Abstract
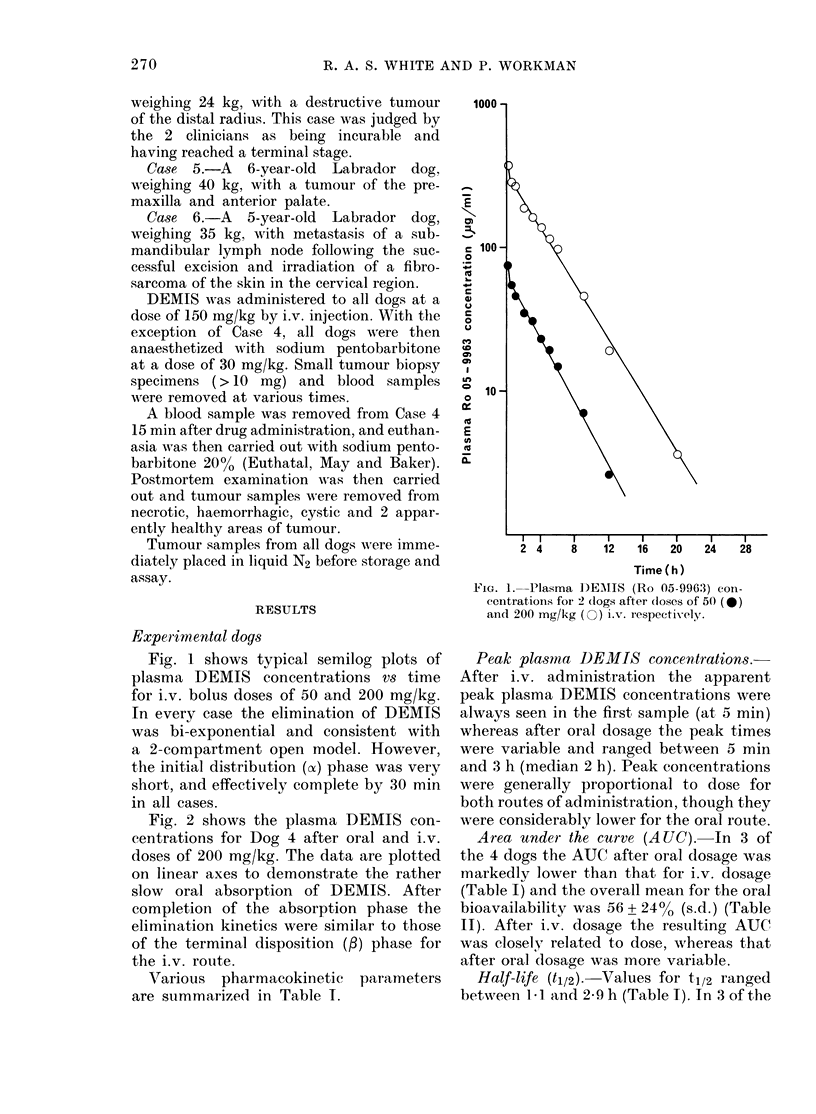
The hypoxic cell radiosensitizer desmethylmisonidazole (1-(2-nitroimidazol-1-yl)-2,3-propandiol; Ro 05-9963; DEMIS) was administered to 4 dogs at doses of 50 and 200 mg/kg by both oral and i.v. routes. The resulting plasma, cerebrospinal fluid and urinary concentrations were measured by HPLC analysis; various pharmacokinetic parameters were obtained and compared with similar data for the parent compound, misonidazole (MISO), in the dog.
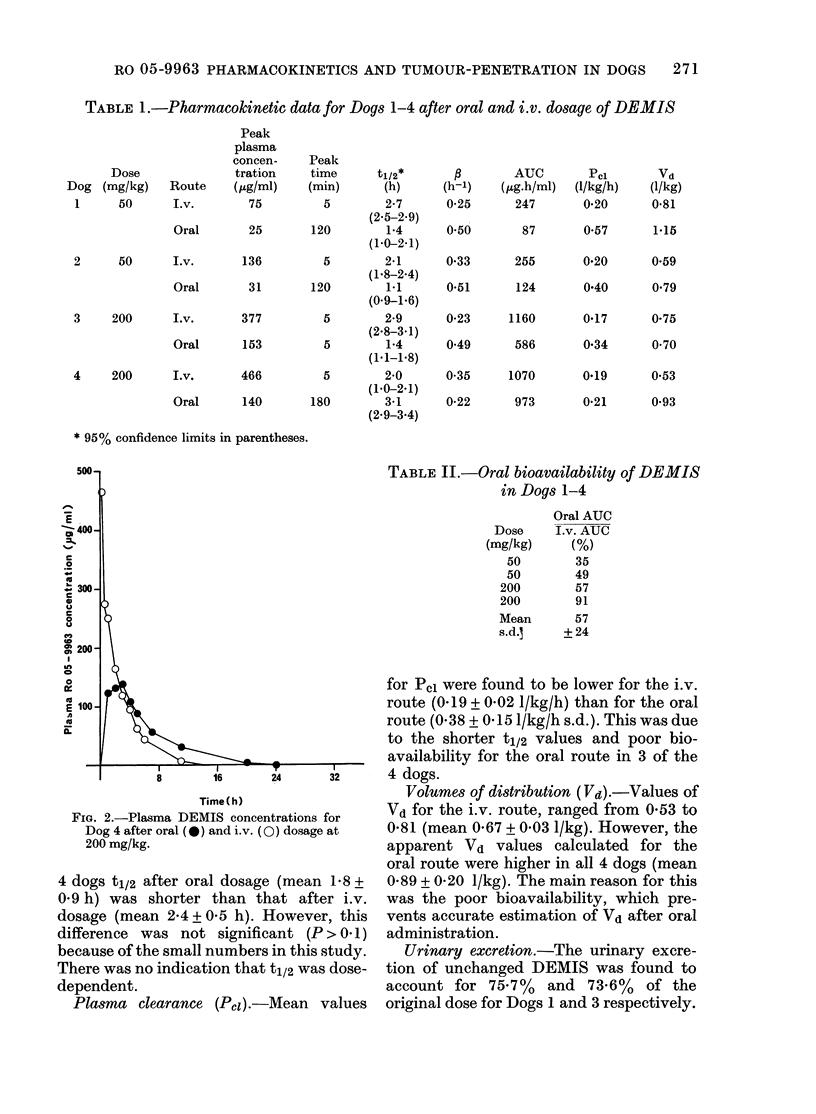
Because of its shorter half-life (2·1 h) the total tissue exposure for DEMIS was only half that for a similar dose of MISO, whereas peak plasma concentrations were 60% higher than those for MISO. Cerebrospinal fluid penetration by DEMIS was limited because of the drug's reduced lipophilicity, and the total cerebrospinal-fluid exposure to the drug during the first 5 h after drug administration was about half that previously recorded for MISO.
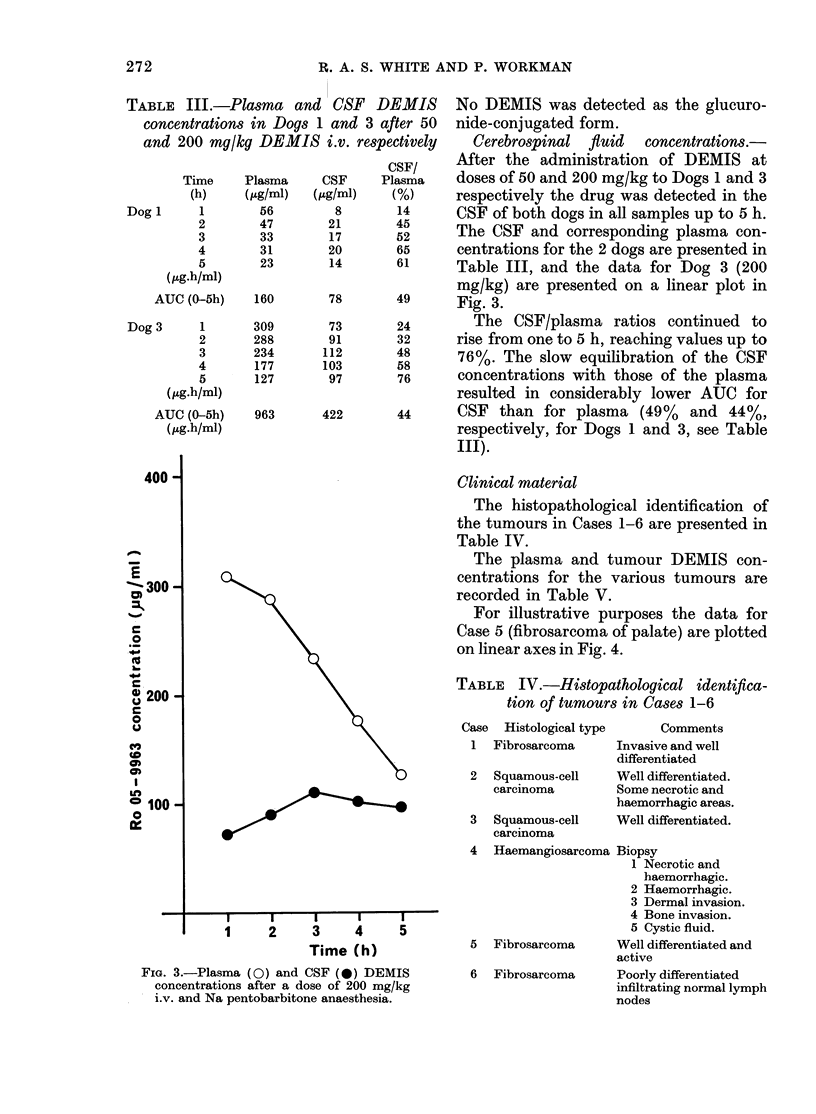
Urinary excretion accounted for 75% of the i.v. dose of unchanged DEMIS, whilst less than 20% of MISO is excreted via this route.
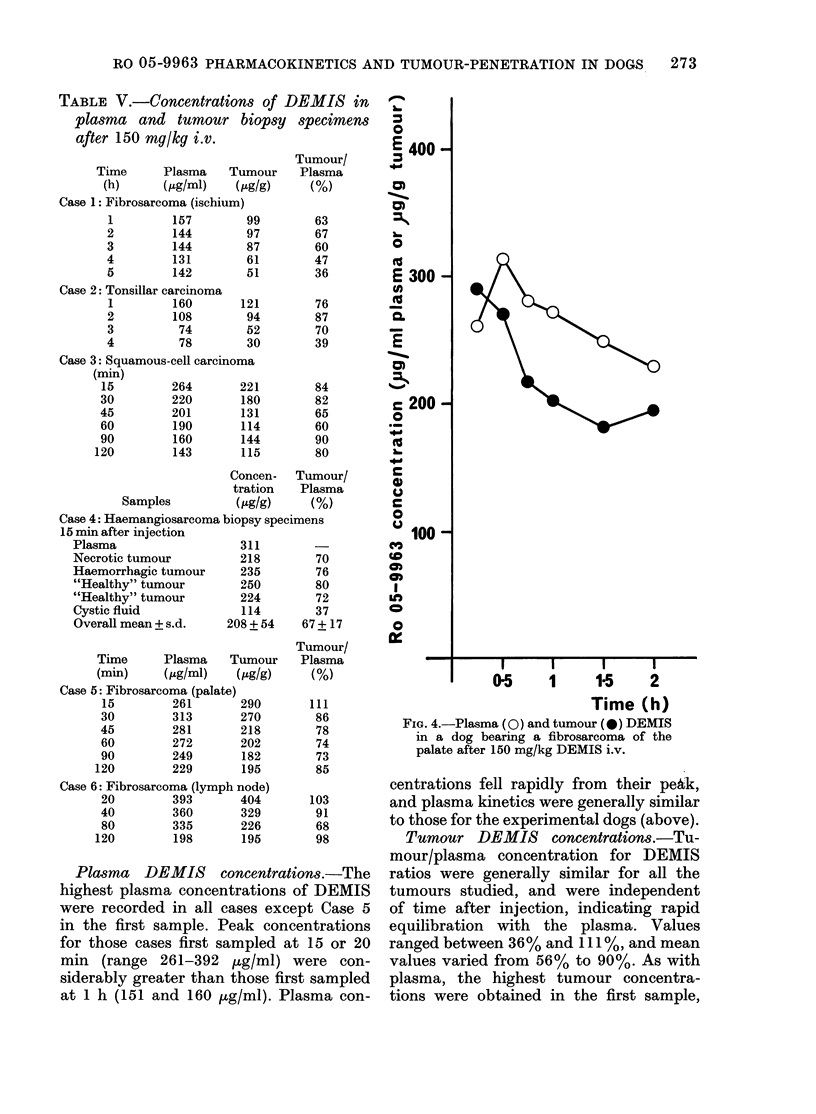
DEMIS was also administered to 6 dogs bearing spontaneous tumours at a dose of 150 mg/kg i.v., and the resulting concentrations were recorded in serial biopsies over a 5h period.
Mean tumour/plasma ratios ranged between 56 and 90%, and were very similar to those previously observed for MISO in canine tumours. Peak DEMIS tumour concentrations, however, occurred rapidly after dosage (15-20 min) and were as much as twice those for MISO, although they declined rapidly from their initial concentration.
We conclude in the light of the reduced tissue exposure, particularly of the nervous tissue, and the improved tumour concentrations, that DEMIS may prove to be a potentially less toxic alternative to MISO.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams G. E., Clarke E. D., Flockhart I. R., Jacobs R. S., Sehmi D. S., Stratford I. J., Wardman P., Watts M. E., Parrick J., Wallace R. G. Structure-activity relationships in the development of hypoxic cell radiosensitizers. I. Sensitization efficiency. Int J Radiat Biol Relat Stud Phys Chem Med. 1979 Feb;35(2):133–150. doi: 10.1080/09553007914550151. [DOI] [PubMed] [Google Scholar]
- Adams G. E., Flockhart I. R., Smithen C. E., Stratford I. J., Wardman P., Watts M. E. Electron-affinic sensitization. VII. A correlation between structures, one-electron reduction potentials, and efficiencies of nitroimidazoles as hypoxic cell radiosensitizers. Radiat Res. 1976 Jul;67(1):9–20. [PubMed] [Google Scholar]
- Ash D. V., Smith M. R., Bugden R. D. Distribution of misonidazole in human tumours and normal tissues. Br J Cancer. 1979 May;39(5):503–509. doi: 10.1038/bjc.1979.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown J. M., Yu N. Y., Cory M. J., Bicknell R. B., Taylor D. L. In vivo evaluation of the radiosensitizing and cytotoxic properties of newly synthesized electron-affinic drugs. Br J Cancer Suppl. 1978 Jun;3:206–211. [PMC free article] [PubMed] [Google Scholar]
- Brown J. M., Yu N. Y., Workman P. Pharmacokinetic considerations in testing hypoxic cell radiosensitizers in mouse tumours. Br J Cancer. 1979 Mar;39(3):310–320. doi: 10.1038/bjc.1979.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dische S., Saunders M. I., Lee M. E., Adams G. E., Flockhart I. R. Clinical testing of the radiosensitizer Ro 07-0582: experience with multiple doses. Br J Cancer. 1977 May;35(5):567–579. doi: 10.1038/bjc.1977.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flockhart I. R., Sheldon P. W., Stratford I. J., Watts M. E. A metabolite of the 2-nitroimidazole misonidazole with radiosensitizing properties. Int J Radiat Biol Relat Stud Phys Chem Med. 1978 Jul;34(1):91–94. doi: 10.1080/09553007814550661. [DOI] [PubMed] [Google Scholar]
- Saunders M. E., Dische S., Anderson P., Flockhart I. R. The neurotoxicity of misonidazole and its relationship to dose, half-life and concentration in the serum. Br J Cancer Suppl. 1978 Jun;3:268–270. [PMC free article] [PubMed] [Google Scholar]
- Urtasun R. C., Band P., Chapman J. D., Rabin H. R., Wilson A. F., Fryer C. G. Clinical phase I study of the hypoxic cell radiosensitizer RO-07-0582, a 2-nitroimidazole derivative. Radiology. 1977 Mar;122(3):801–804. doi: 10.1148/122.3.801. [DOI] [PubMed] [Google Scholar]
- Wardman P., Clarke E. D., Flockhart I. R., Wallace R. G. The rationale for the development of improved hypoxic cell radiosensitizers. Br J Cancer Suppl. 1978 Jun;3:1–5. [PMC free article] [PubMed] [Google Scholar]
- White R. A., Workman P., Freedman L. S., Owen L. N., Bleehen N. M. The pharmacokinetics of misonidazole in the dog. Eur J Cancer. 1979 Oct;15(10):1233–1242. doi: 10.1016/0014-2964(79)90249-4. [DOI] [PubMed] [Google Scholar]
- White R. A., Workman P., Owen L. N., Bleehen N. M. The penetration of misonidazole into spontaneous canine tumours. Br J Cancer. 1979 Aug;40(2):284–294. doi: 10.1038/bjc.1979.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiltshire C. R., Workman P., Watson J. V., Bleehen N. M. Clinical studies with misonidazole. Br J Cancer Suppl. 1978 Jun;3:286–289. [PMC free article] [PubMed] [Google Scholar]
- Workman P. Effects of pretreatment with phenobarbitone and phenytoin on the pharmacokinetics and toxicity of phenytoin on the pharmacokinetics and toxicity of misonidazole in mice. Br J Cancer. 1979 Sep;40(3):335–353. doi: 10.1038/bjc.1979.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Workman P., Little C. J., Marten T. R., Dale A. D., Ruane R. J., Flockhart I. R., Bleehen N. M. Estimation of the hypoxic cell-sensitiser misonidazole and its O-demethylated metabolite in biological materials by reversed-phase high-performance liquid chromatography. J Chromatogr. 1978 May 1;145(3):507–512. doi: 10.1016/s0378-4347(00)81386-9. [DOI] [PubMed] [Google Scholar]



