Abstract
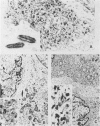
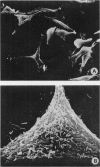
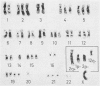
A human melanoma cell line with unusually high growth potential was established from a xenograft growing in athymic mice. When xenograft fragments were cultured in vitro, melanoma cells grew out rapidly without any contamination of mouse stromal cells. An established cell line, FME, derived from this tumour, grew both in monolayer and in shaker suspension culture with doubling times of about 20 h. The cells grew easily at low serum concentrations and could even be cultured in serum-free medium supplemented with insulin and transferrin. The cultured cells were hyperdiploid, as were the cells of the xenograft. The cells grew easily in soft agar and formed tumours in athymic mice. When growing exponentially, the cells were almost unpigmented, but when grown to high density, their melanin content increased. Upon treatment with dimethyl sulphoxide (DMSO), retinoic acid and theophylline, as well as with the tumour promoter 12-O-tetradecanoyl phorbol-13-acetate (TPA), the cells showed growth inhibition and increased melanin synthesis.
Full text
PDF


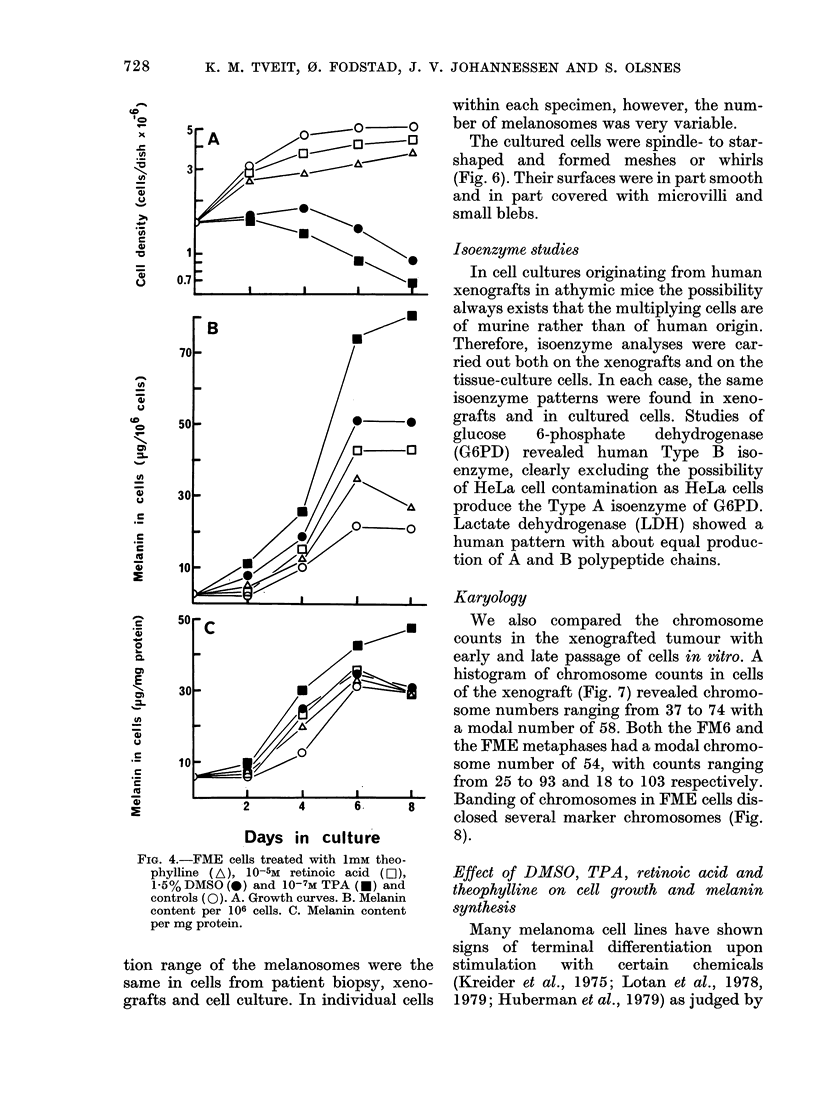
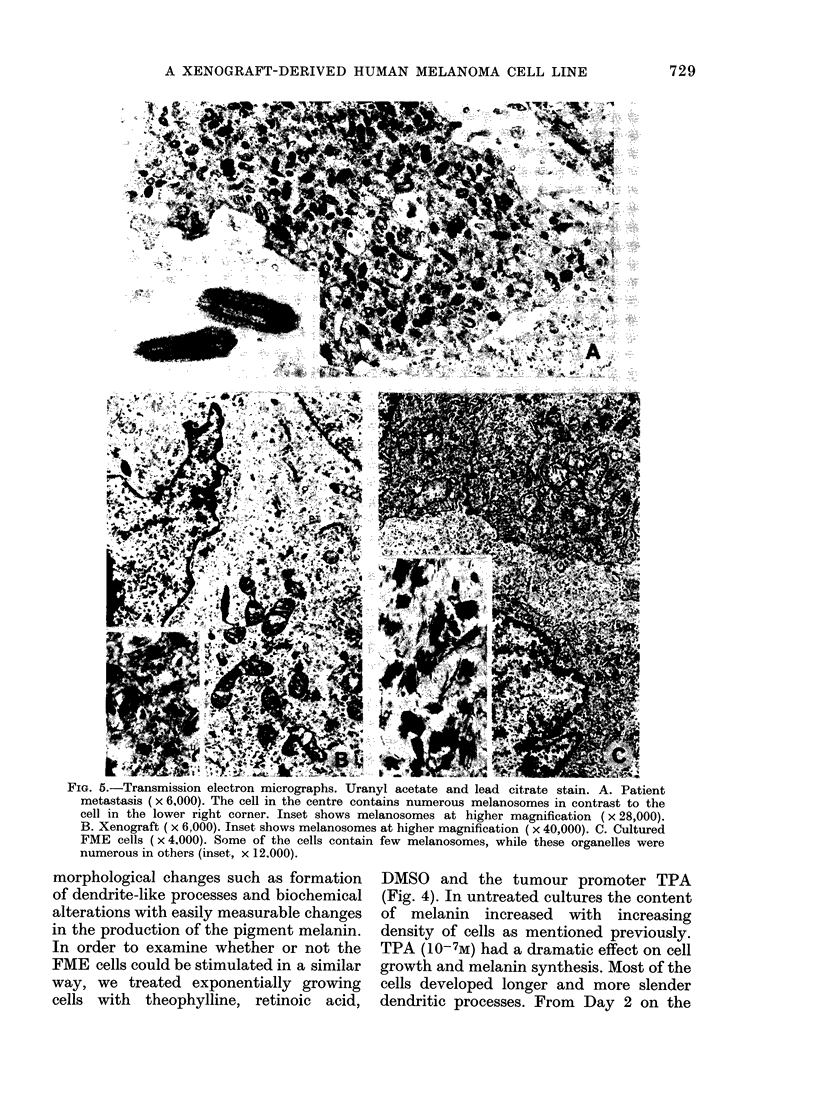
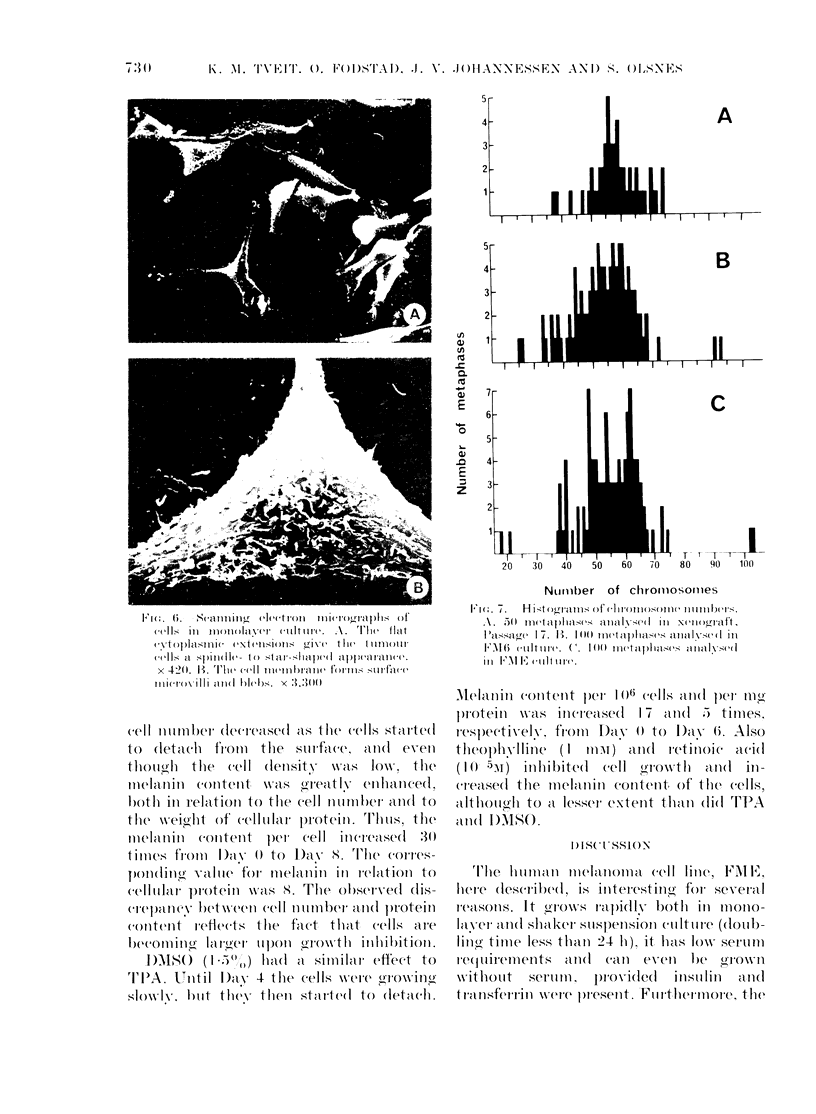
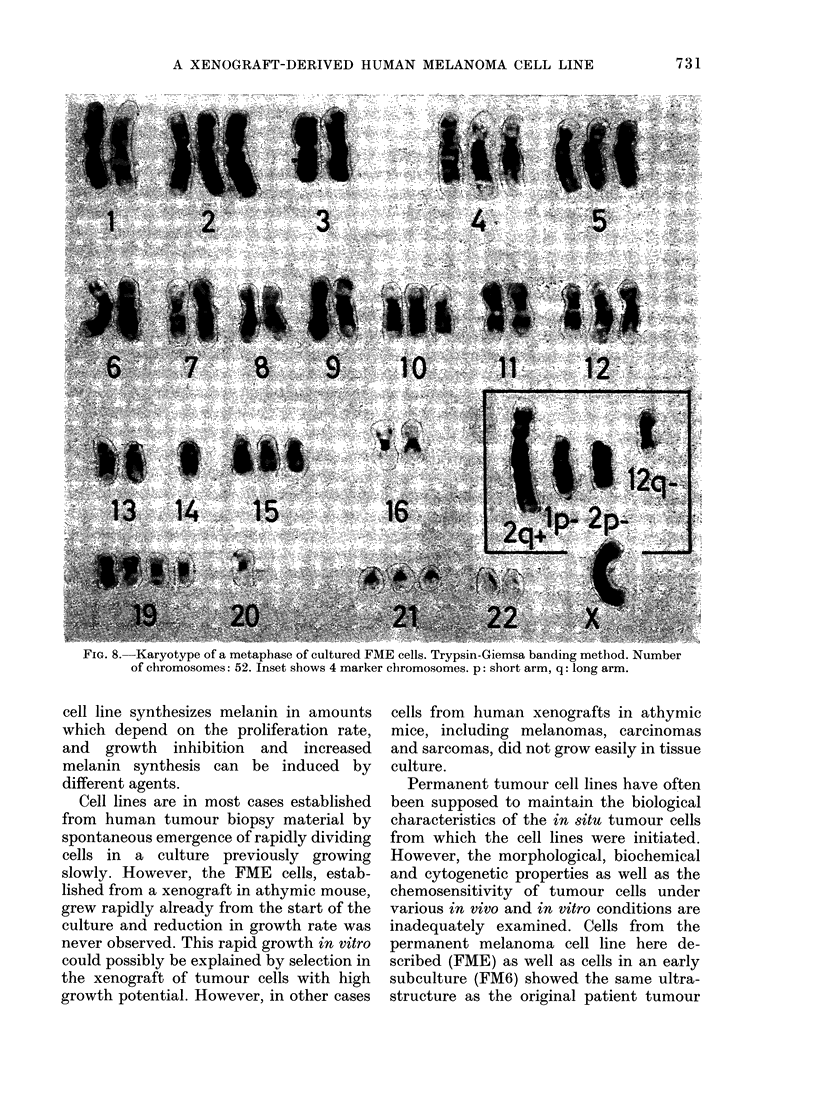


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barnes D., Sato G. Growth of a human mammary tumour cell line in a serum-free medium. Nature. 1979 Oct 4;281(5730):388–389. doi: 10.1038/281388a0. [DOI] [PubMed] [Google Scholar]
- Collins S. J., Ruscetti F. W., Gallagher R. E., Gallo R. C. Terminal differentiation of human promyelocytic leukemia cells induced by dimethyl sulfoxide and other polar compounds. Proc Natl Acad Sci U S A. 1978 May;75(5):2458–2462. doi: 10.1073/pnas.75.5.2458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtenay V. D., Mills J. An in vitro colony assay for human tumours grown in immune-suppressed mice and treated in vivo with cytotoxic agents. Br J Cancer. 1978 Feb;37(2):261–268. doi: 10.1038/bjc.1978.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friend C., Scher W., Holland J. G., Sato T. Hemoglobin synthesis in murine virus-induced leukemic cells in vitro: stimulation of erythroid differentiation by dimethyl sulfoxide. Proc Natl Acad Sci U S A. 1971 Feb;68(2):378–382. doi: 10.1073/pnas.68.2.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerner R. E., Kitamura H., Moore G. E. Studies of tumor cell lines derived from patients with malignant melanoma. Oncology. 1975;31(1):31–43. doi: 10.1159/000225003. [DOI] [PubMed] [Google Scholar]
- Glovanella B. C., Stehlin J. S., Santamaria C., Yim S. O., Morgan A. C., Williams L. J., Jr, Leibovitz A., Fialkow P. J., Mumford D. M. Human neoplastic and normal cells in tissue culture. I. Cell lines derived from malignant melanomas and normal melanocytes. J Natl Cancer Inst. 1976 Jun;56(6):1131–1142. doi: 10.1093/jnci/56.6.1131. [DOI] [PubMed] [Google Scholar]
- Hayashi I., Sato G. H. Replacement of serum by hormones permits growth of cells in a defined medium. Nature. 1976 Jan 15;259(5539):132–134. doi: 10.1038/259132a0. [DOI] [PubMed] [Google Scholar]
- Huberman E., Callaham M. F. Induction of terminal differentiation in human promyelocytic leukemia cells by tumor-promoting agents. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1293–1297. doi: 10.1073/pnas.76.3.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huberman E., Heckman C., Langenbach R. Stimulation of differentiated functions in human melanoma cells by tumor-promoting agents and dimethyl sulfoxide. Cancer Res. 1979 Jul;39(7 Pt 1):2618–2624. [PubMed] [Google Scholar]
- Kitano Y., Hu F. Proliferation and differentiation of pigment cells in vitro. J Invest Dermatol. 1970 Dec;55(6):444–451. doi: 10.1111/1523-1747.ep12260590. [DOI] [PubMed] [Google Scholar]
- Kreider J. W., Wade D. R., Rosenthal M., Densley T. Maturation and differentiation of B16 melanoma cells induced by theophylline treatment. J Natl Cancer Inst. 1975 Jun;54(6):1457–1467. doi: 10.1093/jnci/54.6.1457. [DOI] [PubMed] [Google Scholar]
- Liao S. K., Dent P. B., McCulloch P. B. Characterization of human maligant melanoma cell lines. I. Morphology and growth characteristics in culture. J Natl Cancer Inst. 1975 May;54(5):1037–1044. [PubMed] [Google Scholar]
- Lotan R. Different susceptibilities of human melanoma and breast carcinoma cell lines to retinoic acid-induced growth inhibition. Cancer Res. 1979 Mar;39(3):1014–1019. [PubMed] [Google Scholar]
- Lotan R., Giotta G., Nork E., Nicolson G. L. Characterization of the inhibitory effects of retinoids on the in vitro growth of two malignant murine melanomas. J Natl Cancer Inst. 1978 May;60(5):1035–1041. doi: 10.1093/jnci/60.5.1035. [DOI] [PubMed] [Google Scholar]
- Mather J. P., Sato G. H. The growth of mouse melanoma cells in hormone-supplemented, serum-free medium. Exp Cell Res. 1979 Apr;120(1):191–200. doi: 10.1016/0014-4827(79)90549-4. [DOI] [PubMed] [Google Scholar]
- Oettgen H. F., Aoki T., Old L. J., Boyse E. A., de Harven E., Mills G. M. Suspension culture of a pigment-producing cell line derived from a human malignant melanoma. J Natl Cancer Inst. 1968 Oct;41(4):827–843. [PubMed] [Google Scholar]
- Silagi S. Control of pigment production in mouse melanoma cells in vitro. Evocation and maintenance. J Cell Biol. 1969 Nov;43(2):263–274. doi: 10.1083/jcb.43.2.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strickland S., Mahdavi V. The induction of differentiation in teratocarcinoma stem cells by retinoic acid. Cell. 1978 Oct;15(2):393–403. doi: 10.1016/0092-8674(78)90008-9. [DOI] [PubMed] [Google Scholar]
- Tveit K. M., Fodstad O., Brøgger A., Olsnes S. Human embryonal carcinoma grown in athymic mice and in vitro. Cancer Res. 1980 Mar;40(3):949–953. [PubMed] [Google Scholar]
- WHITTAKER J. R. CHANGES IN MELANOGENESIS DURING THE DEDIFFERENTIATION OF CHICK RETINAL PIGMENT CELLS IN CELL CULTURE. Dev Biol. 1963 Aug;8:99–127. doi: 10.1016/0012-1606(63)90028-9. [DOI] [PubMed] [Google Scholar]