Pancreatic β cells synthesize, store, and secrete insulin in response to appropriate environmental cues. The classic paradigm, as originally proposed by Matschinsky (1), holds that the products of glucose metabolism in β cells control insulin production and release. In recent years, this view has been broadened to include signaling by receptor tyrosine kinases as part of the β cell sensing mechanism, impinging on such diverse functions as insulin release and cellular proliferation (2).
Regulation of β cell mass
Of special interest in this context are mechanisms controlling the adaptation of β cell mass to preserve adequate insulin production. β cell mass results from a dynamic balance of neogenesis, proliferation, and apoptosis (3). Neogenesis of β cells occurs from progenitor cells arising from pancreatic ducts, but the mechanism behind this longstanding observation (4) remains largely unknown (5). β cells can also proliferate in physiologic (growth, pregnancy) and disease conditions (obesity, insulin resistance).
Since alterations of β cell mass can precede the onset of hyperglycemia, I favor the view that initial changes occur independently of glucose concentrations and are mediated by humoral factors (6). However, it would be virtually impossible to exclude the possibility that transient oscillations in glucose levels, occurring prior to the onset of overt hyperglycemia, are sufficient to jump-start β cell growth. Alternatively, glucose may have a permissive effect, as suggested by the work of Rhodes and colleagues , and may act in concert with growth factors to stimulate β cell proliferation (7).
An article in this issue of the JCI (8) and one in the October issue of Nature Medicine (9) report the generation of transgenic mice overexpressing a constitutively active form of the serine/threonine kinase Akt, an important mediator of insulin action. In both instances, the mutant kinase increased β cell mass eight- to tenfold, resulting in increased insulin sensitivity and protection from diabetes caused by the β cell toxin streptozotocin. It is not completely clear how the mutant Akt increased β cell mass. Both reports demonstrate a striking increase in cell size (two- to threefold). Nonetheless, the cellular hypertrophy is insufficient to account for the overall increase in mass. Bernal-Mizrachi et al. (8) ascribe the residual increase to increased proliferation — at least in the postpubertal phase — whereas Tuttle et al. (9) fail to detect changes in proliferation and report a paradoxical increase in apoptosis rates. In the end, both groups suggest that Akt increased neogenesis, a notoriously hard parameter to quantify. Without discounting methodological differences that may account for apparent discrepancies between the two reports, it bears emphasizing that regulation of β cell mass is a genetically heterogeneous process, so that, in different experimental conditions, the relative contributions of neogenesis, replication, and apoptosis are likely to vary.
Making progress, one gene at a time
Clearly, the observation that increasing Akt activity increases β cell mass does not constitute proof that Akt is the physiologic mediator of β cell growth. However, the findings add one more component to the pathway required for β cell neogenesis and proliferation. Other components include Irs-2, an important mediator of insulin action whose ablation impairs β cell growth (10, 11). Ablation of p70s6k1, an Akt substrate, is associated with decreased β cell size (12). Thus, signals upstream and downstream of Akt control β cell size and growth, making it extremely plausible that Akt itself relays those signals. Loss-of-function experiments should now be carried out to complete the picture. Given the complexity of generating knockouts of the three Akt isoforms, I suggest a less squeamish attitude toward dominant negative Akt mutants, a reagent many authors have been loath to use, in view of the vagaries of measuring their effectiveness.
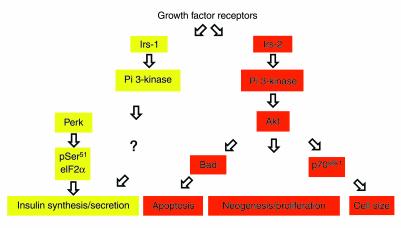
In contrast to mutations affecting the components of the Irs-2→ Akt→p70s6k1 axis, which do not perturb glucose sensing or insulin production, mutations of the Insulin Receptor (13) and Irs1 (14) genes impair insulin synthesis and secretion by a phosphatidylinositol 3-kinase−dependent (PI 3-kinase−dependent) pathway (15). These data suggest that signals regulating β cell growth and those regulating insulin secretion diverge downstream of PI 3-kinase, as suggested for insulin effects on gene expression (16) (Figure 1). The distal effectors of the metabolic stimulus/secretion coupling comprise the eukaryotic translation initiation factor 2α (eIF2α) (17) and its kinase Perk (18). It will be of the utmost interest to determine whether there exists a link between these two sensing mechanisms.
Figure 1.
Regulation of β cell function by tyrosine kinase pathways. There is evidence for participation of tyrosine kinase signaling in different β cell functions. Interestingly, activation of PI 3-kinase is thought to be important for cell size control, neogenesis and proliferation, apoptosis, and insulin synthesis or secretion. However, the pathways activated by PI 3-kinase appear to diverge, with Akt contributing to growth and size control but not to insulin secretion. It is unclear how PI 3-kinase signaling is linked to insulin synthesis or secretion, since insulin regulation is unaffected by loss of p70s6k1. On the other hand, phosphorylation of eIF2α on Ser51 by the kinase gene Perk is thought to play an important role in insulin mRNA translation.
Outstanding questions
This brief survey raises two questions: What activates Akt and what does Akt activate? The latter question is easier to address. Genetic evidence available thus far indicates that multiple Akt substrates are involved in regulating various aspects of β cell function. p70s6k1 is one of them, but the phenotype of p70s6k1–/– mice is considerably milder than that of Irs2–/– mice. Thus, if all the effects of Irs-2 are mediated through Akt, there must be additional players, which are likely to include effectors of the apoptotic cascade, transcription factors, and additional kinases.
The factors that drive β cell proliferation and function under normal or pathological conditions are still unknown. Available data indicate that there exists a plethora of β cell growth factors acting in a genetically heterogeneous and presumably oligogenic fashion (19). My own speculation is that insulin represents the main promoter of β cell neogenesis and that it acts through a paracrine mechanism. As a result of the anatomical association between ducts and islets (20), minute amounts of insulin are released into pancreatic ducts (21), where they can potentially engage insulin-sensitive progenitor cells to differentiate into β cells. If the supply of progenitor cells were genetically limited — in much the same way, for example, as primary ovarian follicles are — insulin resistance would cause diabetes by hastening its depletion. Thus, the two primary causes of type 2 diabetes, insulin resistance and β cell failure, should no longer be viewed as two pathogenetically distinct phenomena, but rather as different facets of the same molecular defect.
Regardless of its mechanisms, the emerging role of tyrosine kinases underscores at once the daunting complexity of β cell physiology and the potential for new approaches to β cell replacement in type 1 diabetes and to propping up faltering β cell function in type 2 diabetes.
Footnotes
See the related article beginning on page 1631.
References
- 1.Matschinsky FM. Banting Lecture 1995. A lesson in metabolic regulation inspired by the glucokinase glucose sensor paradigm. Diabetes. 1996;45:223–241. doi: 10.2337/diab.45.2.223. [DOI] [PubMed] [Google Scholar]
- 2.Nakae J, Kido Y, Kitamura T, Accili D. Glucose homeostasis: lessons from knockout mice. Curr Op Endocrinol Diab. 2001;8:82–87. [Google Scholar]
- 3.Bonner-Weir S. Life and death of the pancreatic beta cells. Trends Endocrinol Metab. 2000;11:375–378. doi: 10.1016/s1043-2760(00)00305-2. [DOI] [PubMed] [Google Scholar]
- 4.Shaw J, Latimer E. Regeneration of pancreatic tissue from the transplanted pancreatic duct in the dog. Am J Physiol. 1925;76:49–53. [Google Scholar]
- 5.Kim SK, Hebrok M. Intercellular signals regulating pancreas development and function. Genes Dev. 2001;15:111–127. doi: 10.1101/gad.859401. [DOI] [PubMed] [Google Scholar]
- 6.Flier SN, Kulkarni RN, Kahn CR. Evidence for a circulating islet cell growth factor in insulin-resistant states. Proc Natl Acad Sci USA. 2001;98:7475–7480. doi: 10.1073/pnas.131192998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rhodes CJ. IGF-I and GH post-receptor signaling mechanisms for pancreatic beta-cell replication. J Mol Endocrinol. 2000;24:303–311. doi: 10.1677/jme.0.0240303. [DOI] [PubMed] [Google Scholar]
- 8.Bernal-Mizrachi E, Wen W, Stahlhut S, Welling CM, Permutt MA. Islet β cell expression of constitutively active Akt1/PKBα induces striking hypertrophy, hyperplasia, and hyperinsulinemia. J Clin Invest. 2001;108:1631–1638. doi: 10.1172/JCI13785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tuttle R, et al. Regulation of pancreatic β-cell growth and survival by the serine/threonine kinase AKt1/PKB. Nat Med. 2001;7:1133–1137. doi: 10.1038/nm1001-1133. [DOI] [PubMed] [Google Scholar]
- 10.Withers DJ, et al. Disruption of IRS-2 causes type 2 diabetes in mice. Nature. 1998;391:900–904. doi: 10.1038/36116. [DOI] [PubMed] [Google Scholar]
- 11.Kubota N, et al. Disruption of insulin receptor substrate 2 causes type 2 diabetes because of liver insulin resistance and lack of compensatory beta-cell hyperplasia. Diabetes. 2000;49:1880–1889. doi: 10.2337/diabetes.49.11.1880. [DOI] [PubMed] [Google Scholar]
- 12.Pende M, et al. Hypoinsulinaemia, glucose intolerance and diminished beta-cell size in S6K1-deficient mice. Nature. 2000;408:994–997. doi: 10.1038/35050135. [DOI] [PubMed] [Google Scholar]
- 13.Kulkarni RN, et al. Tissue-specific knockout of the insulin receptor in pancreatic beta cells creates an insulin secretory defect similar to that in type 2 diabetes. Cell. 1999;96:329–339. doi: 10.1016/s0092-8674(00)80546-2. [DOI] [PubMed] [Google Scholar]
- 14.Kulkarni RN, et al. Altered function of insulin receptor substrate-1-deficient mouse islets and cultured beta-cell lines. J Clin Invest. 1999;104:R69–R75. doi: 10.1172/JCI8339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leibiger B, et al. Selective insulin signaling through A and B insulin receptors regulates transcription of insulin and glucokinase genes in pancreatic beta cells. Mol Cell. 2001;7:559–570. doi: 10.1016/s1097-2765(01)00203-9. [DOI] [PubMed] [Google Scholar]
- 16.Nakae J, Kitamura T, Ogawa W, Kasuga M, Accili D. Insulin regulation of gene expression through the forkhead transcription factor Foxo1 (Fkhr) requires kinases distinct from Akt. Biochemistry. 2001;40:11768–11776. doi: 10.1021/bi015532m. [DOI] [PubMed] [Google Scholar]
- 17.Scheuner D, et al. Translational control is required for the unfolded protein response and in vivo glucose homeostasis. Mol Cell. 2001;7:1165–1176. doi: 10.1016/s1097-2765(01)00265-9. [DOI] [PubMed] [Google Scholar]
- 18.Harding HP, et al. Diabetes mellitus and exocrine pancreatic dysfunction in perk–/– mice reveals a role for translational control in secretory cell survival. Mol Cell. 2001;7:1153–1163. doi: 10.1016/s1097-2765(01)00264-7. [DOI] [PubMed] [Google Scholar]
- 19.Nielsen JH, Svensson C, Galsgaard ED, Moldrup A, Billestrup N. Beta cell proliferation and growth factors. J Mol Med. 1999;77:62–66. doi: 10.1007/s001090050302. [DOI] [PubMed] [Google Scholar]
- 20.Bertelli E, Regoli M, Orazioli D, Bendayan M. Association between islets of Langerhans and pancreatic ductal system in adult rat. Where endocrine and exocrine meet together? Diabetologia. 2001;44:575–584. doi: 10.1007/s001250051663. [DOI] [PubMed] [Google Scholar]
- 21.Conlon JM, Rouiller D, Boden G, Unger RH. Characterization of immunoreactive components of insulin and somatostatin in canine pancreatic juice. FEBS Lett. 1979;105:23–26. doi: 10.1016/0014-5793(79)80879-0. [DOI] [PubMed] [Google Scholar]