Abstract
Background
Short Latency Somatosensory Evoked Potentials (SEPs) may serve to the testing of the somatosensory tract function, which is vulnerable and affected in vascular encephalopathy. The aim of the current study was to search for clinical and neuroimaging correlates of abnormal SEPs in vascular dementia (VD) patients.
Materials and Methods
The study included 14 VD patients, aged 72.93 ± 4.73 years, and 10 controls aged 71.20 ± 4.44 years. All subjects underwent a detailed clinical examination, blood and biochemical testing, brain MRI and were assessed with the MMSE. SEPs were recorded after stimulation from upper and lower limbs. The statistical Analysis included 1 and 2-way MANCOVAs and Factor analysis
Results
The N13 latency was significantly prolonged, the N19 amplitude was lower, the P27 amplitude was lower and the N11-P27 conduction time was prolonged in severely demented patients in comparison to controls. The N19 latency was prolonged in severely demented patients in comparison to both mildly demented and controls. The same was true for the N13-N19 conduction time, and for the P27 latency. Patients with subcortical lesions had all their latencies prolonged and lower P27 amplitude.
Discussion
The results of the current study suggest that there are significant differences between patients suffering from VD and healthy controls in SEPs, but these are detectable only when dementia is severe or there are lesions located in the subcortical regions. The results of the current study locate the abnormal SEPs in the white matter, and are in accord with the literature.
Keywords: vascular dementia, SEPs, MRI, subcortical
Background
Vascular dementia (VD) is the second most frequent type of dementia in the elderly. It may be the result of multiple embolic or thrombotic ishaemic infarcts in the cortex or in subcortical structures. However, it has been well documented that dementia may be caused by hypertension, diffuse cerebral ischaemia or any other cause that may have an adverse effect on cerebral blood flow [1]. Lacunar encephalopathy, due to chronic hypertension or atherosclerosis, may lead to dementia also known as 'subcortical atherosclerotic encephalopathy' (Binswanger's disease) [2].
Although Computerized Tomography (CT) and Magnetic Resonance Imaging (MRI) may provide a detailed image of brain lesions, in many instances their findings are in contrast to the clinical picture [3]. Short Latency Somatosensory Evoked Potentials (SEPs) may serve to the testing of the somatosensory tract function, which is vulnerable and affected in vascular encephalopathy. It has been reported that SEPs are affected to a varied degree in various types of dementia, but the exact cause for this remains elusive [4-8].
The aim of the current study was to search for clinical and neuroimaging correlates of abnormal SEPs in VD patients.
Materials and Methods
The study included 14 patients (6 males, 8 females) that fulfilled criteria for dementia and vascular dementia (VD) according to ICD-10 [9], DSM-IV. [10], and NICNS-AIREN [11-16] criteria and not for Alzheimer's disease (AD) according to NINCDS-ADRDA criteria [17]. Their age was 72.93 ± 4.73 years. The control group included 10 subjects (5 males, 5 females) without symptoms of dementia or any symptoms that could be attributed to a disease affecting the somatosensory tract. Their age was 71.20 ± 4.44 years
All subjects underwent a detailed clinical neurological examination, blood and biochemical testing, brain MRI and were assessed with the Mini-Mental State Examination (MMSE).
The demented patients were classified as mildly demented (MMSE>15) and severely demented (MMSE<16) on the basis of their MMSE scores.
Their upper and lower limbs peripheral conduction was examined (conduction velocity, f-wave) to exclude peripheral problems. SEPs were recorded after stimulation from upper and lower limbs.
In order to elicit and record SEPs, the following method was applied:
i) Upper limbs: Electrical stimulation of the median nerve at the wrist and recording from surface electrodes placed 1. at the Erb point, 2. at the C6–C7 interspinous space and 3. at the somatosensory area of the parietal lobe contralateral to the limb stimulated (C3' or C4' according to the 10–20 system). An electrode placed at Fz served as the reference for all the above recordings.
ii) Lower limbs: Electrical stimulation of the peroneal nerve at the knee and recording from surface electrodes placed 1. for lumbar potentials (LP or N11) at the L1–L2 interspinous space and with the reference electrode placed two interspinous spaces higher, 2. for cortical potentials (P27) at the Cz (scalp) and with the Fz as the reference (according to the 10–20 system)
The duration of the electrical stimulation was 200 μsec, and the frequency 2 p/sec. The intensity was enough to cause constriction of the respective muscles. In order to obtain a better SEPs recording, 512 stimuli were applied. The filters used were set at 0.8 Hz low cut-off (high pass) and at 1KHz high cut-off (low pass). The amplifier gain was set to 20 μV/div. The analysis time was 50 msec for upper limbs and for lower limbs 30 msec for lumbar potentials and 100 msec for cortical potentials. To verify the reliability of the results, all recordings were performed twice.
N9 and LP (N11) were assessed only in order to exclude a peripheral problem that would affect the results. The following waveforms were assessed and measured and subsequently used in the statistical analysis: Upper limbs: N13 and N19. Lower Limbs: P27. Also the conduction time N13-N19 and LP (N11)-P27 were also measured.
In order to consider a recording as abnormal, its latency should exceed 2.5 standard deviations of the controls and its amplitude should be lower than the lowest amplitude found in controls.
Statistical Analysis
The statistical analysis included 1 and 2-way MANCOVAs with age as covariate (three analyses in total), in order to test for differences in the results of the psychophysiological testing in the groups 1. demented vs. controls 2. defined by the severity of dementia (no dementia, mild dementia, severe dementia) as well as 3. by MRI findings (normal, cortical lesions, subcortical lesions, both cortical and subcortical). Because MRI was coded in a 'qualitative' manner, two new dummy variables were created ('cortical lesions' yes/no, 'subcortical lesions' yes/no) and used in the analysis and as covariates. When groups defined by the severity of dementia were tested, MRI findings were added as a covariate; when MRI-defined groups were tested, the severity of dementia was added as a covariate. The Bonferonni correction demanded to set the significance level at p < 0.0166 (0.05/3 = 0.0166).
Also, Factor analysis (principal components analysis) was performed to the data with varimax normalized rotation. The dummy variables for MRI were used. This analysis was performed for exploratory reasons only, since the ratio cases-to-variables was low (24:11 = 2.18)
Results
The composition of the study sample, the size of groups as well as the mean and standard deviation of all the results in the various groups are shown in table 1.
Table 1.
Descriptive statistics. Latency and amplitude of SEPs parameters in controls and in the various patients groups
|
controls N = 10 |
Demented N = 14 |
mild dementia N = 4 |
severe dementia N = 10 |
Subcortical N = 3 |
Cortical N = 3 |
both cortical and subcortical N = 8 |
||||||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | |
| N13 Latency | 12.96 | 0.27 | 13.87 | 1.25 | 13.08 | 0.73 | 14.19 | 1.30 | 13.47 | 0.59 | 13.17 | 0.86 | 14.29 | 1.45 |
| N13 Amplitude | 2.56 | 0.32 | 2.09 | 0.91 | 2.75 | 0.44 | 1.83 | 0.92 | 2.68 | 0.57 | 2.50 | 0.46 | 1.72 | 0.99 |
| N19 Latency | 18.19 | 0.48 | 20.19 | 1.91 | 18.33 | 0.61 | 20.94 | 1.72 | 19.93 | 1.50 | 18.40 | 0.72 | 20.96 | 1.97 |
| N19 Amplitude | 2.27 | 0.22 | 1.16 | 0.97 | 1.86 | 0.98 | 0.88 | 0.86 | 1.73 | 1.32 | 0.95 | 1.01 | 1.03 | 0.88 |
| N13-19 Latency | 5.23 | 0.36 | 6.29 | 0.93 | 5.25 | 0.17 | 6.70 | 0.75 | 6.47 | 0.92 | 5.23 | 0.15 | 6.61 | 0.86 |
| P27 Latency | 27.28 | 0.29 | 30.59 | 2.64 | 28.40 | 1.91 | 31.46 | 2.43 | 31.47 | 3.65 | 27.43 | 0.49 | 31.44 | 1.91 |
| P27 Amplitude | 1.78 | 0.14 | 0.96 | 0.77 | 1.02 | 0.91 | 0.94 | 0.77 | 1.30 | 0.87 | 1.33 | 0.98 | 0.70 | 0.66 |
| N11-P27 Latency | 16.66 | 0.45 | 19.91 | 2.74 | 17.88 | 1.97 | 20.73 | 2.64 | 20.73 | 4.05 | 17.10 | 0.46 | 20.66 | 2.21 |
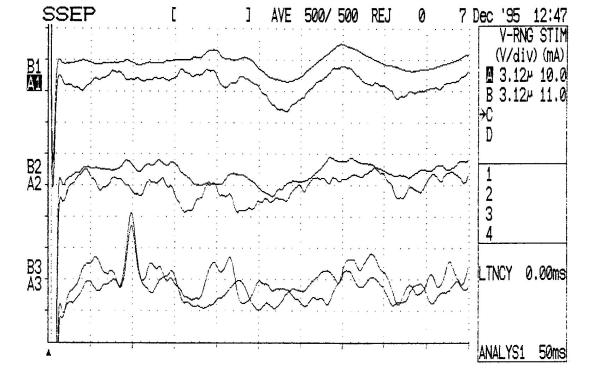
A characteristic MRI of a patient with VD is shown in figure 1, and the respected SEPs curves are shown in figure 2.
Figure 1.
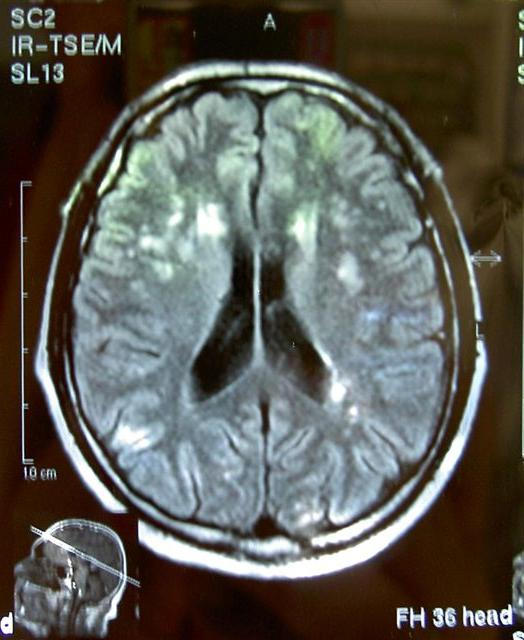
Brain MRI, T2 sequence: multiple cortical and subcortical infracts in a vascular dementia patient
Figure 2.
Abnormal central SSEPs (N13, N19) in a vascular dementia patient
All patients and controls had normal findings (taking into consideration their age) concerning the peripheral nerves. Abnormal SEPs suggesting a central nervous system dysfunction were present in 11 out of 14 (78.57%) VD patients but in none of the control subjects. More specifically, in 3 patients (21.42%) there was an abnormal N13 (both amplitude and latency), in 10 (71.48%) cortical P19 and P27 were abnormal (amplitude and/or latency) and in 10 (71.48%) there was a prolongation of the central conduction both from upper and lower limbs. Finally, in 3 patients with an abnormal N13 and cortical lesions which were detected with the MRI, the central conduction time was especially prolonged.
The quantified analysis suggested that the severity of dementia and the localization of the lesions are significant factors affecting SEPs (tables 2,3,4,5).
Table 2.
1-way MANCOVA with age and MRI findings as covariates for the comparison between the three clinical groups defined by the severity of dementia (no dementia, mild, severe) concerning their psychophysiological assessement. After bonferonni correction: p = 0.003
| Wilks' Lambda | Rao's R | df 1 | df 2 | p-level | |
| 1 | 0.066 | 3.96 | 16 | 22 | 0.001 |
Table 3.
Sheffe Post-hoc test: Only significant results are reported.
| Variable: N13L | Variable: N19A | ||||
| N | MD | N | MD | ||
| N | N | ||||
| MD | 0.9784 | MD | 0.6184 | ||
| SD | 0.0266 | 0.1546 | SD | 0.0010 | 0.0811 |
| Variable: N19L | Variable: N13-19L | ||||
| N | MD | N | MD | ||
| N | N | ||||
| MD | 0.9827 | MD | 0.9982 | ||
| SD | 0.0003 | 0.0065 | SD | 0.0000 | 0.0011 |
| Variable: P27L | Variable: P27A | ||||
| N | MD | N | MD | ||
| N | N | ||||
| MD | 0.5715 | MD | 0.1507 | ||
| SD | 0.0002 | 0.0281 | SD | 0.0261 | 0.9799 |
| Variable: N11-P27L | |||||
| N | MD | ||||
| N | |||||
| MD | 0.5671 | ||||
| SD | 0.0005 | 0.0612 | |||
no dementia: N mild dementia: MD severe dementia: SD L: Latency, A: Amplitude
Table 4.
2-way MANCOVA analysis with age and severity of dementia as covariates for the comparison between the four clinical groups defined by MRI findings concerning their psychophysiological assessment.
| Wilks' Lambda | Rao's R | df 1 | df 2 | p-level | p-level After bonferonni correction | |
| 1 | 0.26 | 3.85 | 8 | 11 | 0.0212 | 0.0636 |
| 2 | 0.13 | 9.23 | 8 | 11 | 0.0006 | 0.0036 |
| 12 | 0.36 | 2.42 | 8 | 11 | 0.0882 | 0.2646 |
factors: 1: MRI coritcal lesions yes/no (1/0) 2: MRI subcortical lesions yes/no (1/0)
Table 5.
Sheffe Post-hoc test. Only significant results are reported.
| Variable | P |
| N13 latency | 0.04974 |
| N13 amplitude | 0.22710 |
| N19 latency | 0.00037 |
| N19 amplitude | 0.40134 |
| N13-N19 latency | 0.00001 |
| P27 latency | <0.00001 |
| P27 amplitude | 0.03572 |
| N11-P27 latency | 0.00006 |
Comparison between demented and controls
No significant differences were detected.
Comparison between controls and two groups of demented patients (mild dementia and severe dementia)
The latency of N13 was significantly prolonged in severely demented patients in comparison to controls. The amplitude of N19 was lower in severely demented patients in comparison to controls, while N19 latency was prolonged in severely demented patients in comparison both to mildly demented and controls. The same was true for the conduction time between N13-N19, and for the P27 latency. The P27 amplitude was lower and the N11-P27 conduction time was prolonged in severely demented patients in comparison to controls (tables 2 and 3).
The above results suggest that mildly demented patients did not differ from controls in any one of the measurements. No group differed from the other two concerning the earliest waves (N9 and LP/N11) parameters. Severely demented patients differed from controls in N13 latency and N19 and P27 amplitude. These patients differed both from mildly demented and from controls concerning N19 and P27 latency and N13-N19 latency time. So these results point to a gradual separation between groups. Latency is the first characteristic to differ, and amplitude follows. Severely demented patients separate first from controls, and latter from mildly demented. Mildly demented patients never separate from controls.
Comparison between controls and three groups of demented patients defined by MRI findings (normals, cortical lesions, subcortical lesions, both cortical and subcortical lesions)
The localization of the lesions seems to be very important. Only subcortical lesions seem to affect SEPs, and more specifically, patients with subcortical lesions had all their latencies prolonged and lower P27 amplitude (tables 4 and 5). It is important that this specific distinction between subjects with subcortical lesions and those without (this group includes both controls and patients with cortical lesions alone) is the only one that emerges.
An important question is whether the above results reflect a general and confounding effect of the 'severity' or of the 'localization of the lesions', since more severely demented patients tended to have both cortical and subcortical lesions and vice versa. However the use of covariates may provide an answer to this question. The results suggest that both the severity and the localization of lesions affect SEPs results in VD patients independently from each other.
The factor analysis (principal components analysis) of data with varimax normalized rotation produced a three-factor model which explained 86% of the total variance (table 6). This analysis confirmed the lack of relationship of N13 to VD. What is very interesting is the fact that amplitudes load at the same factor with cortical lesions while latencies load together with subcortical lesions, a finding which is more or less reasonable.
Table 6.
Factor analysis (principal components analysis) of results with varimax normalized rotation. The two-factor model explains 79% of variance
| Factor 1 | Factor 2 | Factor 3 | |
| N13 latency | 0.40 | 0.86 | 0.18 |
| N13 amplitude | -0.12 | -0.89 | -0.34 |
| N19 latency | 0.67 | 0.68 | 0.25 |
| N19 amplitude | -0.32 | -0.36 | -0.75 |
| N13-19 latency | 0.83 | 0.34 | 0.25 |
| P27 latency | 0.90 | 0.25 | 0.28 |
| P27 amplitude | -0.42 | -0.23 | -0.68 |
| N11-P27 latency | 0.87 | 0.30 | 0.24 |
| Severity of dementia | 0.66 | 0.18 | 0.61 |
| Cortical lesions in MRI | 0.17 | 0.17 | 0.87 |
| Subcortical lesions in MRI | 0.83 | 0.12 | 0.33 |
| Explained Variable | 4.29 | 2.51 | 2.67 |
| Proportion of total variance | 39% | 23% | 24% |
| Total variance explained | 86% |
Discussion
The results of the current study suggest that there are significant differences between patients suffering from VD and healthy controls in SEPs, but these are detectable only when dementia is severe or there are lesions located in the subcortical regions, which, however, is usual in this type of dementia. Milder forms of dementia or cortical lesions may not affect SEPs to a detectable degree.
Dementia is a common condition in the elderly. In spite of the considerable advances in this field, many times it is difficult to diagnose the disorder and to specify the type of dementia. Even the most elaborate methods in neuropsychology may not differentiate between VD and AD (which many times co-exist), while CT and MRI may provide with impressive information concerning brain structures, but many times fail to solve the problem, because although vascular lesions are not uncommon in the elderly, they do not always lead to dementia. This problem of diagnosis and differential diagnosis is much worse concerning earlier stages and milder cases.
The current study assumed that SEPs could be a valuable non-invasive method which may be useful in the assessment of the elderly and may provide important information for the diagnosis and differential diagnosis of dementia [4], since it can assess the functioning of the central somatosensory tract at both at the cortical and subcortical level [18]. However the results of the current study suggest that SEPs can be used to trace mainly subcortical lesions.
The comparison of SEPs with imaging methods (MRI) was not the aim of the current study, since MRI findings were a key element to arrive to the diagnosis of VD. That's why all VD patients had vascular lesions. Therefore, by definition, concerning the current study sample, MRI had 100% sensitivity for dementia cases. The aim of the current study was to locate the possible sources for SEPs disturbances in VD.
The results of the current study locate the abnormal SEPs and especially the prolongation of the central conduction time both from upper and lower limbs in the white matter of the Central Nervous System of VD patients. This is not peculiar however, since imaging studies locate most lesions at the subcortical level [19]. These results are in accord with the literature [5,7,8].
On the other hand, it is reported that SEPs are normal in AD patients [20]. It is also well documented that in AD patients the brain pathology is mainly located in the cortex, in contrast to VD patients. Thus, SEPs may be normal or less affected in AD, in comparison to VD [21-24].
Apart from brain ichaemic lesions, in VD, lesions may be found in the spinal cord. The presence of an abnormal N13 (upper limbs) as well as the prolongation of conduction time from lower limbs in some VD patients are suggestive of the presence of lesions in the dorsal structures of the spinal cord (columns and nuclei) in these patients [25]. In the current study, normal N9 and N11 precluded the presence of a peripheral lesion.
A serious disadvantage of the current study is the small study group. However this disadvantage may be partially compensated by the rigorous methodology and the fact that the results are clear and straightforward. Another drawback is the fact that the patients included in the study were receiving various medications which potentially may affect SEPs recordings. However, no systematic bias was present.
Conclusion
Short-latency Somatosensory Evoked Potentials may constitute a valuable tool for the assessment of demented patients and for the differential diagnosis of dementia. They even constitute an important method for the assessment and early detection of silent subcortical lesions caused by atherosclerotic risk factors. SEPs are more economic than MRI but whether they also constitute a more sensitive tool for the detection of these lesions, needs further and better focused research.
Competing interests
None declared.
Contributor Information
Iacovos Tsiptsios, Email: yianna@law.auth.gr.
Konstantinos N Fountoulakis, Email: kfount@med.auth.gr.
Konstantinos Sitzoglou, Email: kfount@med.auth.gr.
Anastasia Papanicolaou, Email: yianna@law.auth.gr.
Konstantinos Phokas, Email: kfount@med.auth.gr.
Fotis Fotiou, Email: kfount@med.auth.gr.
George St Kaprinis, Email: kaprinis@med.auth.gr.
References
- Chan R, Hachinski V. Current Therapy in Neurologic Disease. fifth. 1997. The other Dementias. pp. 311–314. [Google Scholar]
- Marsen D, Fowler T. Clinical Neurology. Second. 1998. Dementia. pp. 225–243. [Google Scholar]
- Loizou LA, Kendal BE, Marshall J. Subcortical arteriosclerotic encephalopathy; A clinical and radiological investigation. Journal of Neurology Neurosurgery and Psychiatry. 1981;44:294–304. doi: 10.1136/jnnp.44.4.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abbruzzese G, Reni L, Ratto S, Favale F. Short-latency somatosensory evoked potentials in degenerative and vascular dementia. Journal of Neurology Neurosurgery and Psychiatry. 1984;47:1034–1037. doi: 10.1136/jnnp.47.9.1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferri R, Del Gracco S, Elia M, Musumeci SA, Spada R, Stefanini MC. Scalp topographic mapping of middle-latency somatosensory evoked potentials in normal aging and dementia. Neurophysiological Clinics. 1996;26:311–319. doi: 10.1016/S0987-7053(97)85098-8. [DOI] [PubMed] [Google Scholar]
- Ito J. Somatosensory event-related potentials (ERPs) in patients with different types of dementia. Journal of Neurological Science. 1994;121:139–146. doi: 10.1016/0022-510X(94)90342-5. [DOI] [PubMed] [Google Scholar]
- Kato H, Sugawara Y, Ito H, Kogure K. White matter lucencies in multi-infarct dementia: a somatosensory evoked potentials and CT study. Acta Neurologica Scandiinavica. 1990;81:181–183. doi: 10.1111/j.1600-0404.1990.tb00959.x. [DOI] [PubMed] [Google Scholar]
- Okuda B, Tachibana H, Takeda M, Kawabata K, Sugita M. Visual and somatosensory evoked potentials in Parkinson's and Binswanger's disease. Dementia. 1996;7:53–58. doi: 10.1159/000106853. [DOI] [PubMed] [Google Scholar]
- WHO . The ICD-10 Classification of Mental and Behavioural Disorders-Diagnostic Criteria for Research. Geneva; 1993. [Google Scholar]
- American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorder- Fourth Edition (DSM-IV) Washington DC, American Psychiatric Press; 1994. [Google Scholar]
- Roman GC, Tatemichi TK, Erkinjuntti T, Cummings JL, Masdeu JC, Garcia JH, Amaducci L, Orgogozo JM, Brun A, Hofman A, et al. Vascular dementia: diagnostic criteria for research studies. Report of the NINDS-AIREN International Workshop. Neurology. 1993;43:250–260. doi: 10.1212/wnl.43.2.250. [DOI] [PubMed] [Google Scholar]
- Erkinjuntti T. Clinical criteria for vascular dementia: the NINDS-AIREN criteria. Dementia. 1994;5:189–192. doi: 10.1159/000106721. [DOI] [PubMed] [Google Scholar]
- Lopez OL, Larumbe MR, Becker JT, Rezek D, Rosen J, Klunk W, DeKosky ST. Reliability of NINDS-AIREN clinical criteria for the diagnosis of vascular dementia. Neurology. 1994;44:1240–1245. doi: 10.1212/wnl.44.7.1240. [DOI] [PubMed] [Google Scholar]
- van Straaten EC, Scheltens P, Knol DL, van Buchem MA, van Dijk EJ, Hofman PA, Karas G, Kjartansson O, de Leeuw FE, Prins ND, Schmidt R, Visser MC, Weinstein HC, Barkhof F. Operational definitions for the NINDS-AIREN criteria for vascular dementia: an interobserver study. Stroke. 2003;34:1907–1912. doi: 10.1161/01.STR.0000083050.44441.10. [DOI] [PubMed] [Google Scholar]
- Roman GC. Defining dementia: clinical criteria for the diagnosis of vascular dementia. Acta Neurologica Scandinavica Suppllement. 2002;178:6–9. doi: 10.1034/j.1600-0404.106.s178.2.x. [DOI] [PubMed] [Google Scholar]
- Hogervorst E, Bandelow S, Combrinck M, Irani S, Smith AD. The validity and reliability of 6 sets of clinical criteria to classify Alzheimer's disease and vascular dementia in cases confirmed post-mortem: added value of a decision tree approach. Dementia and Geriatric Cognitive Disorders. 2003;16:170–180. doi: 10.1159/000071006. [DOI] [PubMed] [Google Scholar]
- Report of the NINCDS-ADRDA Work Group: Clinical diagnosis of Alzheimer's Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- Tsiptsios I, Fotiou F, Sitzoglou K, K.N. Fountoulakis. Neurophysiological investigation of cervical spondylosis. Electromyography and Clinical Neurophysiology. 2001;41:305–313. [PubMed] [Google Scholar]
- Tomlinson BE. The pathology of dementia. In: Wells CE, editor. Dementia. Philadelphia, Davis; 1977. pp. 113–153. [Google Scholar]
- Desmedt JE, Cheron G. Somatosensory evoked potentials to finger stimulation in healthy octogenarians and in young adults: wave forms. Scalp topography and transit times of parietal and frontal components. Electroencephalography and Clinical Neurophysiology. 1980;50:404–425. doi: 10.1016/0013-4694(80)90007-3. [DOI] [PubMed] [Google Scholar]
- Tachibana H, Takeda M, Okuda B, Kawabata K, Nishimura H, Kodama N, Iwamoto Y, Sugita M. Multimodal evoked potentials in Alzheimer's disease and Binswanger's disease. Journal of Geriatric Psychiatry and Neurology. 1996;9:7–12. doi: 10.1177/089198879600900102. [DOI] [PubMed] [Google Scholar]
- Takeda M, Tachibana H, Sugita M. [Multimodal evoked potentials in patients with dementia] Nippon Ronen Igakkai Zasshi. 1993;30:1058–1067. doi: 10.3143/geriatrics.30.1058. [DOI] [PubMed] [Google Scholar]
- Rosen I, Gustafson L, Risberg J. Multichannel EEG frequency analysis and somatosensory-evoked potentials in patients with different types of organic dementia. Dementia. 1993;4:43–49. doi: 10.1159/000107294. [DOI] [PubMed] [Google Scholar]
- Tachibana H, Kawabata K, Takeda M, Sugita M, Kondo J, Miyauchi M, Matsuoka A. [Electrophysiological comparison between patients with Binswanger's encephalopathy and Alzheimer's disease] Rinsho Byori. 1991;39:999–1004. [PubMed] [Google Scholar]
- Fazio C. Vascular pathology of the spinal cord. In: Minkler J, editor. Pathology of the Nervous System. Vol. 2. New York, McGraw-Hill; 1971. pp. 1548–1567. [Google Scholar]