Abstract
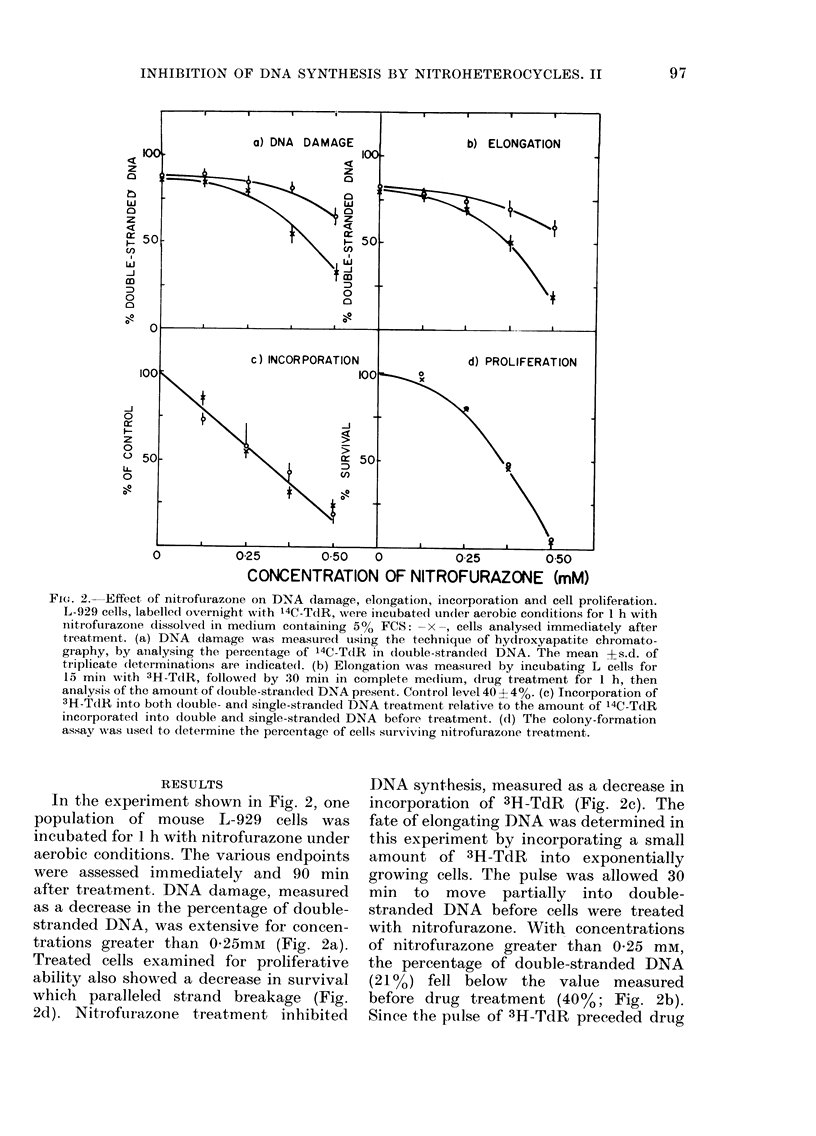
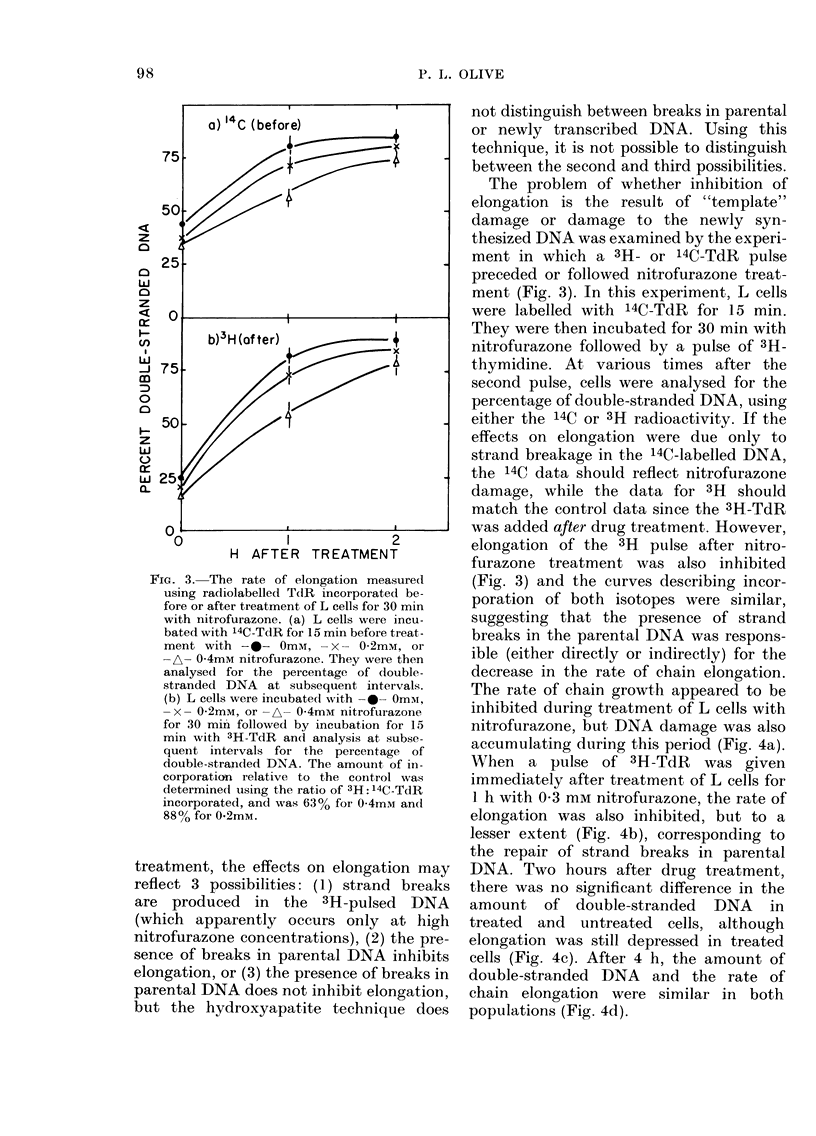
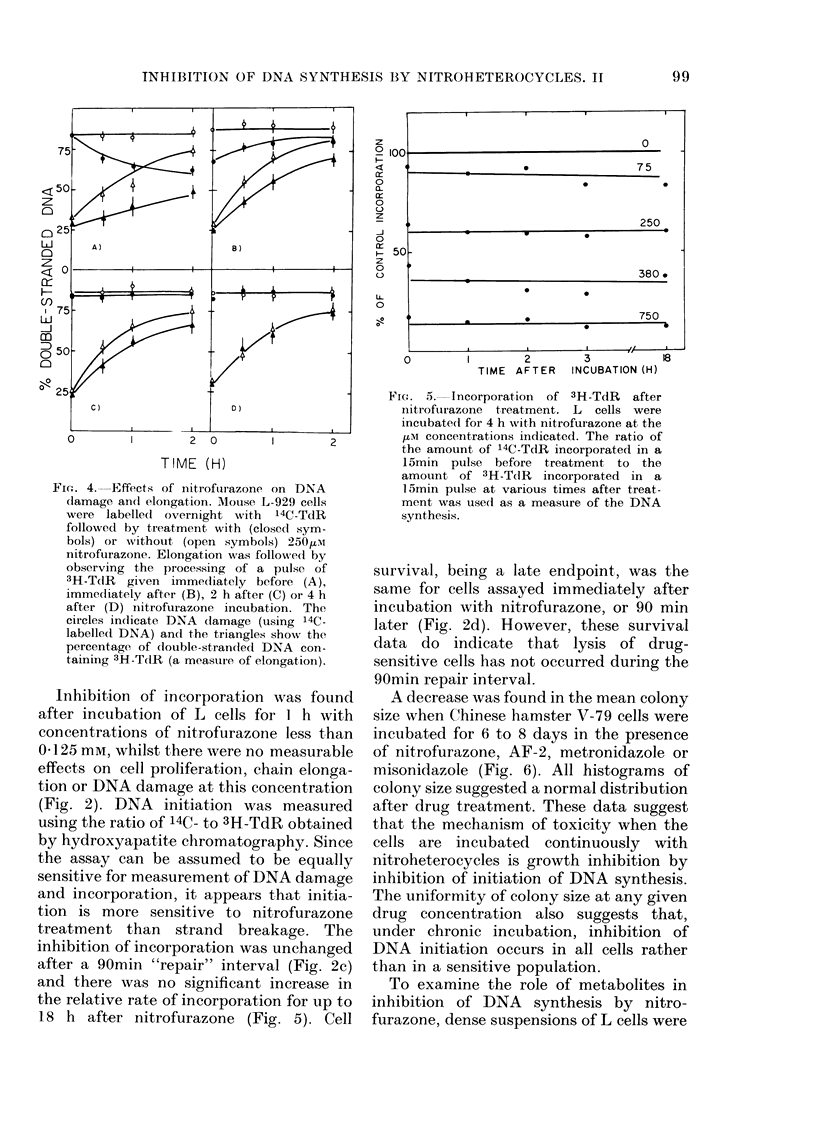
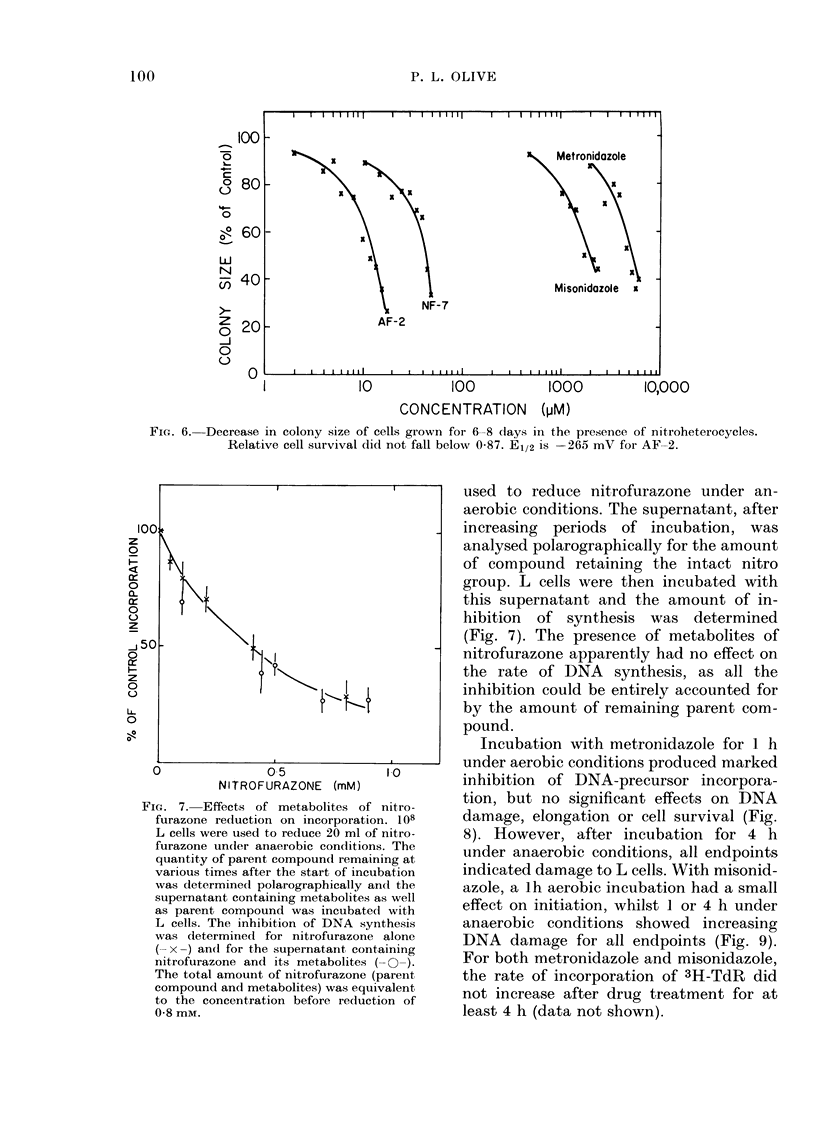
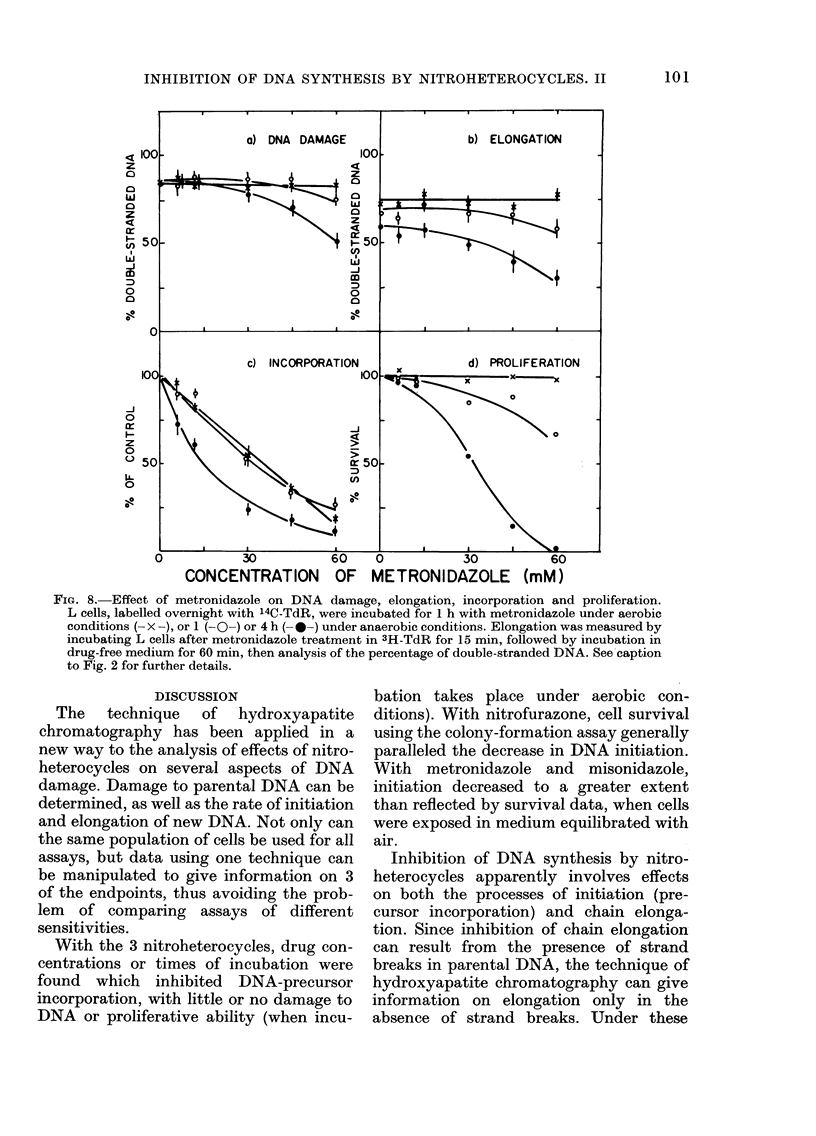
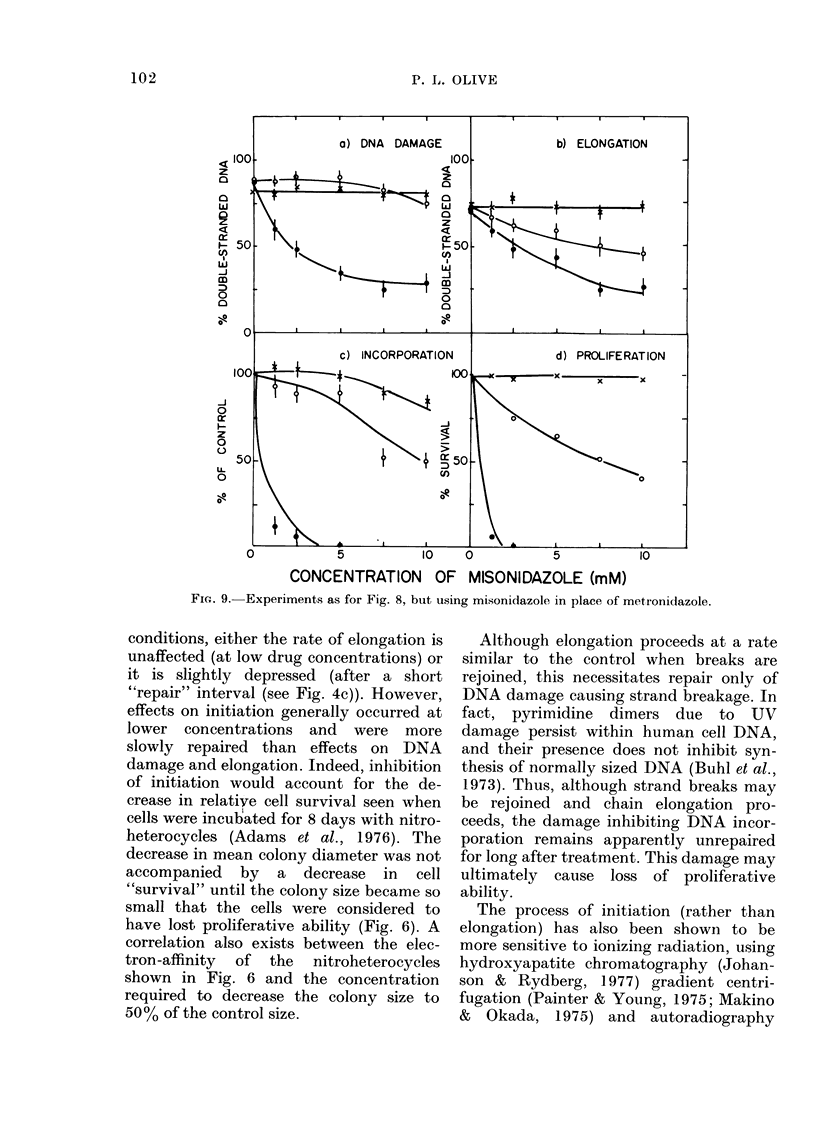
Nitroheterocycles have been shown to inhibit the incorporation of 3H-TdR by cultured L-929 cells, and the degree of inhibition is related to their electron-affinity. On the basis of their chemical reactivity and potential clinical utility, nitrofurazone, misonidazole and metronidazole were selected for more detailed studies of the mechanism of inhibition of DNA synthesis. Double-isotope labelling in conjunction with hydroxyapatite chromatography allowed the evaluation of drug effects on initiation of DNA replicons, DNA chain elongation and DNA damage (single-strand breaks), and their correlation with eventual cell viability. Partial inhibition of initiation of DNA synthesis generally preceded other measurable effects, and was not reversed by incubation in the absence of drug. In the absence of DNA strand breaks (at low drug doses or after a repair interval) the rate of elongation was similar in both treated and untreated cell populations. Measurable DNA damage (strand breaks) was predictive for cytotoxicity. At lower drug doses, or under aerobic conditions, DNA synthesis was not always associated with a decrease in plating efficiency (cytotoxicity) but was reflected in decreased colony size (growth rate) of the cells. Thus the aerobic "toxicity" previously reported for chronic exposure to these agents may be better described as a "cytostatic" effect. Under anaerobic conditions (where cell killing is much greater) inhibition of initiation plays a less important role, and the nitroheterocycles are metabolically reduced to intermediates which are truly cytotoxic.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahnström G., Erixon K. Radiation induced strand breakage in DNA from mammalian cells. Strand separation in alkaline solution. Int J Radiat Biol Relat Stud Phys Chem Med. 1973 Mar;23(3):285–289. doi: 10.1080/09553007314550311. [DOI] [PubMed] [Google Scholar]
- Anderson L. E., McClure W. O. An improved scintillation cocktail of high-solubilizing power. Anal Biochem. 1973 Jan;51(1):173–179. doi: 10.1016/0003-2697(73)90465-x. [DOI] [PubMed] [Google Scholar]
- Fuska J., Fusková A., Jurásek A. Effect of 5-nitrofuran derivatives on the uptake of labelled nucleic acids and protein precursors in the acid-insoluble fraction of Ehrlich ascites tumour cells. Neoplasma. 1973;20(2):171–179. [PubMed] [Google Scholar]
- Johanson K. J., Rydberg B. The effect of 60Co gamma-radiation and hydroxyurea on the in vivo chain growth of DNA in crypt cells of the small intestine of the mouse. Int J Radiat Biol Relat Stud Phys Chem Med. 1977 May;31(5):441–449. doi: 10.1080/09553007714550531. [DOI] [PubMed] [Google Scholar]
- Kikui M. Antitumor activity of the nitrofuran derivatives against Ehrlich ascites tumor. Med J Osaka Univ. 1968 Dec;19(2):127–141. [PubMed] [Google Scholar]
- Lu C., McCalla D. R. Action of some nitrofuran derivatives on glucose metabolism, ATP levels, and macromolecule synthesis in Escherichia coli. Can J Microbiol. 1978 Jun;24(6):650–657. doi: 10.1139/m78-109. [DOI] [PubMed] [Google Scholar]
- Makino F., Okada S. Effects of ionizing radiation on DNA replication in cultured mammalian cells. Radiat Res. 1975 Apr;62(1):37–51. [PubMed] [Google Scholar]
- Nakamura S., Shimizu M. Inhibition of the synthesis of macromolecules in Escherichia coli by nitrofuran derivatives. I. (5-Nitro-2-furyl)vinylpyridines. Chem Pharm Bull (Tokyo) 1973 Jan;21(1):130–136. doi: 10.1248/cpb.21.130. [DOI] [PubMed] [Google Scholar]
- Olive P. L. Inhibition of DNA synthesis by nitroheterocycles. I. Correlation with half-wave reduction potential. Br J Cancer. 1979 Jul;40(1):89–93. doi: 10.1038/bjc.1979.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olive P. L., McCalla D. R. Cytotoxicity and DNA damage to mammalian cells by nitrofurans. Chem Biol Interact. 1977 Feb;16(2):223–233. doi: 10.1016/0009-2797(77)90131-4. [DOI] [PubMed] [Google Scholar]
- Painter R. B. Rapid test to detect agents that damage human DNA. Nature. 1977 Feb 17;265(5595):650–651. doi: 10.1038/265650a0. [DOI] [PubMed] [Google Scholar]
- Painter R. B., Young B. R. X-ray-induced inhibition of DNA synthesis in Chinese hamster ovary, human HeLa, and Mouse L cells. Radiat Res. 1975 Dec;64(3):648–656. [PubMed] [Google Scholar]


