The CNS and the various endocrine organs are linked in a dynamic manner through networks of reciprocal interactions that allow for both long-term adaptation and short-term shifts in environmental conditions. These regulatory systems embrace the hypothalamus, the pituitary gland, and any of several target glands, including the adrenal gland and the thyroid. Because the anterior pituitary gland secretes at least six key hormones — adrenocorticotropin (ACTH), growth hormone (GH), prolactin (PRL), thyrotropin (TSH), and the gonadotropins follicle stimulating hormone and luteinizing hormone — such diverse parameters as cell integrity, stress responses, growth, development, reproduction, and energy homeostasis are all directly or indirectly under central control (1). In addition, largely because of the dominant role of the hypothalamo-pituitary-adrenal (HPA) axis, inflammation and immunity are also subject to neuroendocrine effects. The articles in this Perspective series explore some of the developmental, physiological, and molecular interactions occurring at the immuno-neuroendocrine interface.
Control of pituitary function
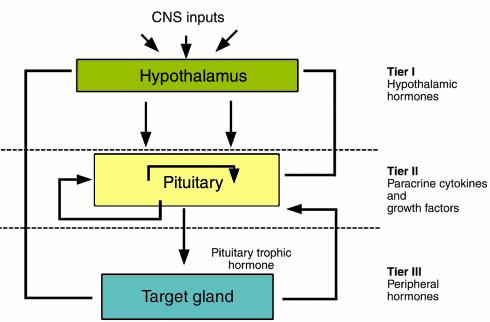
Three tiers of control subserve regulation of anterior pituitary trophic hormone secretion (2). First, the hypothalamus synthesizes and secretes releasing and inhibiting hormones, which traverse the hypothalamo-pituitary portal system and bind to specific anterior pituitary G protein−linked transmembrane cell receptors. Engagement of these receptors regulates trophic hormone synthesis and secretion, as well as pituicyte mitotic activity. Hypothalamic hormones are generated in a pulsatile fashion, in which pituitary hormone secretory profiles depend upon the portal concentrations of these peptides, as well as their controlled temporal variation. Second, autocrine, paracrine, or centrally derived intrapituitary growth factors and cytokines regulate anterior pituitary cell functions. Signaling by these molecules, which may be specific or may overlap in their targets, often occurs in concert with hypothalamic hormones. Intrapituitary cytokines act both on specific hormone gene expression, and to regulate pituitary cell development and replication. Third, trophic hormones stimulate secretion by the adrenal and thyroid glands and by the gonads, and they can also directly induce target hormones such as IGF-I. These peripheral hormones, in turn, participate in feedback regulation (usually negative) of both hypothalamic and pituitary hormone secretion (Figure 1).
Figure 1.
Model for regulation of anterior pituitary hormone secretion by three tiers of control. Hypothalamic hormones traverse the portal system and impinge directly upon their respective target cells. Intrapituitary cytokines and growth factors regulate tropic cell function by paracrine (and autocrine) control. Peripheral hormones exert negative feedback inhibition of respective pituitary trophic hormone synthesis and secretion. Reproduced with permission from Endocrine Reviews (2).
Thus, hypothalamic corticotropin-releasing hormone (CRH) induces pro-opiomelanocortin (POMC) synthesis and ACTH secretion (3). ACTH, in turn, stimulates synthesis and secretion of adrenal steroid hormones, especially cortisol. Growth hormone releasing hormone induces GH synthesis and secretion, while somatostatin (SRIF) inhibits GH release. GH, in turn, induces peripheral IGF-I synthesis. Thyrotropin-releasing hormone (TRH) induces TSH synthesis and secretion. TSH acts as the trophic signal for thyroid hormone synthesis and secretion. Gonadotropin-releasing hormone (GnRH) elicits FSH and LH secretion, and FSH and LH regulate gonadal steroidogenesis and germ cell function. In contrast, hypothalamic dopamine is a tonic suppressor of PRL secretion.
Stress, inflammation, and ACTH
Commitment of anterior pituitary cells to differentiated function follows spatially and temporally regulated transcription factor expression. Cells of the primitive Rathke’s pouch undergo proliferation and determination into functional lineages, which are responsible for cell-specific hormone expression. The T-pit1/Tbx19 transcription factor appears critical for cell-specific determination of POMC-expressing pituitary cells (4, 5), the earliest differentiated pituitary hormone–expressing cells to appear. Fetal POMC expression and ACTH immunoreactivity are detected at 6 weeks.
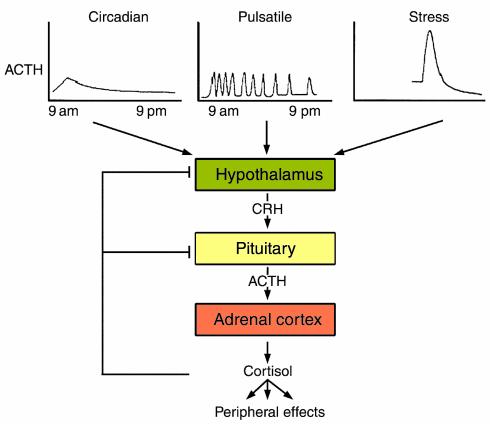
The primary protein product of the POMC gene undergoes posttranslational processing to yield the N-terminal cleavage product, ACTH, as well as β lipoprotein and β endorphin (6). Multiple hypothalamic, pituitary, and peripheral factors allow for stress-mediated or inflammation-induced POMC regulation and ACTH secretion. POMC gene expression is induced by hypothalamic CRH, vasopressin, catecholamines, and cytokines, and is suppressed by glucocorticoids. The CRH type 1 receptor, predominantly expressed on the corticotroph (7), activates cAMP, protein kinase A, and CREB, and also mitogen-activated protein kinase, leading to POMC transcription. Essential cellular functions maintaining metabolic and endocrine control require a homeostatic, nonstressed pattern of ACTH and glucocorticoid secretion, which can readily respond to counteract life-threatening insults. ACTH secretion is characterized by both circadian periodicity and ultradian pulsatility (Figure 2) generated by CRH release and also influenced by peripheral corticosteroids. ACTH secretion peaks before 7 am and nadir adrenal steroid secretion occurs between 11 pm and 3 am, with periodic secretory bursts occurring throughout the day. These rhythms are entrained by visual cues and the light-dark cycle, and stress results in increased ACTH pulse amplitude (8). Adrenal ACTH receptors signal via adenyl cyclase to regulate adrenal gland size, structure, and function. p450 enzyme transcription, cortisol, aldosterone, 17-OH progesterone, and, to a minimal extent, adrenal androgens are induced by ACTH. The neuroendocrine and immune systems also communicate bidirectionally, and stress-induced glucocorticoids, catecholamines, and CRH mediate peripheral immune responses (9).
Figure 2.
Mechanisms regulating the rhythmicity of the HPA axis. The acute stress response of ACTH secretion is reflected by enhanced pulse amplitude that declines rapidly. Adapted from ref. 28.
In their article in this Perspective series, Shanks and Lightman review the impact of intrauterine stress and the neuro-immune interface on HPA axis development. The role of two critical signaling molecules mediating the neuro-immune endocrine interface, gp130 and suppressor of cytokine signalling–3 (SOCS-3), are also discussed in the articles to follow in this series. Cytokine gp130 receptor signaling activates the HPA axis even in the absence of CRH (10), while SOCS-3 appears to play a critical role in inhibiting cytokine-mediated POMC induction and ACTH secretion. Signaling of these two molecules thus serves to transduce immuno-neuroendocrine responses to stress, in concert with other central factors, especially CRH (3).
Neuroendocrine modulation of cytokine signaling
The ubiquitous expression of cytokines and their respective receptors reflects their protean tissue functions, as well as unique cell-specific actions. These cell signaling molecules regulate critical tissue activities including hematopoietic, immune, neural, bone, reproductive, and metabolic systems. Structural and functional overlap also results in redundant cytokine family actions that complicate interpretation of selective transgenic knockout models. Cytokines may function as classic endocrine secretions emanating from a proximal tissue, traversing the circulation and impacting a distal target (11). They also commonly behave as paracrine or autocrine cell regulators mediating neighborhood functions.
As immuno-neuroendocrine modulators, cytokines act to transduce signals interfacing peripheral and central stress and inflammation with the hypothalamo-pituitary axes (12). Cytokines and their receptors are expressed centrally in the hypothalamus, as well as within anterior pituitary cells. Transduction of signals at this interface is mediated by cytokines acting as auto/paracrine factors to regulate pituitary development, cell proliferation, and hormone secretion. The interplay of central cytokines is complex and can involve the mutual stimulation of systemic as well as brain-derived cytokines. Specifically, the proinflammatory gp130 cytokine family participates in regulation of ACTH and mediates the immuno-neuroendocrine interface (11, 13). As discussed by Arzt in this Perspective series, ligands for the gp130 receptor cytokine family (13) signal via common intracellular molecules and often exert redundant functions. The two inducers of corticotroph POMC, CRH and the gp130 cytokines, act in synergy and signal through the cAMP and the JAK/STAT/SOCS pathways, respectively. The neuroendocrine stress response thus is reflective of complex intrapituitary cross-talk between signaling pathways.
The neuro-immune interface
HPA activation during inflammation is an important protective mechanism, as the resultant induction of endogenous corticosteroids restricts the immune reaction (6) and mediates lymphocyte compartmentalization. Cytokines, including IL-1, IL-6, leukemia inhibitory factor (LIF), and TNF, participate as mediators of the complex HPA response to inflammation (12). LIF, a member of the gp130 receptor cytokine family (14), mediates the HPA response to stress and inflammation by inducing POMC expression and ACTH secretion in the course of the inflammatory process (13). Mice in whom the gene for LIF has been deleted (LIFKO; see ref. 15) mount an attenuated ACTH and corticosterone response to stressors. LIF replacement, conversely, restores ACTH levels in stressed LIFKO mice (13). LIF administered prior to lethal Escherichia coli–induced septic shock has a protective effect, preventing sepsis-induced tissue injury and lowering mortality (16). LIF is also required for appropriate inflammatory responses to injury (17).
Signals transducing neuroendocrine stress response originate from several sources. Peripherally derived cytokines cross the blood-brain barrier and act upon the hypothalamus and pituitary, where they regulate pituitary POMC and ACTH expression in response to peripheral inflammation. The diffusible form of murine LIF is induced in the hypothalamus and pituitary after LPS injection (18), and brain IL-6 and gp130 are similarly induced after LPS or IL-1 injections (19). LIF is expressed in human corticotropes, further suggesting a paracrine role for pituitary cytokines. Peripheral inflammation may therefore directly or indirectly induce central hypothalamic and/or pituitary production of IL-6–like cytokines (11). Hypothalamic cytokines may also migrate through portal vessels and stimulate pituitary POMC gene expression and ACTH secretion in synergy with hypothalamic CRH. In addition, POMC is activated in LIFKO mice after intraperitoneal LIF treatment, suggesting that circulating cytokines can affect the ACTH axis during inflammation. Although elevated blood LIF levels have been described in patients with giant cell arteritis and septic shock (20), it is unclear whether elevated circulating LIF levels are sufficient to account for triggering the HPA response. Regardless of the source of pituitary-active cytokines, peripheral inflammatory cytokines clearly impact on central HPA function.
Inflammatory stressors also directly affect CRH and vasopressin, as well as inducible proinflammatory cytokines, and also function as ACTH regulators. For example, intramuscular turpentine injection in rats increases circulating IL-6 levels and elevates ACTH (12). Inhibitors of POMC likewise participate in the inflammatory responses. In particular, glucocorticoids, which feed back to regulate CRH and POMC, directly modulate the release of nitric oxide and other central secondary messengers (9, 21). Changes in hypothalamic CRH also occur as a result of negative feedback regulation by stress-induced circulating glucocorticoids. For this reason, hypothalamic CRH declines on day 14 after the onset of chronic autoimmune CFA-induced arthritis (22).
By stimulating the HPA axis, cytokines also antagonize their own peripheral proinflammatory action (23). Excessive HPA axis stimulation leads to immunosuppression and therefore to increased susceptibility to infection. Hypothalamic cAMP-inducing neuropeptides, such as CRH, PACAP, or epinephrine, whose production is enhanced after stress stimuli, also induce SOCS-3 expression and counterbalance the excessive stimulatory effect of cytokines on the HPA axis, thus helping to maintain immuno-neuroendocrine homeostasis (24). Long-term physiologic stress responses are achieved by integration of the autonomic nervous system, HPA, cardiovascular, metabolic, and immune protective responses. Achieving this dynamic stability, or stress allostasis, requires considerable tissue “wear and tear” (25) resulting from sustained overactivity (or underactivity) of these systems. Thus, an appropriate stress response is initiated by a stressor, sustained for an appropriate time interval, and then switched off, allowing for a recovery period. Repeated stressors may result in lack of adaptation, prolonged responses with no recovery period, or inadequate responses (25). Negative regulation of pituitary cytokine function is therefore critical to prevent excess or prolonged HPA stimulation. Several regulators have been characterized that act at different levels of the cytokine-induced JAK2-STAT3-POMC-ACTH cascade (13). SOCS-3 (26), an inhibitor of cytokine action, is potently induced by LIF in the pituitary (27), providing a mechanism enabling corticotrope plasticity with fast induction and suppression of ACTH secretion. The central role of SOCS-3 is the topic of Auernhammer and Melmed in the final article in this Perspective series.
References
- 1.Melmed, S. 2001. Disorders of the anterior pituitary and hypothalamus. In Harrison’s principles of internal medicine. E. Braunwald et al., editors. McGraw-Hill. New York, New York, USA. 2029–2052.
- 2.Ray D, Melmed S. Pituitary cytokine and growth factor expression and action. Endocr Rev. 1997;18:206–228. doi: 10.1210/edrv.18.2.0297. [DOI] [PubMed] [Google Scholar]
- 3.Vale W, Spiess J, Rivier C, Rivier J. Characterization of a 41-residue ovine hypothalamic peptide that stimulates secretion of corticotropin and beta-endorphin. Science. 1981;213:1394–1397. doi: 10.1126/science.6267699. [DOI] [PubMed] [Google Scholar]
- 4.Lamolet B, et al. A pituitary cell-restricted T box factor, Tpit, activates POMC transcription in cooperation with Pitx homeoproteins. Cell. 2001;104:849–859. doi: 10.1016/s0092-8674(01)00282-3. [DOI] [PubMed] [Google Scholar]
- 5.Liu J, et al. Tbx19, a tissue-selective regulator of POMC gene expression. Proc Natl Acad Sci USA. 2001;98:8674–8679. doi: 10.1073/pnas.141234898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chrousos GP. The hypothalamic-pituitary-adrenal axis and immune-mediated inflammation. N Engl J Med. 1995;332:1351–1362. doi: 10.1056/NEJM199505183322008. [DOI] [PubMed] [Google Scholar]
- 7.Arai M, Assil IQ, Abou-Samra AB. Characterization of three corticotropin-releasing factor receptors in catfish: a novel third receptor is predominantly expressed in pituitary and urophysis. Endocrinology. 2001;142:446–454. doi: 10.1210/endo.142.1.7879. [DOI] [PubMed] [Google Scholar]
- 8.Dorin RI, et al. Assessment of stimulated and spontaneous adrenocorticotropin secretory dynamics identifies distinct components of cortisol feedback inhibition in healthy humans. J Clin Endocrinol Metab. 1996;81:3883–3891. doi: 10.1210/jcem.81.11.8923833. [DOI] [PubMed] [Google Scholar]
- 9.Karalis K, Muglia LJ, Bae D, Hilderbrand H, Majzoub JA. CRH and the immune system. J Neuroimmunol. 1997;72:131–136. doi: 10.1016/s0165-5728(96)00178-6. [DOI] [PubMed] [Google Scholar]
- 10.Bethin KE, Vogt SK, Muglia LJ. Interleukin-6 is an essential, corticotropin-releasing hormone-releasing hormone-independent stimulator of the adrenal axis during immune system activation. Proc Natl Acad Sci USA. 2000;97:9317–9322. doi: 10.1073/pnas.97.16.9317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reichlin S. What’s in a name or what does leukemia inhibitory factor have to do with the pituitary gland? Endocrinology. 1998;139:2199–2200. doi: 10.1210/endo.139.5.6079. [DOI] [PubMed] [Google Scholar]
- 12.Turnbull AV, Rivier CL. Regulation of the hypothalamic-pituitary-adrenal axis by cytokines: actions and mechanisms of action. Physiol Rev. 1999;79:1–71. doi: 10.1152/physrev.1999.79.1.1. [DOI] [PubMed] [Google Scholar]
- 13.Auernhammer CJ, Melmed S. Leukemia-inhibitory factor-neuroimmune modulator of endocrine function. Endocr Rev. 2000;21:313–345. doi: 10.1210/edrv.21.3.0400. [DOI] [PubMed] [Google Scholar]
- 14.Gearing DP, et al. Molecular cloning and expression of cDNA encoding a murine myeloid leukemia inhibitory factor (LIF) EMBO J. 1987;6:3995–4002. doi: 10.1002/j.1460-2075.1987.tb02742.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stewart CL, et al. Blastocyst implantation depends on maternal expression of leukaemia inhibitory factor. Nature. 1992;359:76–79. doi: 10.1038/359076a0. [DOI] [PubMed] [Google Scholar]
- 16.Waring PM, Waring LJ, Billington T, Metcalf D. Leukemia inhibitory factor protects against experimental lethal Escherichia coli septic shock in mice. Proc Natl Acad Sci USA. 1995;92:1337–1341. doi: 10.1073/pnas.92.5.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sugiura S, et al. Leukaemia inhibitory factor is required for normal inflammatory responses to injury in the peripheral and central nervous systems in vivo and is chemotactic for macrophages in vitro. Eur J Neurosci. 2000;12:457. doi: 10.1046/j.1460-9568.2000.00922.x. [DOI] [PubMed] [Google Scholar]
- 18.Wang Z, Ren SG, Melmed S. Hypothalamic and pituitary leukemia inhibitory factor gene expression in vivo: a novel endotoxin-inducible neuro-endocrine interface. Endocrinology. 1996;137:2947–2953. doi: 10.1210/endo.137.7.8770918. [DOI] [PubMed] [Google Scholar]
- 19.Lebel E, Vallieres L, Rivest S. Selective involvement of interleukin-6 in the transcriptional activation of the suppressor of cytokine signaling-3 in the brain during systemic immune challenges. Endocrinology. 2000;141:3749–3763. doi: 10.1210/endo.141.10.7695. [DOI] [PubMed] [Google Scholar]
- 20.Waring P, Wycherley K, Cary D, Nicola N, Metcalf D. Leukemia inhibitory factor levels are elevated in septic shock and various inflammatory body fluids. J Clin Invest. 1995;90:2031–2037. doi: 10.1172/JCI116083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Turnbull AV, Rivier C. Corticotropin-releasing factor, vasopressin, and prostaglandins mediate, and nitric oxide restrains, the hypothalamic-pituitary-adrenal response to acute local inflammation in the rat. Endocrinology. 1996;137:455–463. doi: 10.1210/endo.137.2.8593789. [DOI] [PubMed] [Google Scholar]
- 22.Harbuz MS, et al. Evidence for altered control of hypothalamic CRF in immune-mediated diseases. Ann NY Acad Sci. 1995;771:449–458. doi: 10.1111/j.1749-6632.1995.tb44701.x. [DOI] [PubMed] [Google Scholar]
- 23.Sternberg EM. Neural-immune interactions in health and disease. J Clin Invest. 1997;100:2641–2647. doi: 10.1172/JCI119807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bousquet C, Chesnokova V, Kariagina A, Ferrand A, Melmed S. cAMP neuropeptide agonists induce pituitary suppressor of cytokine signaling-novel negative feedback mechanism for corticotroph cytokine action. Mol Endocrinol. 2001;3:1880–1890. doi: 10.1210/mend.15.11.0733. [DOI] [PubMed] [Google Scholar]
- 25.McEwen BS. Protective and damaging effects of stress mediators. N Engl J Med. 1998;338:171–179. doi: 10.1056/NEJM199801153380307. [DOI] [PubMed] [Google Scholar]
- 26.Hilton DJ, et al. Twenty proteins containing a C-terminal SOCS box form five structural classes. Proc Natl Acad Sci USA. 1998;95:114–119. doi: 10.1073/pnas.95.1.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Auernhammer CJ, Bousquet C, Melmed S. Autoregulation of pituitary corticotroph SOCS-3 expression: characterization of the murine SOCS-3 promoter. Proc Natl Acad Sci USA. 1999;96:6964–6969. doi: 10.1073/pnas.96.12.6964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.White, A., and Ray, D. 2001. Adrenocorticotropic hormone. In Endocrinology. L.J. DeGroot and J.L. Jameson, editors. W.B. Saunders Co. Philadelphia, Pennsylvania, USA. 221–234.