of B cells from PPARγ+/– mice.
Interestingly, a dramatic difference was observed in the viability of B cells from PPARγ+/+ versus PPARγ+/– mice irrespective of the absence or presence of PPARγ agonists (a significant difference, P < 0.01, was observed in the cell number over all time points). Since the cell number of PPARγ+/– increased slightly at the 24-hour point, the increased basal proliferative response might contribute in the increased cell viability. The counting of dead cells revealed that B cells from PPARγ+/– mice are less susceptible to cell death, since the proportion of dead cells is around 40% in PPARγ+/+ B cells and 25% in PPARγ+/– B cells, respectively, in the absence of the agonists (compare Figure 4, c and d). The activation of B cells with either LPS or IgM F(ab′)2 stimuli did not alter the ratio of dead cells in B cells from both mice (data not shown). The increased cell survival might contribute in the increased thymidine incorporation of PPARγ+/– B cells. Taken together, these results indicate the involvement of PPARγ in the B cell proliferative response and that the reduced amount of PPARγ results in a hyperproliferative response of B cells.
Spontaneous activation of NF-κB in B cells of PPARγ+/− mice.
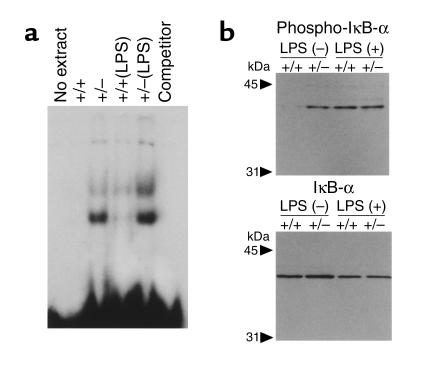
To investigate the mechanism of hyperreactivity in B cells from the mutant mice, we examined nuclear translocation of NF-κB, which is proposed to be regulated by PPARγ (7, 8). Nuclear extracts of B cells from PPARγ+/– mice that were stimulated with LPS exhibited more enhanced binding activity to NF-κB oligonucleotides in EMSA (Figure 5a). Strikingly, NF-κB activity was observed even in the resting B cells without stimulation in PPARγ+/– mice. The activation of NF-κB is associated with phosphorylation of IκB, followed by degradation of IκB by the proteasome and nuclear translocation of NF-κB (35). Therefore, in order to investigate the mechanism of NF-κB activation, we examined the phosphorylation of IκBα in B cells. While the total amount of IκBα was not significantly altered, immunoblotting revealed that the phosphorylated form of IκBα increased in B cells from unprimed PPARγ+/– mice (Figure 5b). These results indicate that in the mutants NF-κB was spontaneously activated without addition of any stimulus.
Figure 5.
Activation of NF-κB pathway in B cells from PPARγ+/– mice. (a) Nuclear extracts from B cells were analyzed by EMSA for the ability to bind to an NF-κB consensus probe. Lane 1: No extract, with labeled oligonucleotide; lane 2: resting B cells from wild-type mice (+/+); lane 3: resting B cells from PPARγ+/– mice (+/–); lane 4: stimulated B cells by LPS for 5 minutes from wild-type mice; lane 5: stimulated B cells from PPARγ+/– mice; lane 6: lane 5 with excess of cold oligonucleotide; lane 7: lane 5 without labeled oligonucleotide. (b) Phosphorylation of IκBα. Cell lysates from B cells were probed with anti-phosphorylated IκBα (lanes 1–4) and anti- IκBα (lanes 5–8). Lanes 1 and 5 were resting B cells from wild-type mice. Lanes 2 and 6 were resting B cells from PPARγ+/– mice. Lanes 3 and lane 7 were B cells from wild-type mice, stimulated with LPS for 5 minutes. Lanes 4 and 8 were stimulated B cells from PPARγ+/– mice.
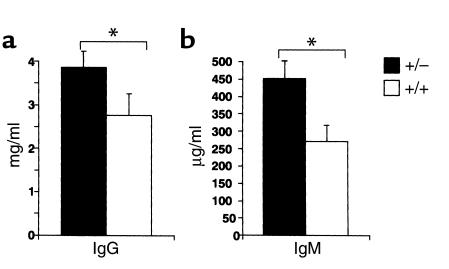
A significant increase in the serum Ig level was observed in the unprimed PPARγ+/– mice (Figure 6). These results indicate that B cells from PPARγ+/– mice are spontaneously activated.
Figure 6.
Serum IgG and IgM levels were increased in PPARγ+/– mice. Serum IgG (a) and IgM (b) levels in PPARγ+/– mice (closed column, +/–) were higher than in wild-type mice (open column, +/+). Both PPARγ+/– and wild-type 12-week-old mice were bled, and serum IgG and IgM levels were measured by ELISA. The mean Ab titers (± SD) of each group of ten mice are shown. The statistical differences between PPARγ+/– and PPARγ+/+ littermates derived from the same litter were significant (*P < 0.01).
Antigen-specific immune response is enhanced in PPARγ haploinsufficient mice.
B cells differentiate into Ab-producing cells by T cell help. Ab enhances antigen-presentation of the immune system by facilitating antigen uptake by forming immune complex. Conversely, B cells are valuable antigen-presenting cells for circulating antigens that are ingested into B cells via their antigen-specific receptor (36). Thus, B cells contribute to efficient generation of antigen-specific immune responses. Therefore, we examined whether the antigen-specific immune response is altered in PPARγ+/–.
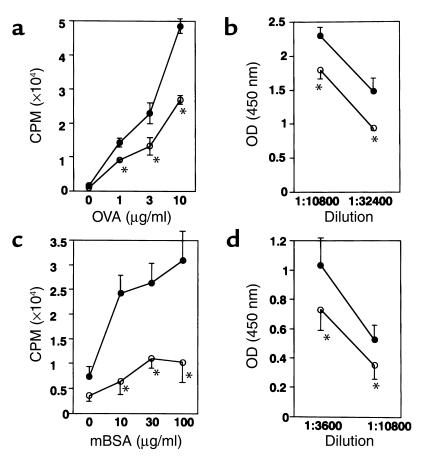
Splenocytes from PPARγ+/– immunized with either OVA or mBSA demonstrated an enhanced antigen-specific immune response against the immunized antigen (Figure 7, a and c). The level of Ab against either OVA or mBSA in the sera from the immunized mice was also significantly increased in PPARγ+/– mice (Figure 7, b and d). These results indicate that the antigen-specific immune response is probably enhanced due to B cell hyperreactivity in PPARγ+/– mice.
Figure 7.
Antigen-specific immune response was enhanced in PPARγ+/– mice. (a) Proliferation of splenocytes from wild-type mice (open circles, +/+) and PPARγ+/– mice (filled circles, +/–), both of which were primed with OVA. Proliferation was measured by [3H]thymidine incorporation in the presence of various concentrations of OVA. Bars show the mean ± SD (n = 10 per group). The difference between the two groups was significant (*P < 0.01). (b) The level of Ab against OVA in sera from mice primed with OVA. Mice were bled 30 days after priming with OVA and assayed for the anti-OVA Ab level by ELISA. The mean Ab titers (± SD) of each group of ten mice are shown. The difference between the two groups was significant (*P < 0.01). (c) Proliferation against mBSA of splenocytes from mBSA-primed wild-type control mice (open circles, +/+) and PPARγ+/– mice (filled circles, +/–), respectively. Proliferation was measured by [3H]thymidine incorporation in the presence of various concentrations of mBSA. Bars show the mean ± SD (n = 10 per group).The difference between the two groups was significant (*P < 0.001). (d) Mice were bled 30 days after intra-articular challenge with mBSA and assayed for the anti-mBSA Ab level by ELISA. The mean Ab titers (± SD) of each group of the total ten mice are shown. The statistical difference between PPARγ+/– and PPARγ+/+ littermates derived from the same litter were significant (*P < 0.01).
The production of T cell–derived cytokines was also enhanced, since higher amounts of both IL-2 and IFN-γ were detected in the supernatant of antigen-stimulated splenocytes from PPARγ+/– mice in comparison with the wild-type littermate mice (our unpublished observation). However, we could not evaluate deviation toward a Th1 response, since IL-4 in the supernatant was below the detectable level from either the mutant or control mice and since no significant difference in the amount of Ig isotypes in the sera was noted (data not shown). We also could not discern whether the enhanced total production of cytokines in the supernatant is due to the increased production by each antigen-specific T cell or simply reflects the increased frequency of antigen-specific T cells generated by the enhanced response.
PPARγ+/− mice developed more severe antigen-induced arthritis.
B cell hyperreactivity is proposed to be involved in the pathogenesis of autoimmune diseases, including RA. Therefore, to explore the association of B cell hyperreactivity in PPARγ+/– with the disease, we employed an antigen-induced arthritis model and evaluated the arthritis severity in terms of the ankle joint thickness. In the wild-type littermate mice used as a control, the maximal increase of joint thickness was almost 0.8 mm at 24–48 hours after the antigen challenge, and it began to decline thereafter. By day 10, only slight joint swelling remained. In contrast, in PPARγ+/– mice, more severe arthritis was induced: the maximal increase of joint thickness was more than 1.3 mm at 24 hours after the challenge (Figure 8). Although joint swelling in the PPARγ+/– mice started earlier than in the control, arthritis in the PPARγ+/– mice was not prolonged, and on day 8 it was almost the same as in the wild-type controls. Histological examination of the ankle joints revealed synovial lining cell hyperplasia, fibrin deposits, and formation of pannus-like tissues (Figure 9). Hyperplasia of synovial lining cells was accompanied by the accumulation of numerous mononuclear cells filled into tarsal-tibial joint space, which was more vigorous in PPARγ+/– than PPARγ+/+ mice. A greater number of inflammatory cells also invaded into periarticular soft tissues in PPARγ+/– than in PPARγ+/+ mice. Thus, the histological change was more severe in PPARγ+/– than in PPARγ+/+ mice.
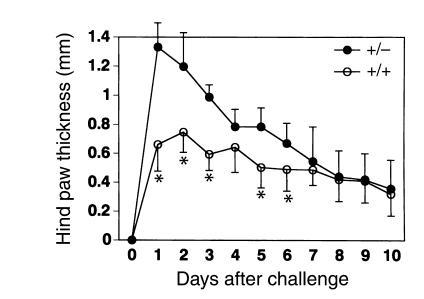
Figure 8.
PPARγ+/– mice showed more severe antigen-induced arthritis. Mice primed with mBSA were challenged intra-articularly with the antigen on day 0. The increase in hind paw thickness during the course of the disease was monitored using a dial-gauge calipers. Bars show the mean ± SD (n = 10 per group). The statistical difference (*P < 0.01) between the PPARγ+/– and PPARγ+/+ mice from day 1 to day 5 was calculated using data of all mice as well as every littermate group.
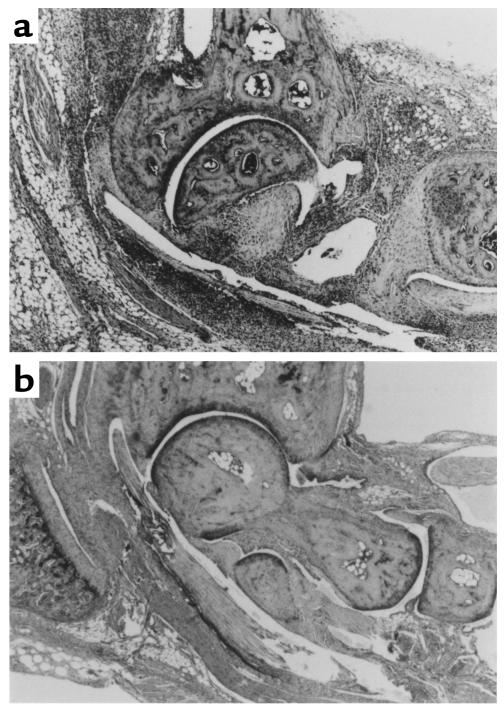
Figure 9.
Histological examination of ankles from arthritic mice. Hematoxylin and eosin staining of the ankle joint revealed increased infiltration of inflammatory mononuclear cells in PPARγ+/– mice (a) compared with wild-type mice (b).
Taken together, these findings indicate that PPARγ haploinsufficiency results in dysregulation of NF-κB and hyperreactivity of B cells. The reduced expression of PPARγ in physiological level affects B cells more profoundly than T cells. The hyperreactivity of B cells might be related to the exacerbation of arthritis in PPARγ+/– mice.
Discussion
In the present study, we showed that PPARγ haploinsufficiency affects the B cell proliferative response. The increased B cell proliferative response may be involved in the enhanced antigen-specific immune response, which contributes to the exacerbation of antigen-induced arthritis. We conclude that the enhanced immune response in PPARγ+/– mice is attributable, at least in part, to hyperreactivity of B cells. Our reasoning is discussed below.
B cells from PPARγ+/– mice expand more rapidly and more aggressively in response to proliferative stimuli. Therefore, during immunization, the frequency of B cells specific for the antigen should increase. The increased number of antigen-specific B cells enhances the T cell response specific for the antigen via antigen-presentation by B cells. Conversely, the increased number of antigen-specific T cells promotes clonal expansion of antigen-specific B cells and vice versa. Thus, hyperreactivity of B cells should be a positive driving force for the antigen-specific immune response.
This assumption could be confirmed by adoptive transfer experiments or in vitro reconstitution assays using B cells from PPARγ+/– mice and antigen-specific T cells from wild-type mice. However, the heterogeneous genetic background, especially regarding MHC, of ICR mice that we employed in this study does not allow these kinds of experiments.
The genetic heterogeneity of ICR mice also prevented us from examining antigen-presentation including MHC class II expression. This point is important since not only B cells but also other types of cells, including macrophages and synoviocytes, are involved in antigen presentation. Since their function is known to be altered by PPARγ agonists, it is possible that other cell types also contribute in enhancement of antigen-specific immune response and arthritis, via modification of the antigen-presentation or inflammatory process.
The increased cell viability could contribute in the increased thymidine incorporation of B cells from PPARγ+/– mice, since the increased thymidine incorporation depends on both the number of cell cycle that each cell undergoes and the number of cells that undergo mitosis. Nevertheless, since the increased basal proliferative response reflects the increased viability in addition to the increased proliferation, the increase of SI, which takes into account the increased basal proliferation in B cells from PPARγ+/– mice, strongly suggest the hyperproliferative response of the B cells. In addition, although PI staining does not distinguish apoptosis-caused cell death from necrosis-induced cell death (33), it is possible that haploinsufficiency of PPARγ render B cells less susceptible to apoptosis. It would be interesting to examine whether the haploinsufficiency of PPARγ is related to prevention of apoptosis.
Toll-like receptor 4 stimulated with LPS is known to involve the NF-κB pathway via MyD88/IRAK (37, 38). Signaling via B cell antigen receptor cross-linking is also involved in NF-κB (39). It is well known that PPARγ agonistic ligands negatively regulate macrophage activation through transcriptional suppression via NF-κB, AP-1, and STAT (7, 8), although a recent paper demonstrated a PPARγ-independent anti-inflammatory effect of PPARγ agonistic ligands (18). These findings support our idea that hyperreactivity of B cells can be explained at least by activation of NF-κB, which should be regulated by PPARγ. However, the exact mechanism of NF-κB pathway suppression by PPARγ remains unknown. A direct interaction between NF-κB and PPARγ has been reported (40). PPARα, another PPAR isotype, was reported to suppress the NF-κB pathway by increasing the expression of IκBα (41). Since we found that phosphorylation of IκBα is accelerated in the mutant while the total amount of IκBα does not differ significantly, it is possible that PPARγ regulates upstream of IκBα, such as IKK in B cells. Alternatively, the haploinsufficiency of PPARγ might cause suppression of other regulatory molecules.
NF-κB is also indispensable for T cell proliferation (42). PKC-θ is located upstream of the NF-κB pathway. The reason why the phenotype is affected in B cells but not in T cells is unclear. It is possible that regulation of NF-κB by PPARγ is redundant or dispensable in T cells.
It is known that B cell hyperreactivity is one of the characteristics of autoimmune disease patients. An elevated titer of immunoglobulins, an enhanced proliferative response to various stimuli, and reduced susceptibility to apoptosis were reported (43–45). Therefore, our findings that PPAR-γ haploinsufficiency especially affects B cell reactivity provide new insight into the association between PPAR-γ and immunological disease.
Acknowledgments
We are grateful to Naoko Sato, Kazumi Abe, and Atumi Ohkubo for their excellent technical assistance. This work was supported by grants from the Ministry of Health, Labor and Welfare, and Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
References
- 1.Tontonoz P, Hu E, Spiegelman BM. Stimulation of adipogenesis in fibroblasts by PPAR gamma 2, a lipid-activated transcription factor. Cell. 1994;79:1147–1156. doi: 10.1016/0092-8674(94)90006-x. [DOI] [PubMed] [Google Scholar]
- 2.Kliewer SA, et al. Differential expression and activation of a family of murine peroxisome proliferator-activated receptors. Proc Natl Acad Sci USA. 1994;91:7355–7359. doi: 10.1073/pnas.91.15.7355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Forman BM, et al. 15-Deoxy-delta 12, 14-prostaglandin J2 is a ligand for the adipocyte determination factor PPAR gamma. Cell. 1995;83:803–812. doi: 10.1016/0092-8674(95)90193-0. [DOI] [PubMed] [Google Scholar]
- 4.Kliewer SA, et al. A prostaglandin J2 metabolite binds peroxisome proliferator-activated receptor gamma and promotes adipocyte differentiation. Cell. 1995;83:813–819. doi: 10.1016/0092-8674(95)90194-9. [DOI] [PubMed] [Google Scholar]
- 5.Lemberger T, Desvergne B, Wahli W. Peroxisome proliferator-activated receptors: a nuclear receptor signaling pathway in lipid physiology. Annu Rev Cell Dev Biol. 1996;12:335–363. doi: 10.1146/annurev.cellbio.12.1.335. [DOI] [PubMed] [Google Scholar]
- 6.Kersten S, Desvergne B, Wahli W. Roles of PPARs in health and disease. Nature. 2000;405:421–424. doi: 10.1038/35013000. [DOI] [PubMed] [Google Scholar]
- 7.Ricote M, Li AC, Willson TM, Kelly CJ, Glass CK. The peroxisome proliferator-activated receptor-gamma is a negative regulator of macrophage activation. Nature. 1998;391:79–82. doi: 10.1038/34178. [DOI] [PubMed] [Google Scholar]
- 8.Jiang C, Ting AT, Seed B. PPAR-gamma agonists inhibit production of monocyte inflammatory cytokines. Nature. 1998;391:82–86. doi: 10.1038/34184. [DOI] [PubMed] [Google Scholar]
- 9.Clark RB, et al. The nuclear receptor PPAR gamma and immunoregulation: PPAR gamma mediates inhibition of helper T cell responses. J Immunol. 2000;164:1364–1371. doi: 10.4049/jimmunol.164.3.1364. [DOI] [PubMed] [Google Scholar]
- 10.Harris SG, Phipps RP. Peroxisome proliferator-activated receptor gamma (PPAR-gamma) activation in naive mouse T cells induces cell death. Ann N Y Acad Sci. 2000;905:297–300. doi: 10.1111/j.1749-6632.2000.tb06565.x. [DOI] [PubMed] [Google Scholar]
- 11.Yang XY, et al. Activation of human T lymphocytes is inhibited by peroxisome proliferator-activated receptor gamma (PPARgamma) agonists. PPARgamma co-association with transcription factor NFAT. J Biol Chem. 2000;275:4541–4544. doi: 10.1074/jbc.275.7.4541. [DOI] [PubMed] [Google Scholar]
- 12.Padilla J, Kaur K, Harris SG, Phipps RP. PPAR-gamma-mediated regulation of normal and malignant B lineage cells. Ann N Y Acad Sci. 2000;905:97–109. doi: 10.1111/j.1749-6632.2000.tb06542.x. [DOI] [PubMed] [Google Scholar]
- 13.Padilla J, Kaur K, Cao HJ, Smith TJ, Phipps RP. Peroxisome proliferator activator receptor-gamma agonists and 15-deoxy-Delta(12, 14)(12, 14)-PGJ(2) induce apoptosis in normal and malignant B-lineage cells. J Immunol. 2000;165:6941–6948. doi: 10.4049/jimmunol.165.12.6941. [DOI] [PubMed] [Google Scholar]
- 14.Lehmann JM, et al. An antidiabetic thiazolidinedione is a high affinity ligand for peroxisome proliferator-activated receptor gamma (PPAR gamma) J Biol Chem. 1995;270:12953–12956. doi: 10.1074/jbc.270.22.12953. [DOI] [PubMed] [Google Scholar]
- 15.Nolte RT, et al. Ligand binding and co-activator assembly of the peroxisome proliferator-activated receptor-gamma. Nature. 1998;395:137–143. doi: 10.1038/25931. [DOI] [PubMed] [Google Scholar]
- 16.Straus DS, et al. 15-deoxy-delta 12,14-prostaglandin J2 inhibits multiple steps in the NF-kappa B signaling pathway. Proc Natl Acad Sci USA. 2000;97:4844–4849. doi: 10.1073/pnas.97.9.4844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rossi A, et al. Anti-inflammatory cyclopentenone prostaglandins are direct inhibitors of IkappaB kinase. Nature. 2000;403:103–108. doi: 10.1038/47520. [DOI] [PubMed] [Google Scholar]
- 18.Chawla A, et al. PPAR-gamma dependent and independent effects on macrophage-gene expression in lipid metabolism and inflammation. Nat Med. 2001;7:48–52. doi: 10.1038/83336. [DOI] [PubMed] [Google Scholar]
- 19.Hale, L.P., and Haynes, B.F. 2001. Pathology of rheumatoid arthritis and associated disorders. In Arthritis and allied conditions. A textbook of rheumatology. 14th edition. Volume 1. W.J. Koopman, editor. Williams & Wilkins Press. Baltimore, Maryland, USA. 1103–1127.
- 20.Weyand CM, Goronzy JJ. Pathogenesis of rheumatoid arthritis. Med Clin North Am. 1997;81:29–55. doi: 10.1016/s0025-7125(05)70504-6. [DOI] [PubMed] [Google Scholar]
- 21.Misaki Y, Ezaki I, Yamamoto K. T cell clonality in the rheumatic diseases. APLAR Journal of Rheumatology. 1998;1:194–197. [Google Scholar]
- 22.Carson DA, et al. Rheumatoid factor and immune networks. Annu Rev Immunol. 1987;5:109–126. doi: 10.1146/annurev.iy.05.040187.000545. [DOI] [PubMed] [Google Scholar]
- 23.Kawahito Y, et al. 15-deoxy-delta(12, 14)- PGJ(2) induces synoviocyte apoptosis and suppresses adjuvant-induced arthritis in rats. J Clin Invest. 2000;106:189–197. doi: 10.1172/JCI9652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kubota N, et al. PPAR gamma mediates high-fat diet-induced adipocyte hypertrophy and insulin resistance. Mol Cell. 1999;4:597–609. doi: 10.1016/s1097-2765(00)80210-5. [DOI] [PubMed] [Google Scholar]
- 25.Kadowaki T. Insights into insulin resistance and type 2 diabetes from knockout mouse models. J Clin Invest. 2000;106:459–465. doi: 10.1172/JCI10830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barak Y, et al. PPAR gamma is required for placental, cardiac, and adipose tissue development. Mol Cell. 1999;4:585–595. doi: 10.1016/s1097-2765(00)80209-9. [DOI] [PubMed] [Google Scholar]
- 27.Rosen ED, et al. PPAR gamma is required for the differentiation of adipose tissue in vivo and in vitro. Mol Cell. 1999;4:611–617. doi: 10.1016/s1097-2765(00)80211-7. [DOI] [PubMed] [Google Scholar]
- 28.Kawahata K, et al. Altered expression level of a systemic nuclear autoantigen determines the fate of immune response to self. J Immunol. 1999;162:6482–6491. [PubMed] [Google Scholar]
- 29.Setoguchi K, et al. Antigen-specific T cells transduced with IL-10 ameliorate experimentally induced arthritis without impairing the systemic immune response to the antigen. J Immunol. 2000;165:5980–5986. doi: 10.4049/jimmunol.165.10.5980. [DOI] [PubMed] [Google Scholar]
- 30.Li L, Yee C, Beavo JA. CD3- and CD28-dependent induction of PDE7 required for T cell activation. Science. 1999;283:848–851. doi: 10.1126/science.283.5403.848. [DOI] [PubMed] [Google Scholar]
- 31.Takai T, Li M, Sylvestre D, Clynes R, Ravetch JV. FcR gamma chain deletion results in pleiotrophic effector cell defects. Cell. 1994;76:519–529. doi: 10.1016/0092-8674(94)90115-5. [DOI] [PubMed] [Google Scholar]
- 32.Nicoletti I, Migliorati G, Pagliacci MC, Grignani F, Riccardi C. A rapid and simple method for measuring thymocyte apoptosis by propidium iodide staining and flow cytometry. J Immunol Methods. 1991;139:271–279. doi: 10.1016/0022-1759(91)90198-o. [DOI] [PubMed] [Google Scholar]
- 33.Hu X, Tang M, Fisher AB, Olashaw N, Zuckerman KS. TNF-alpha-induced growth suppression of CD34+ myeloid leukemic cell lines signals through TNF receptor type I and is associated with NF-kappa B activation. J Immunol. 1999;163:3106–3115. [PubMed] [Google Scholar]
- 34.Liou HC, Sha WC, Scott ML, Baltimore D. Sequential induction of NF-kappa B/Rel family proteins during B-cell terminal differentiation. Mol Cell Biol. 1994;14:5349–5359. doi: 10.1128/mcb.14.8.5349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Karin M, Ben-Neriah Y. Phosphorylation meets ubiquitination: the control of NF-kappa B activity. Annu Rev Immunol. 2000;18:621–663. doi: 10.1146/annurev.immunol.18.1.621. [DOI] [PubMed] [Google Scholar]
- 36.Lanzavecchia A. Receptor-mediated antigen uptake and its effect on antigen presentation to class II-restricted T lymphocytes. Annu Rev Immunol. 1990;8:773–793. doi: 10.1146/annurev.iy.08.040190.004013. [DOI] [PubMed] [Google Scholar]
- 37.Aderem A, Ulevitch RJ. Toll-like receptors in the induction of the innate immune response. Nature. 2000;406:782–787. doi: 10.1038/35021228. [DOI] [PubMed] [Google Scholar]
- 38.Zhang G, Ghosh S. Toll-like receptor-mediated NF-κB activation: a phylogenetically conserved paradigm in innate immunity. J Clin Invest. 2001;107:13–19. doi: 10.1172/JCI11837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Reth M, Wienands J. Initiation and processing of signals from the B cell antigen receptor. Annu Rev Immunol. 1997;15:453–479. doi: 10.1146/annurev.immunol.15.1.453. [DOI] [PubMed] [Google Scholar]
- 40.Chung SW, et al. Oxidized low density lipoprotein inhibits interleukin-12 production in lipopolysaccharide-activated mouse macrophages via direct interactions between peroxisome proliferator-activated receptor-gamma and nuclear factor-kappa B. J Biol Chem. 2000;275:32681–32687. doi: 10.1074/jbc.M002577200. [DOI] [PubMed] [Google Scholar]
- 41.Delerive P, Gervois P, Fruchart JC, Staels B. Induction of IκBα expression as a mechanism contributing to the anti-inflammatory activities of peroxisome proliferator-activated receptor-alpha activators. J Biol Chem. 2000;75:36703–36707. doi: 10.1074/jbc.M004045200. [DOI] [PubMed] [Google Scholar]
- 42.Sun Z, et al. PKC-theta is required for TCR-induced NF-kappaB activation in mature but not immature T lymphocytes. Nature. 2000;404:402–407. doi: 10.1038/35006090. [DOI] [PubMed] [Google Scholar]
- 43.Lipsky PE. Systemic lupus erythematosus: an autoimmune disease of B cell hyperactivity. Nat Immunol. 2001;2:764–766. doi: 10.1038/ni0901-764. [DOI] [PubMed] [Google Scholar]
- 44.Diamond, B. 2001. B cells. In Kelly’s Textbook of Rheumatology. 6th edition. S. Ruddy, E.D. Harris, C.B. Sledge, editors. W.B. Saunders Company. Philadelphia, Pennsylvania, USA. 131–149.
- 45.Klinman DM, Steinberg AD. Systemic autoimmune disease arises from polyclonal B cell activation. J Exp Med. 1987;165:1755–1760. doi: 10.1084/jem.165.6.1755. [DOI] [PMC free article] [PubMed] [Google Scholar]