Abstract
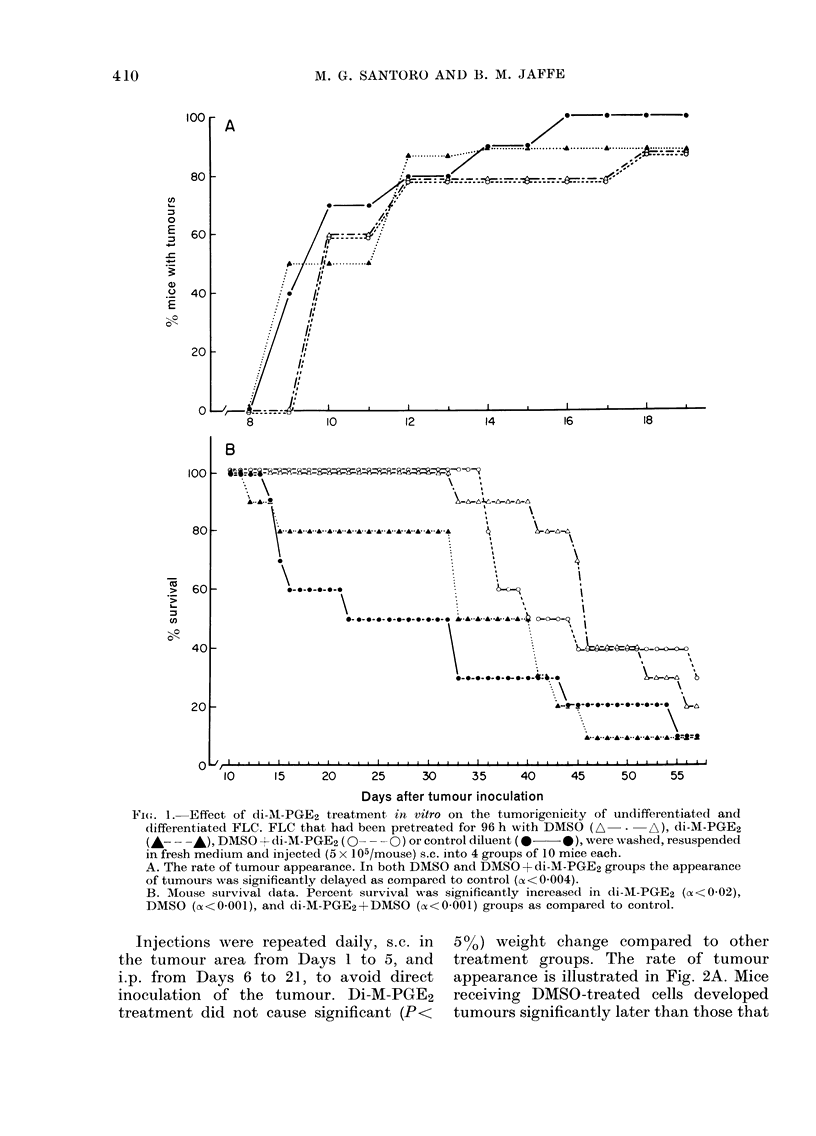
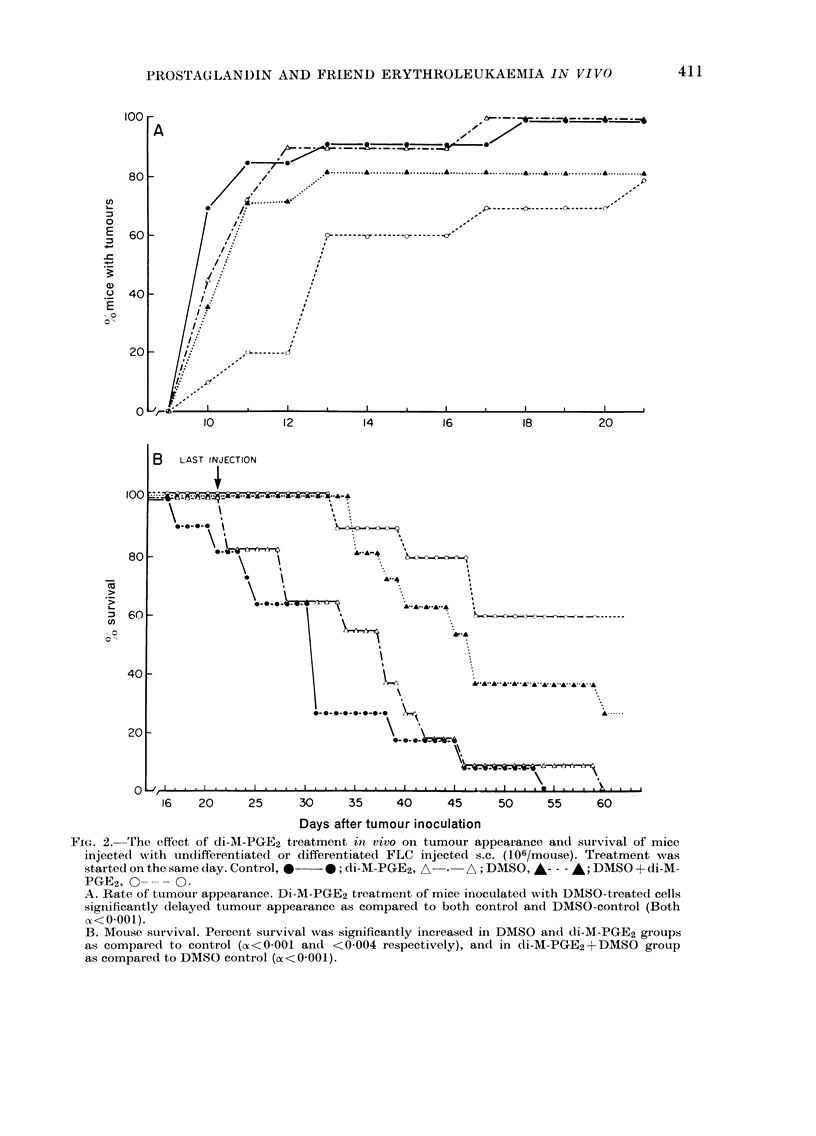
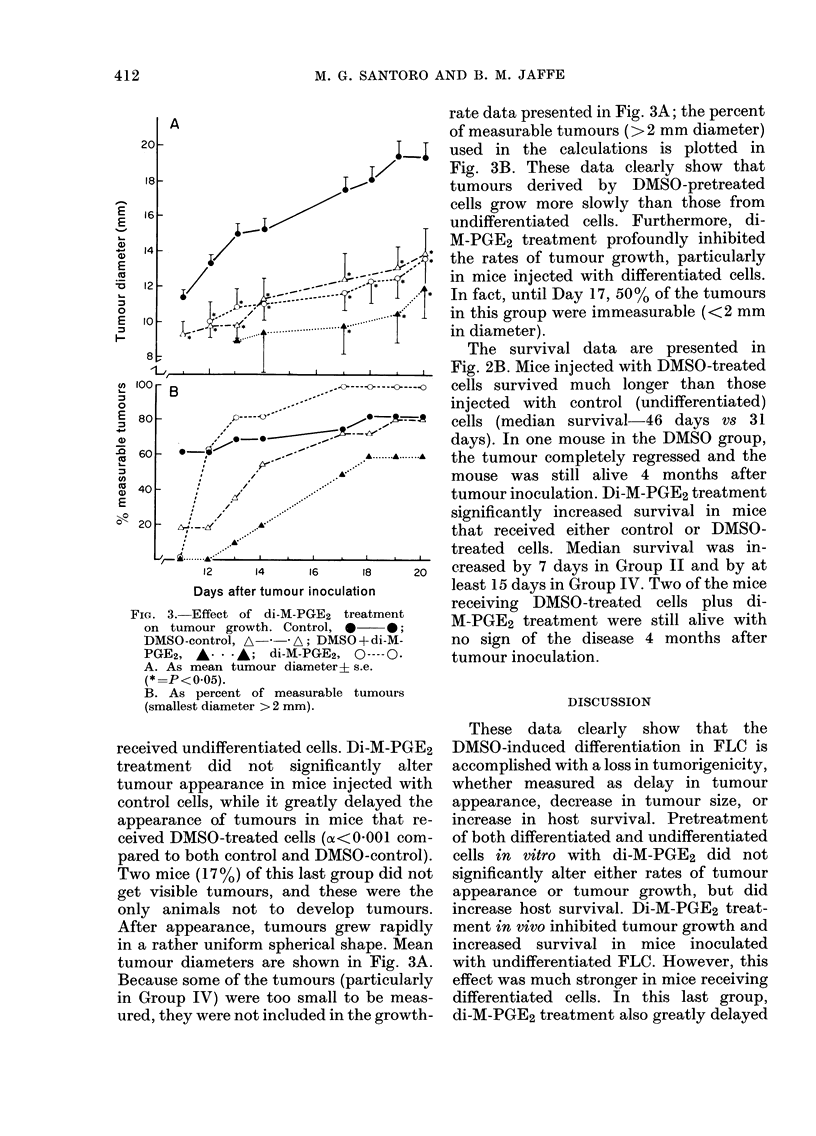
The effect of 16,16-dimethyl-PGE2-methyl ester (di-M-PGE2), a long-acting synthetic analogue of prostaglandin E2, on the replication of Friend erythroleukaemia cells (FLC) in vivo has been studied. Pre-treatment in vitro of both undifferentiated and differentiated FLC with di-M-PGE2 (1 microgram/ml) did not alter rates of tumour appearance or growth, but increased the median survival of DBA/2J mice. Systemic administration of di-M-PGE2 (10 microgram/mouse/day) was not toxic to the mice, but significantly inhibited tumour growth and increased median survival in mice injected s.c. with undifferentiated FLC. These effects of di-M-PGE2 were much more pronounced in mice receiving differentiated (DMSO-treated) FLC. In this latter group, the appearance of tumour was also significantly delayed by di-M-PGE2. The different effects of di-M-PGE2 treatment on tumours derived from undifferentiated and differentiated cells suggest that the analogue is acting directly on tumour-cell replication rather than on factors related to the host response.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson C. B., Jaffe B. M. Circulating prostaglandin E and canine skin allografts. Surg Forum. 1974;25(0):287–289. [PubMed] [Google Scholar]
- CROSBY W. H., FURTH F. W. A modification of the benzidine method for measurement of hemoglobin in plasma and urine. Blood. 1956 Apr;11(4):380–383. [PubMed] [Google Scholar]
- Ebert P. S., Wars I., Buell D. N. Erythroid differentiation in cultured Friend leukemia cells treated with metabolic inhibitors. Cancer Res. 1976 May;36(5):1809–1813. [PubMed] [Google Scholar]
- Friend C., Scher W., Holland J. G., Sato T. Hemoglobin synthesis in murine virus-induced leukemic cells in vitro: stimulation of erythroid differentiation by dimethyl sulfoxide. Proc Natl Acad Sci U S A. 1971 Feb;68(2):378–382. doi: 10.1073/pnas.68.2.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamprecht B., Jaffee B. M., Philpott G. W. Prostaglandin production by neuroblastoma, glioma and fibroblast cell lines; stimulation by N6,02'-dibutyryl adenosine 3':5'-cyclic monophosphate. FEBS Lett. 1973 Oct 15;36(2):193–198. doi: 10.1016/0014-5793(73)80367-9. [DOI] [PubMed] [Google Scholar]
- Johnson G. S., Pastan I. Change in growth and morphology of fibroblasts by prostaglandins. J Natl Cancer Inst. 1971 Dec;47(6):1357–1364. [PubMed] [Google Scholar]
- Kakita A., Blanchard J., Fortner J. G. Effectiveness of prostaglandin E1 and procarbazine hydrochloride in prolonging the survival of vascularized cardiac hamster-to-rat xenograft. Transplantation. 1975 Dec;20(6):439–442. doi: 10.1097/00007890-197512000-00001. [DOI] [PubMed] [Google Scholar]
- Leder A., Leder P. Butyric acid, a potent inducer of erythroid differentiation in cultured erythroleukemic cells. Cell. 1975 Jul;5(3):319–322. doi: 10.1016/0092-8674(75)90107-5. [DOI] [PubMed] [Google Scholar]
- Prasad K. N. Morphological differentiation induced by prostaglandin in mouse neuroblastoma cells in culture. Nat New Biol. 1972 Mar 15;236(63):49–52. doi: 10.1038/newbio236049a0. [DOI] [PubMed] [Google Scholar]
- Prasad K. N. Neuroblastoma clones: prostaglandin versus dibutyryl cyclic AMP, 8-benzylthio-cyclic AMP, phosphodiesterase inhibitors and x-rays. Proc Soc Exp Biol Med. 1972 May;140(1):126–129. doi: 10.3181/00379727-140-36408. [DOI] [PubMed] [Google Scholar]
- Preisler H. D., Bjornsson S., Mori M., Lyman G. H. Inducers of Friend leukaemic cell differentiation in vitro--effects of in vivo administration. Br J Cancer. 1976 Jun;33(6):634–645. doi: 10.1038/bjc.1976.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuben R. C., Wife R. L., Breslow R., Rifkind R. A., Marks P. A. A new group of potent inducers of differentiation in murine erythroleukemia cells. Proc Natl Acad Sci U S A. 1976 Mar;73(3):862–866. doi: 10.1073/pnas.73.3.862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santoro M. G., Benedetto A., Jaffe B. M. Effect of endogenous and exogenous prostaglandin E on Friend erythroleukaemia cell growth and differentiation. Br J Cancer. 1979 Mar;39(3):259–267. doi: 10.1038/bjc.1979.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santoro M. G., Philpott G. W., Jaffe B. M. Dose dependent inhibition of B-16 melanoma growth in vivo by a synthetic analogue of PGE2. Prostaglandins. 1977 Oct;14(4):645–651. doi: 10.1016/0090-6980(77)90191-5. [DOI] [PubMed] [Google Scholar]
- Santoro M. G., Philpott G. W., Jaffe B. M. Inhibition of B-16 melanoma growth in vivo by a synthetic analog of prostaglandin E2. Cancer Res. 1977 Oct;37(10):3774–3779. [PubMed] [Google Scholar]
- Santoro M. G., Philpott G. W., Jaffe B. M. Inhibition of tumour growth in vivo and in vitro by prostaglandin E. Nature. 1976 Oct 28;263(5580):777–779. doi: 10.1038/263777a0. [DOI] [PubMed] [Google Scholar]
- Thomas D. R., Philpott G. W., Jaffe B. M. The relationship between concentration of prostaglandin E and rates of cell replication. Exp Cell Res. 1974 Mar 15;84(1):40–46. doi: 10.1016/0014-4827(74)90377-2. [DOI] [PubMed] [Google Scholar]


