Abstract
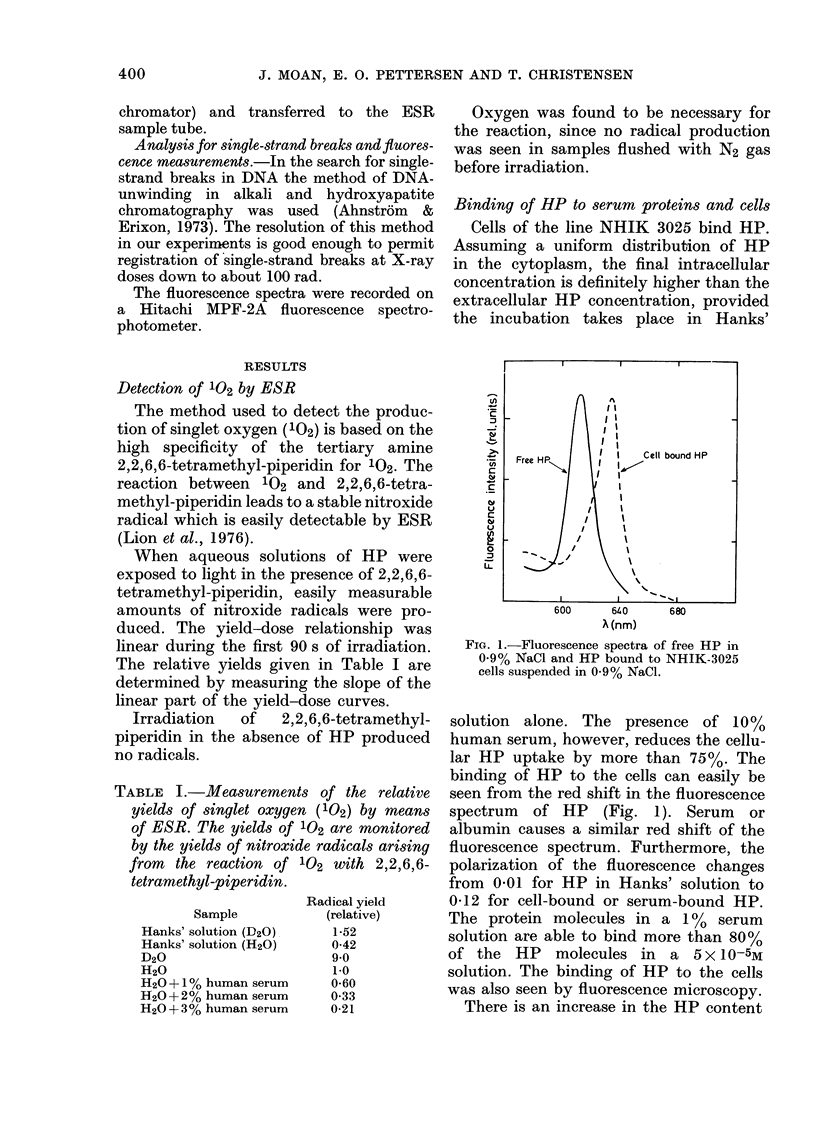
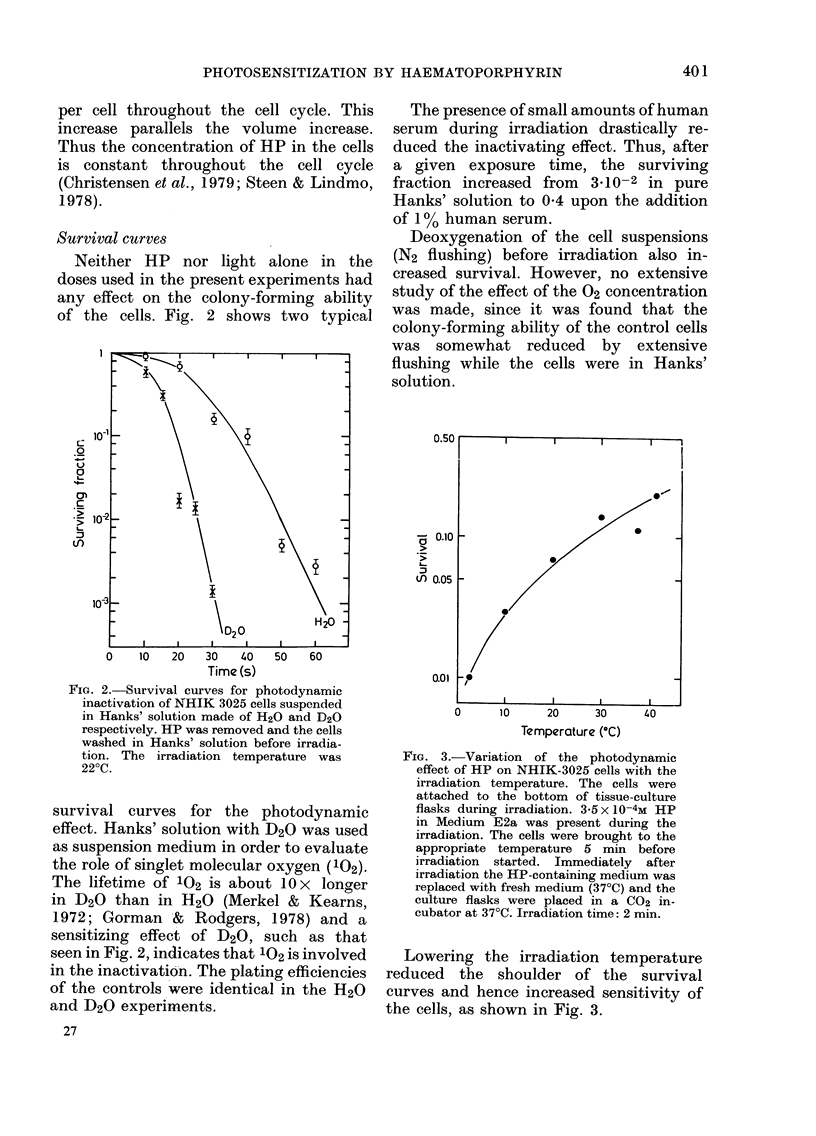
The photosensitizing effect of haematoporphyrin (HP) on human cells of the established line NHIK 3025 has been studied. Fluorescence measurements show that HP is bound to these cells. Serum proteins also bind HP, and the presence of 10% human serum during incubation with HP (3 X 10(-4)M) reduces the cellular uptake of HP by 75% or more. The photosensitized inactivation is enhanced when the cells are suspended in D2O-buffer during irradiation. This indicates that singlet oxygen is involved in the inactivation. Two findings indicate that the photoinduced damage is repairable: firstly, the fraction of cells surviving a given light dose decreases with decreasing irradiation temperature, and secondly, the survival curves have a shoulder at low exposures of light.
Full text
PDF

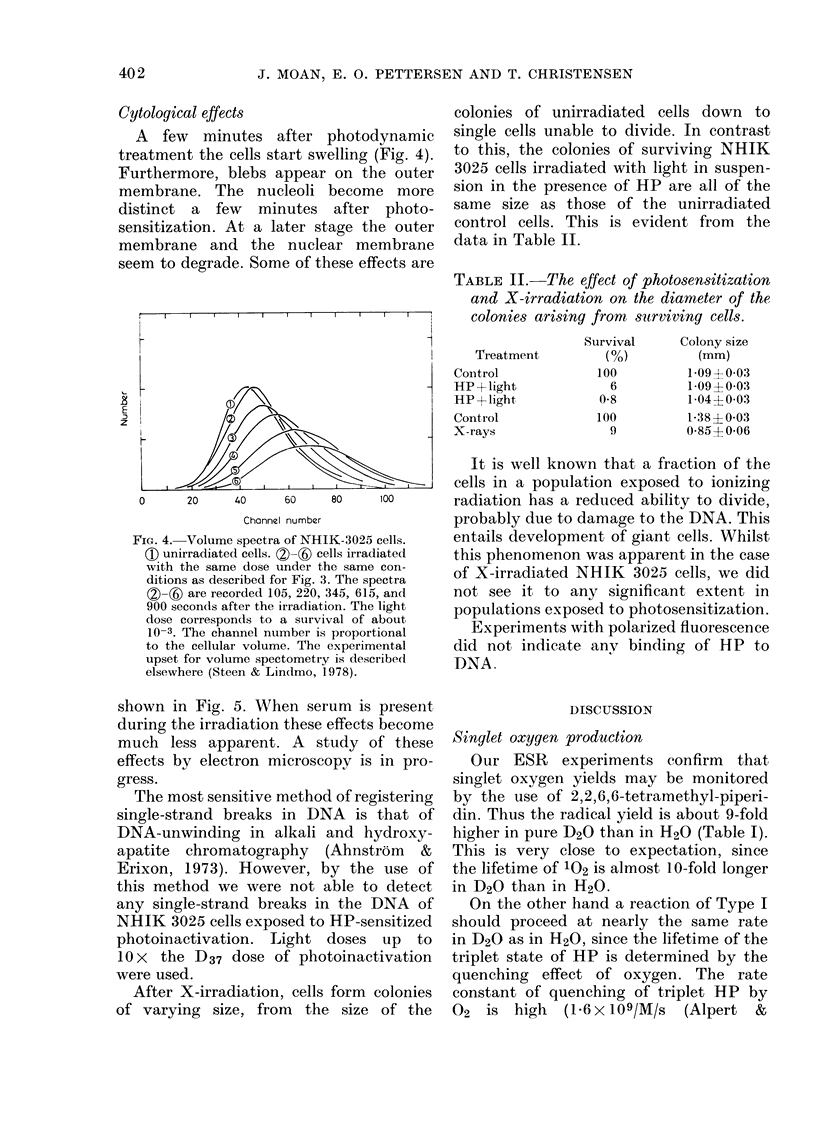
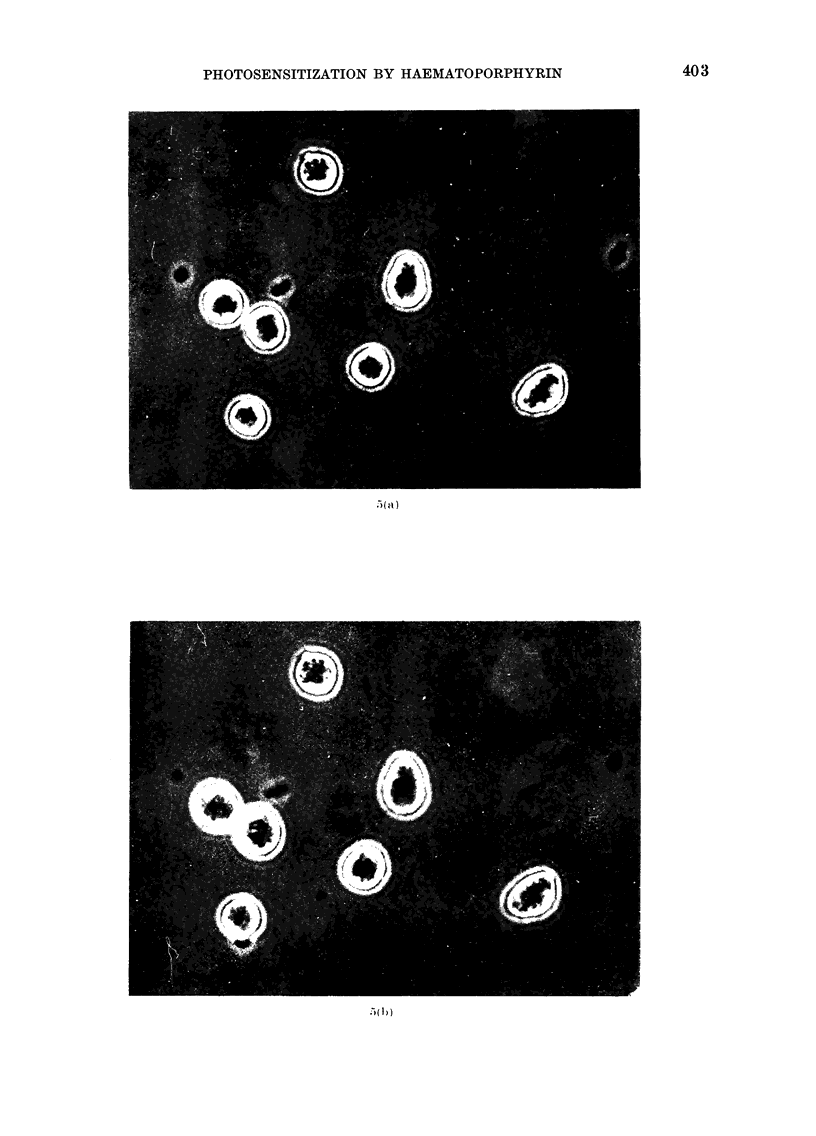
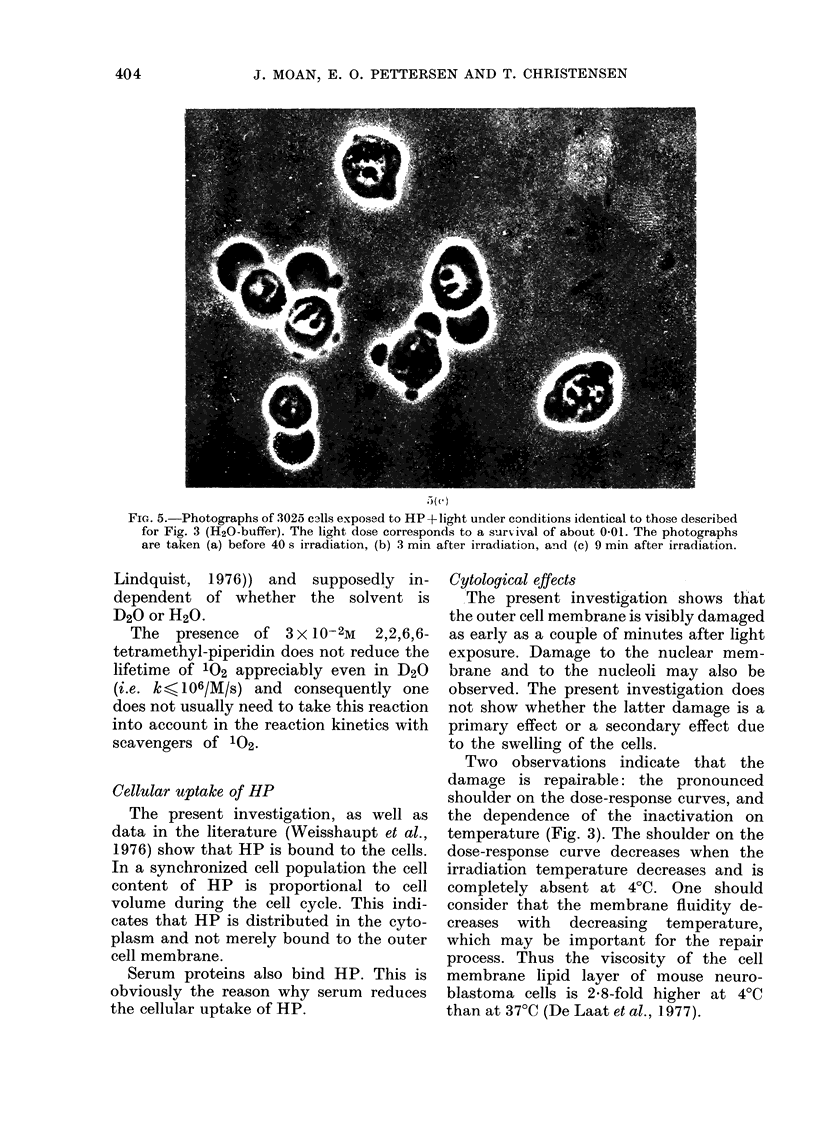



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahnström G., Erixon K. Radiation induced strand breakage in DNA from mammalian cells. Strand separation in alkaline solution. Int J Radiat Biol Relat Stud Phys Chem Med. 1973 Mar;23(3):285–289. doi: 10.1080/09553007314550311. [DOI] [PubMed] [Google Scholar]
- Allison A. C., Magnus I. A., Young M. R. Role of lysosomes and of cell membranes in photosensitization. Nature. 1966 Feb 26;209(5026):874–878. doi: 10.1038/209874a0. [DOI] [PubMed] [Google Scholar]
- Cauzzo G., Gennari G., Jori G., Spikes J. D. The effect of chemical structure on the photosensitizing efficiencies of porphyrins. Photochem Photobiol. 1977 Apr;25(4):389–395. doi: 10.1111/j.1751-1097.1977.tb07358.x. [DOI] [PubMed] [Google Scholar]
- Christensen T., Moan J., Wibe E., Oftebro R. Photodynamic effect of haematoporphyrin throughout the cell cycle of the human cell line NHIK 3025 cultivated in vitro. Br J Cancer. 1979 Jan;39(1):64–68. doi: 10.1038/bjc.1979.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delmelle M. Retinal sensitized photodynamic damage to liposomes. Photochem Photobiol. 1978 Sep;28(3):357–360. doi: 10.1111/j.1751-1097.1978.tb07718.x. [DOI] [PubMed] [Google Scholar]
- Dewey W. C., Hopwood L. E., Sapareto S. A., Gerweck L. E. Cellular responses to combinations of hyperthermia and radiation. Radiology. 1977 May;123(2):463–474. doi: 10.1148/123.2.463. [DOI] [PubMed] [Google Scholar]
- Dubbelman T. M., de Goeij A. F., van Steveninck J. Protoporphyrin-sensitized photodynamic modification of proteins in isolated human red blood cell membranes. Photochem Photobiol. 1978 Aug;28(2):197–204. doi: 10.1111/j.1751-1097.1978.tb07695.x. [DOI] [PubMed] [Google Scholar]
- Granelli S. G., Diamond I., McDonagh A. F., Wilson C. B., Nielsen S. L. Photochemotherapy of glioma cells by visible light and hematoporphyrin. Cancer Res. 1975 Sep;35(9):2567–2570. [PubMed] [Google Scholar]
- Gregorie H. B., Jr, Horger E. O., Ward J. L., Green J. F., Richards T., Robertson H. C., Jr, Stevenson T. B. Hematoporphyrin-derivative fluorescence in malignant neoplasms. Ann Surg. 1968 Jun;167(6):820–828. doi: 10.1097/00000658-196806000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly J. F., Snell M. E., Berenbaum M. C. Photodynamic destruction of human bladder carcinoma. Br J Cancer. 1975 Feb;31(2):237–244. doi: 10.1038/bjc.1975.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S. H., Kim J. H., Hahn E. W. The enhanced killing of irradiated HeLa cells in synchronous culture by hyperthermia. Radiat Res. 1976 May;66(2):337–345. [PubMed] [Google Scholar]
- LIPSON R. L., BALDES E. J., OLSEN A. M. The use of a derivative of hematoporhyrin in tumor detection. J Natl Cancer Inst. 1961 Jan;26:1–11. [PubMed] [Google Scholar]
- Lion Y., Delmelle M., van de Vorst A. New method of detecting singlet oxygen production. Nature. 1976 Sep 30;263(5576):442–443. doi: 10.1038/263442a0. [DOI] [PubMed] [Google Scholar]
- Matheson I. B., Etheridge R. D., Kratowich N. R., Lee J. The quenching of singlet oxygen by amino acids and proteins. Photochem Photobiol. 1975 Mar;21(3):165–171. doi: 10.1111/j.1751-1097.1975.tb06647.x. [DOI] [PubMed] [Google Scholar]
- Moore C., Wallis C., Melnick J. L., Kuns M. D. Photodynamic treatment of herpes keratitis. Infect Immun. 1972 Feb;5(2):169–171. doi: 10.1128/iai.5.2.169-171.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettersen E. O., Bakke O., Lindmo T., Oftebro R. Cell cycle characteristics of synchronized and asynchronous populations of human cells and effect of cooling of selected mitotic cells. Cell Tissue Kinet. 1977 Nov;10(6):511–522. doi: 10.1111/j.1365-2184.1977.tb00309.x. [DOI] [PubMed] [Google Scholar]
- Steen H. B., Lindmo T. Cellular and nuclear volume during the cell cycle of NHIK 3025 cells. Cell Tissue Kinet. 1978 Jan;11(1):69–81. doi: 10.1111/j.1365-2184.1978.tb00876.x. [DOI] [PubMed] [Google Scholar]
- Tannock I. F. Oxygen diffusion and the distribution of cellular radiosensitivity in tumours. Br J Radiol. 1972 Jul;45(535):515–524. doi: 10.1259/0007-1285-45-535-515. [DOI] [PubMed] [Google Scholar]
- WINKELMAN J., RASMUSSEN-TAXDAL D. S. Quantitative determination of porphyrin uptake by tumor tissue following parenteral administration. Bull Johns Hopkins Hosp. 1960 Oct;107:228–233. [PubMed] [Google Scholar]
- Wallis C., Melnick J. L. Photodynamic inactivation of animal viruses: a review. Photochem Photobiol. 1966 Mar;4(2):159–170. doi: 10.1111/j.1751-1097.1965.tb05733.x. [DOI] [PubMed] [Google Scholar]
- Weishaupt K. R., Gomer C. J., Dougherty T. J. Identification of singlet oxygen as the cytotoxic agent in photoinactivation of a murine tumor. Cancer Res. 1976 Jul;36(7 Pt 1):2326–2329. [PubMed] [Google Scholar]
- de Laat S. W., van der Saag P. T., Shinitzky M. Microviscosity modulation during the cell cycle of neuroblastoma cells. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4458–4461. doi: 10.1073/pnas.74.10.4458. [DOI] [PMC free article] [PubMed] [Google Scholar]