Abstract
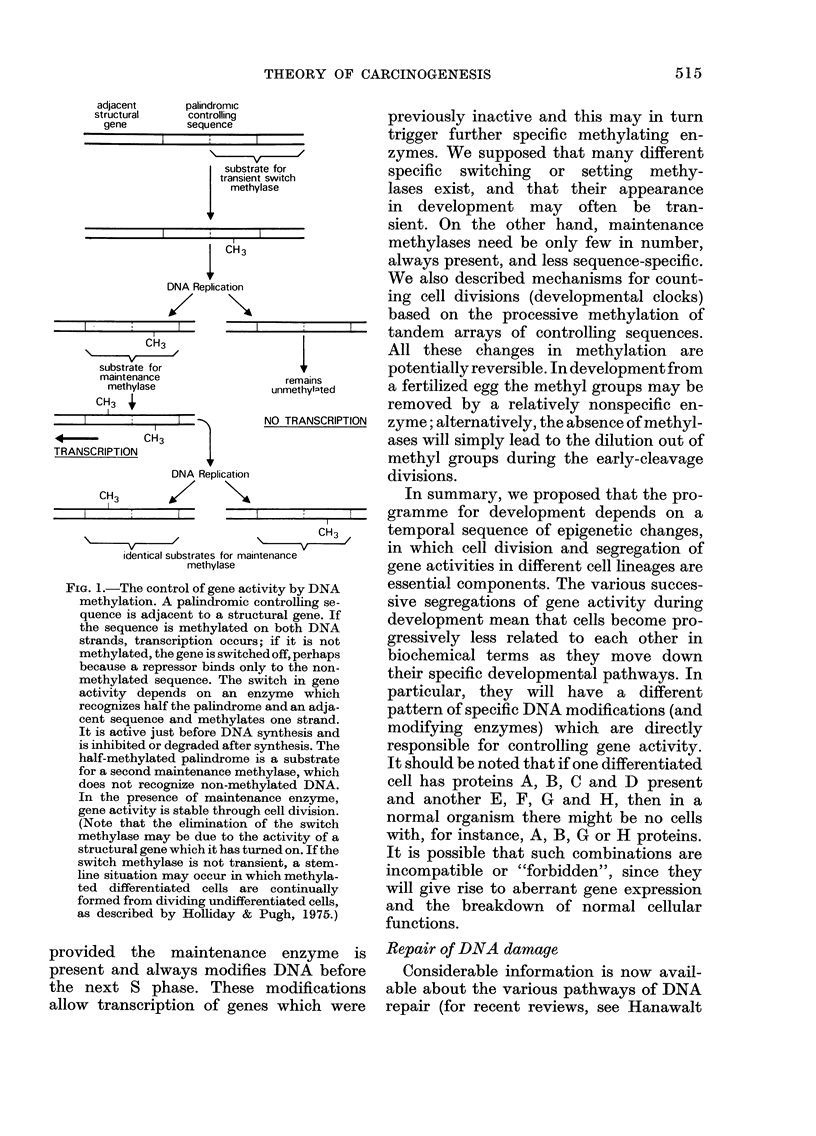
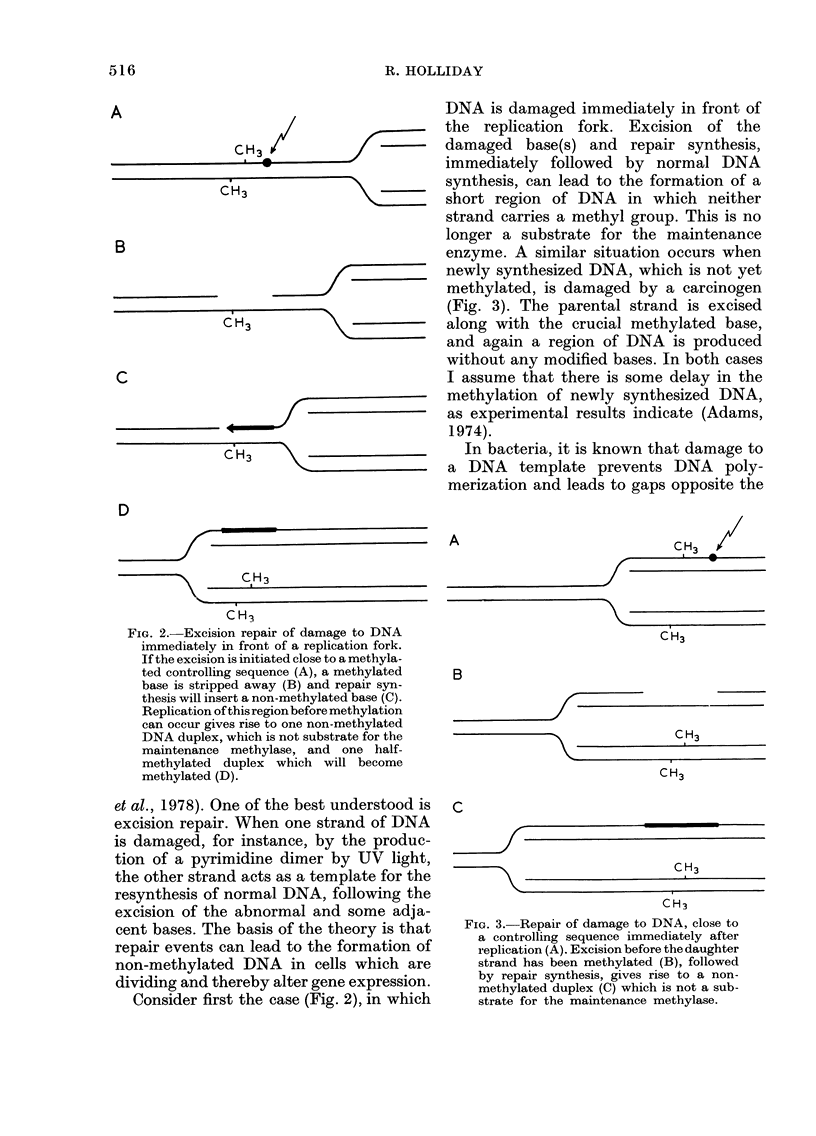
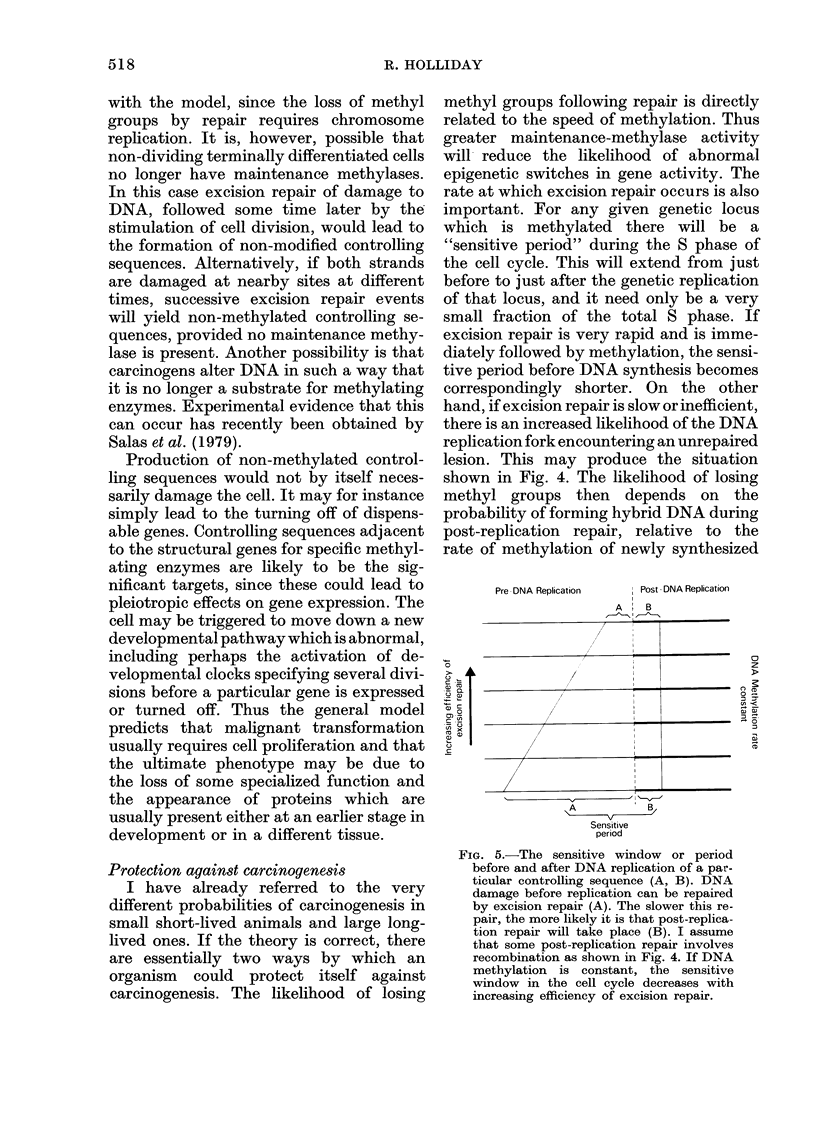
Although many carcinogens are mutagens, there is no direct evidence that the cancer-cell phenotype is the result of gene mutation. Transplantation experiments have strongly indicated that malignant cells can arise or revert to the normal phenotype in the absence of mutation. It is suggested that damage to DNA followed by repair triggers the epigenetic changes in gene expression which are responsible for malignancy. We previously proposed that methylation of specific DNA sequences adjacent to structural genes determines whether or not transcription will occur. Specific methylases are required for the switching on of genes and for the stable maintenance of the methylated state, which provides a basis for the control of gene expression in differentiated cells. It is now seen that damage to DNA followed by repair, just before or just after DNA replication, can lead to the loss of methyl groups. This can induce a switch in gene activity which is heritable, but potentially reversible. The known large difference in the probability of malignant transformation in cells of rodents and large mammals is hard to explain if mutation is responsible. On the other hand, this new theory provides an explanation for this difference, since the probability of epigenetic changes in gene activity will depend on the activity of methylating enzymes and the rate of excision repair. The theory is supported by the evidence that excision repair is more efficient in cultured fibroblasts from large long-lived animals than from small short-lived ones.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ames B. N., Durston W. E., Yamasaki E., Lee F. D. Carcinogens are mutagens: a simple test system combining liver homogenates for activation and bacteria for detection. Proc Natl Acad Sci U S A. 1973 Aug;70(8):2281–2285. doi: 10.1073/pnas.70.8.2281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christman J. K., Price P., Pedrinan L., Acs G. Correlation between hypomethylation of DNA and expression of globin genes in Friend erythroleukemia cells. Eur J Biochem. 1977 Nov 15;81(1):53–61. doi: 10.1111/j.1432-1033.1977.tb11926.x. [DOI] [PubMed] [Google Scholar]
- Cleaver J. E., Bootsma D. Xeroderma pigmentosum: biochemical and genetic characteristics. Annu Rev Genet. 1975;9:19–38. doi: 10.1146/annurev.ge.09.120175.000315. [DOI] [PubMed] [Google Scholar]
- Cohen R., Pacifici M., Rubinstein N., Biehl J., Holtzer H. Effect of a tumour promoter on myogenesis. Nature. 1977 Apr 7;266(5602):538–540. doi: 10.1038/266538a0. [DOI] [PubMed] [Google Scholar]
- De Weerd-Kastelein E. A., Keijzer W., Rainaldi G., Bootsma D. Induction of sister chromatid exchanges in xeroderma pigmentosum cells after exposure to ultraviolet light. Mutat Res. 1977 Nov;45(2):253–261. doi: 10.1016/0027-5107(77)90025-2. [DOI] [PubMed] [Google Scholar]
- FARBER E. ETHIONINE CARCINOGENESIS. Adv Cancer Res. 1963;7:383–474. doi: 10.1016/s0065-230x(08)60986-0. [DOI] [PubMed] [Google Scholar]
- Goth R., Rajewsky M. F. Persistence of O6-ethylguanine in rat-brain DNA: correlation with nervous system-specific carcinogenesis by ethylnitrosourea. Proc Natl Acad Sci U S A. 1974 Mar;71(3):639–643. doi: 10.1073/pnas.71.3.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart R. W., Setlow R. B. Correlation between deoxyribonucleic acid excision-repair and life-span in a number of mammalian species. Proc Natl Acad Sci U S A. 1974 Jun;71(6):2169–2173. doi: 10.1073/pnas.71.6.2169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holliday R., Pugh J. E. DNA modification mechanisms and gene activity during development. Science. 1975 Jan 24;187(4173):226–232. [PubMed] [Google Scholar]
- Huberman E., Mager R., Sachs L. Mutagenesis and transformation of normal cells by chemical carcinogens. Nature. 1976 Nov 25;264(5584):360–361. doi: 10.1038/264360a0. [DOI] [PubMed] [Google Scholar]
- Kauffman S. A. Control circuits for determination and transdetermination. Science. 1973 Jul 27;181(4097):310–318. doi: 10.1126/science.181.4097.310. [DOI] [PubMed] [Google Scholar]
- King T. J., DiBerardino M. A. Transplantation of nuclei from the frog renal adenocarcinoma. I. Development of tumor nuclear-transplant embryos. Ann N Y Acad Sci. 1965 Aug 10;126(1):115–126. doi: 10.1111/j.1749-6632.1965.tb14271.x. [DOI] [PubMed] [Google Scholar]
- Kinsella A. R., Radman M. Tumor promoter induces sister chromatid exchanges: relevance to mechanisms of carcinogenesis. Proc Natl Acad Sci U S A. 1978 Dec;75(12):6149–6153. doi: 10.1073/pnas.75.12.6149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawley P. D., Martin C. N. Molecular mechanisms in alkylation mutagenesis. Induced reversion of bacteriophage T4rII AP72 by ethyl methanesulphonate in relation to extent and mode of ethylation of purines in bacteriophage deoxyribonucleic acid. Biochem J. 1975 Jan;145(1):85–91. doi: 10.1042/bj1450085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann A. R. Postreplication repair of DNA in ultraviolet-irradiated mammalian cells. J Mol Biol. 1972 May 28;66(3):319–337. doi: 10.1016/0022-2836(72)90418-4. [DOI] [PubMed] [Google Scholar]
- Loveless A. Possible relevance of O-6 alkylation of deoxyguanosine to the mutagenicity and carcinogenicity of nitrosamines and nitrosamides. Nature. 1969 Jul 12;223(5202):206–207. doi: 10.1038/223206a0. [DOI] [PubMed] [Google Scholar]
- Markert C. L. Neoplasia: a disease of cell differentiation. Cancer Res. 1968 Sep;28(9):1908–1914. [PubMed] [Google Scholar]
- Marquardt H. Cell cycle dependence of chemically induced malignant transformation in vitro. Cancer Res. 1974 Jul;34(7):1612–1615. [PubMed] [Google Scholar]
- McCann J., Ames B. N. Detection of carcinogens as mutagens in the Salmonella/microsome test: assay of 300 chemicals: discussion. Proc Natl Acad Sci U S A. 1976 Mar;73(3):950–954. doi: 10.1073/pnas.73.3.950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCann J., Choi E., Yamasaki E., Ames B. N. Detection of carcinogens as mutagens in the Salmonella/microsome test: assay of 300 chemicals. Proc Natl Acad Sci U S A. 1975 Dec;72(12):5135–5139. doi: 10.1073/pnas.72.12.5135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinnell R. G., Deggins B. A., Labat D. D. Transplantation of pluripotential nuclei from triploid frog tumors. Science. 1969 Jul 25;165(3891):394–396. doi: 10.1126/science.165.3891.394. [DOI] [PubMed] [Google Scholar]
- Medawar P. Anaplasia rediviva. Ann Intern Med. 1977 Jul;87(1):100–102. doi: 10.7326/0003-4819-87-1-100. [DOI] [PubMed] [Google Scholar]
- Meneghini R., Hanawalt P. T4-endonuclease V-sensitive sites in DNA from ultraviolet-irradiated human cells. Biochim Biophys Acta. 1976 Apr 2;425(4):428–437. doi: 10.1016/0005-2787(76)90007-1. [DOI] [PubMed] [Google Scholar]
- Meselson M. S., Radding C. M. A general model for genetic recombination. Proc Natl Acad Sci U S A. 1975 Jan;72(1):358–361. doi: 10.1073/pnas.72.1.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mintz B., Illmensee K. Normal genetically mosaic mice produced from malignant teratocarcinoma cells. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3585–3589. doi: 10.1073/pnas.72.9.3585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore P. D., Holliday R. Evidence for the formation of hybrid DNA during mitotic recombination in Chinese hamster cells. Cell. 1976 Aug;8(4):573–579. doi: 10.1016/0092-8674(76)90225-7. [DOI] [PubMed] [Google Scholar]
- Papaioannou V. E., McBurney M. W., Gardner R. L., Evans M. J. Fate of teratocarcinoma cells injected into early mouse embryos. Nature. 1975 Nov 6;258(5530):70–73. doi: 10.1038/258070a0. [DOI] [PubMed] [Google Scholar]
- Pegg A. E. Formation and metabolism of alkylated nucleosides: possible role in carcinogenesis by nitroso compounds and alkylating agents. Adv Cancer Res. 1977;25:195–269. doi: 10.1016/s0065-230x(08)60635-1. [DOI] [PubMed] [Google Scholar]
- Perry P., Evans H. J. Cytological detection of mutagen-carcinogen exposure by sister chromatid exchange. Nature. 1975 Nov 13;258(5531):121–125. doi: 10.1038/258121a0. [DOI] [PubMed] [Google Scholar]
- Peto R., Roe F. J., Lee P. N., Levy L., Clack J. Cancer and ageing in mice and men. Br J Cancer. 1975 Oct;32(4):411–426. doi: 10.1038/bjc.1975.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter H., Dressler D. On the mechanism of genetic recombination: electron microscopic observation of recombination intermediates. Proc Natl Acad Sci U S A. 1976 Sep;73(9):3000–3004. doi: 10.1073/pnas.73.9.3000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rommelaere J., Miller-Faurès A. Detection by density equilibrium centrifugation of recombinant-like DNA molecules in somatic mammalian cells. J Mol Biol. 1975 Oct 15;98(1):195–218. doi: 10.1016/s0022-2836(75)80109-4. [DOI] [PubMed] [Google Scholar]
- Rosenstraus M. J., Chasin L. A. Separation of linked markers in Chinese hamster cell hybrids: mitotic recombination is not involved. Genetics. 1978 Dec;90(4):735–760. doi: 10.1093/genetics/90.4.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rovera G., O'Brien T. G., Diamond L. Tumor promoters inhibit spontaneous differentiation of Friend erythroleukemia cells in culture. Proc Natl Acad Sci U S A. 1977 Jul;74(7):2894–2898. doi: 10.1073/pnas.74.7.2894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rupp W. D., Wilde C. E., 3rd, Reno D. L., Howard-Flanders P. Exchanges between DNA strands in ultraviolet-irradiated Escherichia coli. J Mol Biol. 1971 Oct 14;61(1):25–44. doi: 10.1016/0022-2836(71)90204-x. [DOI] [PubMed] [Google Scholar]
- Rutter W. J., Pictet R. L., Morris P. W. Toward molecular mechanisms of developmental processes. Annu Rev Biochem. 1973;42:601–646. doi: 10.1146/annurev.bi.42.070173.003125. [DOI] [PubMed] [Google Scholar]
- Salas C. E., Pfohl-Leszkowicz A., Lang M. C., Dirheimer G. Effect of modification by N-acetoxy-N-2-acetylaminofluorene on the level of DNA methylation. Nature. 1979 Mar 1;278(5699):71–72. doi: 10.1038/278071a0. [DOI] [PubMed] [Google Scholar]
- Scarano E. The control of gene function in cell differentiation and in embryogenesis. Adv Cytopharmacol. 1971 May;1:13–24. [PubMed] [Google Scholar]
- Sigal N., Alberts B. Genetic recombination: the nature of a crossed strand-exchange between two homologous DNA molecules. J Mol Biol. 1972 Nov 28;71(3):789–793. doi: 10.1016/s0022-2836(72)80039-1. [DOI] [PubMed] [Google Scholar]
- Swann P. F., Pegg A. E., Hawks A., Farber E., Magee P. N. Evidence for ethylation of rat liver deoxyribonucleic acid after administration of ethionine. Biochem J. 1971 Jun;123(2):175–181. doi: 10.1042/bj1230175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swann P. F. The effect of ethionine on ribonucleic acid synthesis in rat liver. Biochem J. 1975 Sep;150(3):335–344. doi: 10.1042/bj1500335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarrant G. M., Holliday R. A search for allelic recombination in Chinese hamster cell hybrids. Mol Gen Genet. 1977 Nov 18;156(3):273–279. doi: 10.1007/BF00267182. [DOI] [PubMed] [Google Scholar]
- Venner H., Reinert H. Possible role of methylated DNA bases for the transcription of the genetic information. Z Allg Mikrobiol. 1973;13(7):613–624. doi: 10.1002/jobm.3630130711. [DOI] [PubMed] [Google Scholar]
- Yamasaki H., Fibach E., Nudel U., Weinstein I. B., Rifkind R. A., Marks P. A. Tumor promoters inhibit spontaneous and induced differentiation of murine erythroleukemia cells in culture. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3451–3455. doi: 10.1073/pnas.74.8.3451. [DOI] [PMC free article] [PubMed] [Google Scholar]



