Abstract
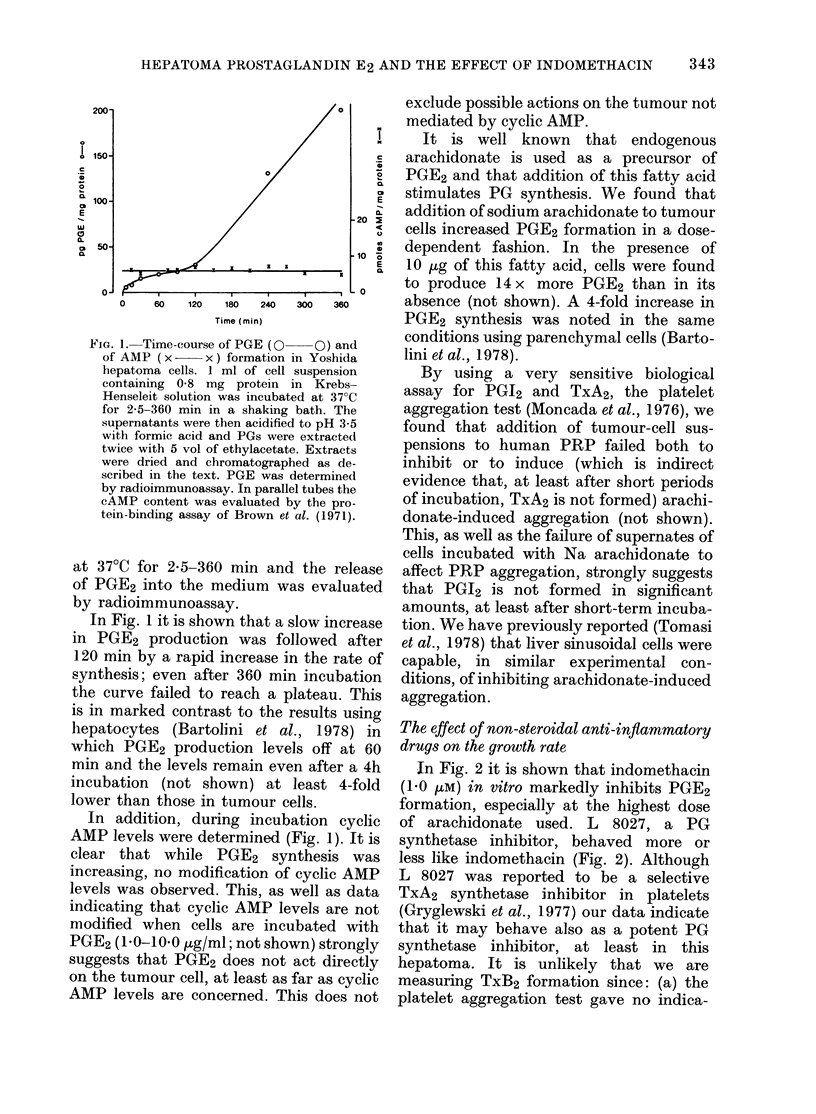
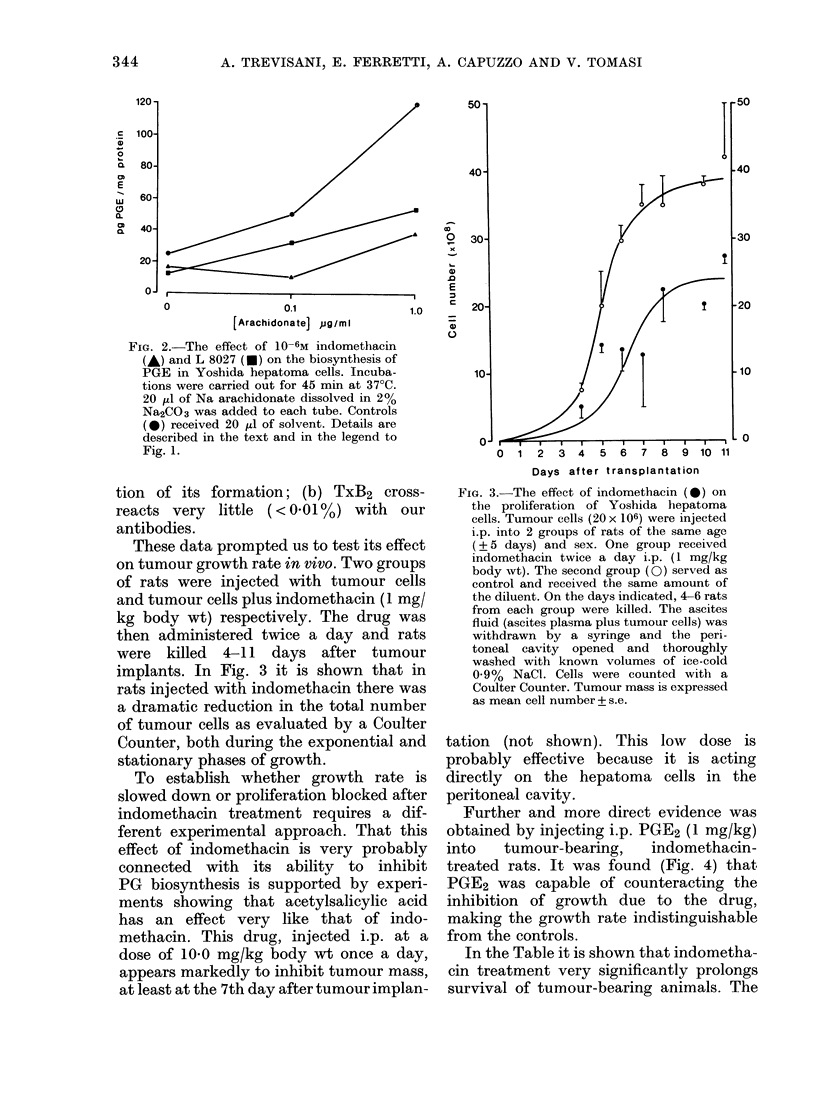
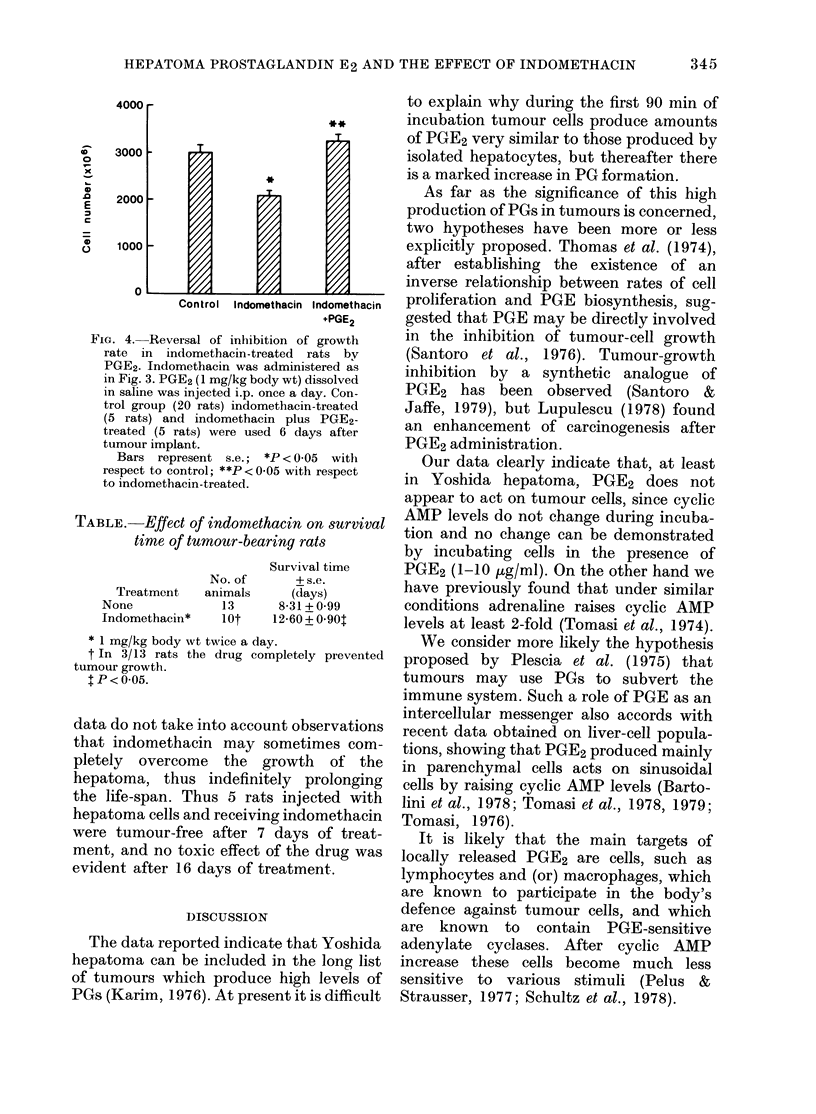
Prostaglandin (PG) E2 biosynthesis in Yoshida hepatoma (AH 130) was evaluated by radioimmunoassay. When hepatoma cells were incubated in vitro, the levels of PGE2 in the medium were similar to those found in hepatocytes for the first 2 h; this was followed by a rapid increase in PGE2 formation, and the 6h incubation levels were 4-fold higher than in hepatocytes. Addition of sodium arachidonate markedly and dose-dependently stimulated PGE2 synthesis; the increase was largely prevented by the addition of indomethacin (1 microM) or L 8027, a prostaglandin synthetase inhibitor. Experiments in vivo indicated that indomethacin treatment of tumour-bearing rats significantly reduced the tumour mass. When rats were injected with PGE2 after receiving the drug, the number of tumour cells was very similar to that of untreated animals. This, as well as the inhibition of tumour growth by acetylsalicylic acid, strongly suggests that the inhibition of PG biosynthesis by anti-inflammatory drugs and the inhibition of tumour proliferation may be closely associated events. It was also found that injections of indomethacin very significantly prolonged survival of hepatoma-bearing rats. Since PGE2 does not appear to affect the cyclic AMP levels of hepatoma cells, it is possible that hepatoma may use PGE2 to subvert the immune system. This could help to explain the effectiveness of anti-inflammatory drugs in the control of tumour growth.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bennett A., Houghton J., Leaper D. J., Stamford I. F. Cancer growth, response to treatment and survival time in mice: beneficial effect of the prostaglandin synthesis inhibitor flurbiprofen. Prostaglandins. 1979 Feb;17(2):179–191. doi: 10.1016/0090-6980(79)90037-6. [DOI] [PubMed] [Google Scholar]
- Brown B. L., Albano J. D., Ekins R. P., Sgherzi A. M. A simple and sensitive saturation assay method for the measurement of adenosine 3':5'-cyclic monophosphate. Biochem J. 1971 Feb;121(3):561–562. doi: 10.1042/bj1210561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flower R. J. Drugs which inhibit prostaglandin biosynthesis. Pharmacol Rev. 1974 Mar;26(1):33–67. [PubMed] [Google Scholar]
- Grinwich K. D., Plescia O. J. Tumor-mediated immunosuppression: prevention by inhibitors of prostaglandin synthesis. Prostaglandins. 1977;14(6):1175–1182. doi: 10.1016/0090-6980(77)90294-5. [DOI] [PubMed] [Google Scholar]
- Gryglewski R. J., Zmuda A., Korbut R., Krecioch E., Bieron K. Selective inhibition of thromboxane A2 biosynthesis in blood platelets. Nature. 1977 Jun 16;267(5612):627–628. doi: 10.1038/267627a0. [DOI] [PubMed] [Google Scholar]
- Hammarström S. Prostaglandin production by normal and transformed 3T3 fibroblasts in cell culture. Eur J Biochem. 1977 Mar 15;74(1):7–12. doi: 10.1111/j.1432-1033.1977.tb11360.x. [DOI] [PubMed] [Google Scholar]
- Hong S. L., Wheless C. M., Levine L. Elevated prostaglandins synthetase activity in methylcholanthrene-transformed mouse BALB/3T3. Prostaglandins. 1977 Feb;13(2):271–279. doi: 10.1016/0090-6980(77)90008-9. [DOI] [PubMed] [Google Scholar]
- Kantor H. S., Hampton M. Indomethacin in submicromolar concentrations inhibits cyclic AMP-dependent protein kinase. Nature. 1978 Dec 21;276(5690):841–842. doi: 10.1038/276841a0. [DOI] [PubMed] [Google Scholar]
- Levine L., Hong S. L. Analogues of anthracene, phenanthrene, and benzoflavone inhibit prostaglandin biosynthesis by cells in culture. Prostaglandins. 1977 Jul;14(1):1–9. doi: 10.1016/0090-6980(77)90152-6. [DOI] [PubMed] [Google Scholar]
- Lupulescu A. Enhancement of carcinogenesis by prostaglandins in male albino Swiss mice. J Natl Cancer Inst. 1978 Jul;61(1):97–106. doi: 10.1093/jnci/61.1.97. [DOI] [PubMed] [Google Scholar]
- Lynch N. R., Salomon J. C. Tumor growth inhibition and potentiation of immunotherapy by indomethacin in mice. J Natl Cancer Inst. 1979 Jan;62(1):117–121. [PubMed] [Google Scholar]
- Moncada S., Gryglewski R., Bunting S., Vane J. R. An enzyme isolated from arteries transforms prostaglandin endoperoxides to an unstable substance that inhibits platelet aggregation. Nature. 1976 Oct 21;263(5579):663–665. doi: 10.1038/263663a0. [DOI] [PubMed] [Google Scholar]
- Pelus L. M., Strausser H. R. Prostaglandins and the immune response. Life Sci. 1977 Mar 15;20(6):903–913. doi: 10.1016/0024-3205(77)90274-0. [DOI] [PubMed] [Google Scholar]
- Plescia O. J., Smith A. H., Grinwich K. Subversion of immune system by tumor cells and role of prostaglandins. Proc Natl Acad Sci U S A. 1975 May;72(5):1848–1851. doi: 10.1073/pnas.72.5.1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson R. P., Baylink D. J., Metz S. A., Cummings K. B. Plasma prostaglandin E in patients with cancer with and without hypercalcemia. J Clin Endocrinol Metab. 1976 Dec;43(6):1330–1335. doi: 10.1210/jcem-43-6-1330. [DOI] [PubMed] [Google Scholar]
- Santoro M. G., Jaffe B. M. Inhibition of Friend erythroleukaemia-cell tumours in vivo by a synthetic analogue of prostaglandin E2. Br J Cancer. 1979 Apr;39(4):408–413. doi: 10.1038/bjc.1979.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santoro M. G., Philpott G. W., Jaffe B. M. Inhibition of tumour growth in vivo and in vitro by prostaglandin E. Nature. 1976 Oct 28;263(5580):777–779. doi: 10.1038/263777a0. [DOI] [PubMed] [Google Scholar]
- Schultz R. M., Pavlidis N. A., Stylos W. A., Chirigos M. A. Regulation of macrophage tumoricidal function: a role for prostaglandins of the E series. Science. 1978 Oct 20;202(4365):320–321. doi: 10.1126/science.694537. [DOI] [PubMed] [Google Scholar]
- Seyberth H. W., Segre G. V., Morgan J. L., Sweetman B. J., Potts J. T., Jr, Oates J. A. Prostaglandins as mediators of hypercalcemia associated with certain types of cancer. N Engl J Med. 1975 Dec 18;293(25):1278–1283. doi: 10.1056/NEJM197512182932502. [DOI] [PubMed] [Google Scholar]
- Tashjian A. H., Jr, Voelkel E. F., Goldhaber P., Levine L. Successful treatment of hypercalcemia by indomethacin in mice bearing a prostaglandin-producing fibrosarcoma. Prostaglandins. 1973 Apr;3(4):515–524. doi: 10.1016/0090-6980(73)90161-5. [DOI] [PubMed] [Google Scholar]
- Thomas D. R., Philpott G. W., Jaffe B. M. The relationship between concentration of prostaglandin E and rates of cell replication. Exp Cell Res. 1974 Mar 15;84(1):40–46. doi: 10.1016/0014-4827(74)90377-2. [DOI] [PubMed] [Google Scholar]
- Tomasi V., Meringolo C., Bartolini G., Orlandi M. Biosynthesis of prostacyclin in rat liver endothelial cells and its control by prostaglandin E2. Nature. 1978 Jun 22;273(5664):670–671. doi: 10.1038/273670a0. [DOI] [PubMed] [Google Scholar]
- Tomasi V. Prostaglandin E1 as an intercellular regulator of cyclic AMP levels. Exp Cell Biol. 1976;44(3-6):260–277. doi: 10.1159/000163116. [DOI] [PubMed] [Google Scholar]



