Abstract
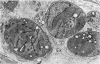
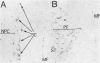
An implantation model has been used to investigate the cellular progression of chemically induced subcutaneous neoplasia in the mouse. Implantation of 3,4-benzpyrene induced persistent changes in the normal process of connective tissue formation around the implant. Light-microscope and autoradiographic studies have shown a temporal progression from aberrant filter- or muscle-associated cells through proliferative foci to large invasive sarcoma. Electron microscopy revealed that presarcomatous cell foci consisted of one of two different cell types. These were either spindle cells with ultrastructural characteristics similar to foreign-body-induced sarcoma, or cells with the ultrastructural features of rhabdomyosarcoma. The subsequent appearance of two histological groups of sarcoma that were ultrastructurally similar to the cells of the early proliferative foci indicated that both elements may progress to form tumours. However, the constituent cells of both groups of tumours displayed a broad histological and ultrastructural spectrum and the marked similarity between the undifferentiated cells of each suggested that both may have arisen from diverse differentiation of a common pluripotential cell such as the pericyte.
Full text
PDF











Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berczi I., Sehon A. H. Tumor inhibition by effector cells cultured from progressing sarcomas. Immunol Commun. 1977;6(6):617–632. doi: 10.3109/08820137709093471. [DOI] [PubMed] [Google Scholar]
- Brand K. G., Johnson K. H., Buoen L. C. Foreign body tumorigenesis. CRC Crit Rev Toxicol. 1976 Oct;4(4):353–394. doi: 10.1080/10408447609164018. [DOI] [PubMed] [Google Scholar]
- CHAMBERS V. C., WEISER R. S. ANNULATE LAMELLAE IN SARCOMA I CELLS. J Cell Biol. 1964 Apr;21:133–139. doi: 10.1083/jcb.21.1.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter R. L. Early development of injection-site sarcomas in rats: a study of tumours induced by iron-dextran. Br J Cancer. 1969 Sep;23(3):559–566. doi: 10.1038/bjc.1969.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornog J. L. The ultrastructure of leiomyoblastoma. With comments on the light microscopic morphology. Arch Pathol. 1969 Apr;87(4):404–410. [PubMed] [Google Scholar]
- Crocker D. J., Murad T. M. Ultrastructure of fibrosarcoma in a male breast. Cancer. 1969 Apr;23(4):891–899. doi: 10.1002/1097-0142(196904)23:4<891::aid-cncr2820230426>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Curtis G. L., Ryan W. L., Stenbäck F. Antibody stimulation of benzo(a)pyrene carcinogenesis. Cancer Lett. 1978 Apr;4(4):223–228. doi: 10.1016/s0304-3835(78)94677-3. [DOI] [PubMed] [Google Scholar]
- EVENSEN A. Changes in the synthesis of deoxyribonucleic acid (DNA) and in mitotic count in epidermis of hairless mice after a single application of one per cent 3:methyl-cholanthrene in benzene. A preliminary report. Acta Pathol Microbiol Scand Suppl. 1961;Suppl 148:43–52. [PubMed] [Google Scholar]
- Frable W. J., Kay S., Lawrence W., Schatzki P. F. Epithelioid sarcoma. An electron microscopic study. Arch Pathol. 1973 Jan;95(1):8–12. [PubMed] [Google Scholar]
- Friedmann I., Bird E. S. Electron-microscope investigation of experimental rhabdomyosarcoma. J Pathol. 1969 Feb;97(2):375–382. doi: 10.1002/path.1710970224. [DOI] [PubMed] [Google Scholar]
- Grasso P., Golberg L. Early changes at the site of repeated subcutaneous injection of food colourings. Food Cosmet Toxicol. 1966 Jun;4(3):269–282. doi: 10.1016/s0015-6264(66)80537-0. [DOI] [PubMed] [Google Scholar]
- Hagopian M., Spiro D. The sarcoplasmic reticulum and its association with the T system in an insect. J Cell Biol. 1967 Mar;32(3):535–545. doi: 10.1083/jcb.32.3.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hard G. C., Butler W. H. Ultrastructural analysis of renal mesenchymal tumor induced in the rat by dimethylnitrosamine. Cancer Res. 1971 Mar;31(3):348–365. [PubMed] [Google Scholar]
- Hooson J., Grasso P., Gangolli S. D. Early reactions of the subcutaneous tissue to repeated injections of carcinogens in aqueous solutions. Br J Cancer. 1971 Sep;25(3):505–515. doi: 10.1038/bjc.1971.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooson J., Grasso P., Gangolli S. D. Injection site tumours and preceding pathological changes in rats treated subcutaneously with surfactants and carcinogens. Br J Cancer. 1973 Mar;27(3):230–244. doi: 10.1038/bjc.1973.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson K. H., Ghobrial H. K., Buoen L. C., Brand I., Brand K. G. Nonfibroblastic origin of foreign body sarcomas implicated by histological and electron microscopic studies. Cancer Res. 1973 Dec;33(12):3139–3154. [PubMed] [Google Scholar]
- Karp R. D., Johnson K. H., Buoen L. C., Ghobrial H. K., Brand I., Brand K. G. Tumorigenesis by Millipore filters in mice: histology and ultrastructure of tissue reactions as related to pore size. J Natl Cancer Inst. 1973 Oct;51(4):1275–1285. doi: 10.1093/jnci/51.4.1275. [DOI] [PubMed] [Google Scholar]
- Lavelle S. M. Action of histone on neoplastic and normal growth. Ir J Med Sci. 1973 Mar;142(2):58–65. doi: 10.1007/BF02949991. [DOI] [PubMed] [Google Scholar]
- Merkow L. P., Epstein S. M., Caito B. J., Bartus B. The cellular analysis of liver carcinogenesis: ultrastructural alterations within hyperplastic liver nodules induced by 2-fluorenylacetamide. Cancer Res. 1967 Sep;27(9):1712–1721. [PubMed] [Google Scholar]
- Murad T. M., Von Haam E. Ultrastructure of myoepithelial cells in human mammary gland tumors. Cancer. 1968 Jun;21(6):1137–1149. doi: 10.1002/1097-0142(196806)21:6<1137::aid-cncr2820210615>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Purchase I. F., Longstaff E., Ashby J., Styles J. A., Anderson D., Lefevre P. A., Westwood F. R. An evaluation of 6 short-term tests for detecting organic chemical carcinogens. Br J Cancer. 1978 Jun;37(6):873–903. doi: 10.1038/bjc.1978.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purchase I. F., Longstaff E., Ashby J., Styles J. A., Anderson D., Lefevre P. A., Westwood F. R. Evaluation of six short term tests for detecting organic chemical carcinogens and recommendations for their use. Nature. 1976 Dec 16;264(5587):624–627. doi: 10.1038/264624a0. [DOI] [PubMed] [Google Scholar]
- VASILIEV J. M. Early changes in the subcutaneous connective tissue of rats after implantation of pellets containing carcinogenic polycyclic hydrocarbons. J Natl Cancer Inst. 1959 Sep;23:441–485. [PubMed] [Google Scholar]
- VASILIEV J. M., GUELSTEIN V. I. SENSITIVITY OF NORMAL AND NEOPLASTIC CELLS TO THE DAMAGING ACTION OF CARCINOGENIC SUBSTANCES: A REVIEW. J Natl Cancer Inst. 1963 Nov;31:1123–1151. [PubMed] [Google Scholar]
- VASILIEV J. M., OLSHEVSKAJA L. V., RAIKHLIN N. T., IVANOVA O. J. Comparative study of alterations induced by 7,12-dimethylbenz[a]anthracene and polymer films in the subcutaneous connective tissue of rats. J Natl Cancer Inst. 1962 Mar;28:515–559. [PubMed] [Google Scholar]