Abstract
Streptococcus suis infection is considered to be a major problem in the swine industry worldwide. Most virulent Canadian isolates of S. suis serotype 2 do not produce the known virulence markers for this pathogen. PCR-based subtraction hybridization was adapted to isolate unique DNA sequences which were specific to virulent strains of S. suis isolated in Canada. Analysis of some subtracted DNA clones revealed significant homology with bacteriophages of gram-positive bacteria. An inducible phage (named Ss1) was observed in S. suis following the incubation of the virulent strain 89-999 with mitomycin C. Phage Ss1 has a long noncontractile tail and a small isometric nucleocapsid and is a member of the Siphoviridae family. Ss1 phage DNA appears to be present in most Canadian S. suis strains tested in this study, which were isolated from diseased pigs or had proven virulence in mouse or pig models. To our knowledge, this is the first report of the isolation of a phage in S. suis.
Infections caused by Streptococcus suis have been considered a major problem in the swine industry worldwide, particularly over the past 10 years. This gram-positive bacterium is responsible for pathological conditions such as meningitis, endocarditis, arthritis, pneumonia, and septicemia followed by sudden death (13). Thirty-five capsular types have been described for S. suis, with type 2 being the most prevalent serotype recovered from diseased animals in most countries, including Canada (2, 12, 32). S. suis serotypes 2 and 14 are also recognized as zoonotic agents (3).
Virulence markers such as muramidase-released protein (MRP), extracellular factor (EF), and suilysin (11, 14, 31) have been identified for S. suis serotype 2. Even if MRP, EF, and suilysin are strongly associated with highly virulent isolates in Europe, they do not seem to be critical for virulence, as demonstrated by gene knockout analysis (1, 24). Moreover, most S. suis serotype 2 strains isolated from diseased pigs in Canada do not produce these virulence markers (10).
It has previously been established, by using ribotyping and random amplified polymorphic DNA analysis assays, that strains of S. suis serotype 2 isolated from diseased pigs are closely related whereas those isolated from healthy animals are genetically diverse (7, 26). Therefore, comparison of genomes between virulent and avirulent bacterial strains could yield information about DNA sequences specific to virulent strains. Two different approaches were used to identify and characterize DNA regions unique to pathogenic S. suis strains. Phage display technology was employed to characterize epitopes specific to a pathogenic S. suis serotype 2 strain (8). However, the only distinct phage antibody found was directed against EF. In the second approach, the in vivo complementation method was used to isolate from a pathogenic strain a 3-kb fragment that increased the virulence of two weakly pathogenic strains (23). Two potential open reading frames were identified by bioinformatic analysis of the 3-kb fragment, but no significant similarities to proteins in databases were found.
Another available strategy to identify DNA sequences specific to virulent strains is known as suppressive subtractive hybridization (SSH), which is based on PCR amplification. This method can amplify DNA sequences that are specifically present in the virulent but not the avirulent strain. Homologous genes in both types of strains are subtracted, and the DNA fragments unique to the virulent strain are cloned and characterized. Here, we report the use of SSH to identify such unique DNA regions present in virulent S. suis serotype 2 strains isolated in Canada, which lead to the detection of a prophage in this bacterial species for the first time.
SSH.
The virulent S. suis serotype 2 strain 89-999 (EF− MRP− hemolysin−), isolated from a diseased pig in Canada and causing septicemia in experimentally inoculated pigs (20), was selected as the DNA tester source. The driver DNA was extracted from the avirulent S. suis serotype 2 strain 90-1330 (20). Standard methods were used for growth of and genomic DNA extraction from S. suis (5). Adaptor ligation, SSH, and PCR amplification were performed essentially as recommended by the supplier of the Clontech PCR-select bacterial genome subtraction kit (BD Biosciences Clontech, Mississauga, Canada). Nested-PCR products were inserted into pBluescript II KS (Stratagene, La Jolla, Calif.) or into pCR2.1 vector by using a T/A cloning kit (Invitrogen, Burlington, Canada). Plasmid DNAs were prepared by a using QIAprep Spin Miniprep kit (Qiagen Inc., Mississauga, Canada) according to the manufacturer's protocol. The cloned SSH products were amplified by PCR in 25 μl of reaction mixture containing 1 μl of diluted recombinant plasmid, 10 pM concentrations of the M13F and M13R primers, 200 μM concentrations of deoxynucleoside triphosphates (Amersham Biosciences Corp., Baie d'Urfé, Canada), 2.5 μl of 10× PCR buffer, and 1 U of Taq polymerase (Amersham Biosciences Corp.). The PCR was performed in GeneAmp PCR system 9700 (Perkin-Elmer Applied Biosystems, Foster City, Calif.) using the following parameters: 94°C for 3 min; 30 cycles at 94°C for 30 s, 55°C for 30 s, and 72°C for 1 min; and a final extension at 72°C for 7 min. Biotin labeling of the inserts was conducted as described above but with the addition of biotin-21-dUTP (BD Biosciences Clontech). For filter hybridization purposes, total genomic DNAs of S. suis strains were randomly labeled with 32P by using Ready-To-Go DNA labeling beads (Amersham Biosciences Corp.). To assess the specificity of the tester-subtractive clones, we carried out the hybridization in both directions. First, PCR products were placed on a nylon membrane and probed with 32P-labeled DNA of tester (S. suis serotype 2 virulent strain 89-999) and driver (S. suis serotype 2 avirulent strain 90-1330) strains (Fig. 1). Second, the clones hybridizing only with the tester DNA were biotin labeled and used to probe dot blot and Southern membranes containing immobilized DNA of tester and driver strains. Thus, after eliminating the clones that hybridized with 90-1390 DNA, the clones harboring sequence specific and unique to the virulent strain 89-999 were recovered.
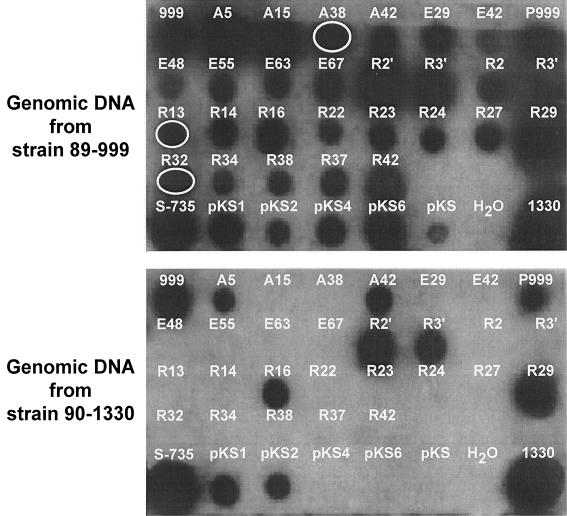
FIG. 1.
Representative dot blot hybridization of SSH products. Cloned SSH products were PCR amplified and probed with 32P randomly labeled genomic DNAs of S. suis serotype 2 strains 89-999 (virulent) and 90-1330 (avirulent). A, E, and R, clones from AluI-, EcoRV-, and RsaI-subtracted libraries, respectively; pKS1, -2, -4, and -6, RsaI-subtracted fragments cloned in pBluescript II KS; 999, DNA from virulent Canadian strain 89-999; 1330, DNA from avirulent Canadian strain 90-1330; S-735, DNA from virulent European strain S-735. Circles indicate the PCR products belonging to phage sequences.
DNA sequences and analysis of the selected clones.
Only clones hybridizing to the genome of the virulent strain 89-999 were selected, giving a total of 35 clones. These clones were also confirmed by hybridization with the genome of S. suis strain 89-1591, another virulent strain from Canada (20). Fifteen of the 35 clones gave a stronger hybridization signal with both virulent strains, 89-999 and 89-1591, and hence were selected for further analysis. DNA sequencing of the clones was performed at the Department of Biochemistry, Microbiology, and Molecular Biology, University of Maine, Orono. Nucleotide homology searches were performed using the BLASTX program of the National Center for Biotechnology Information (http://www.ncbi.nlm.nih.gov/BLAST/). The average size of the subtracted fragments was approximately 600 to 700 bp. Eleven of 15 clones studied were unique.
Bioinformatic analysis.
Nine of the 11 clones showed significant database matches with accessory genetic elements such as transposons, plasmids, and bacteriophages of gram-positive bacteria (Table 1). For instance, two clones (A#3 and A#15) shared homology with deduced proteins found in plasmids of the pMV158 family, including the small cryptic plasmid pSSU1 previously isolated from a S. suis serotype 2 strain (29, 30). This finding suggests that the plasmid carried by strain 89-999 was closely related to pSSU1.
TABLE 1.
Features of subtracted specific clones from S. suis strain 89-999
| Clone | Accession no. | Description
|
||
|---|---|---|---|---|
| Protein | Length (no. of amino acids)a | % Identityb | ||
| A#3 | AY198216 | Replication protein from Streptococcus ferus plasmid pVA380-1 | 110/122 | 90 |
| A#4 | AY198222 | Putative transposase from Lactococcus lactis subsp. lactis | 102/312 | 32 |
| A#15 | AY198217 | Unknown protein (ORF6) from S. suis plasmid pSSU1 | 83/93 | 89 |
| A#16 | AY198215 | No significant homology with proteins in databases | ||
| A#38 | AY198218 | Hypothetical phage protein from S. pyogenes MGAS8232 serotype M18 | 38/110 | 34 |
| E#67 | AY198225 | No significant homology with proteins in databases | ||
| R#13 | AY198220 | Conserved hypothetical protein, phage-associated protein from bacteriophage SF370.2 from S. pyogenes SF370 serotype M1 | 16/29 | 55 |
| R#14 | AY198223 | Relaxase from Streptococcus agalactiae 2603V/R transposon Tn5252 | 131/194 | 67 |
| R#23 | AY198224 | Hydrolase, haloacid dehalogenase-like family from S. agalactiae 2603V/R | 100/207 | 48 |
| R#32 | AY198219 | Conserved hypothetical protein, phage-associated protein from S. pyogenes MGAS315 serotype M3 | 124/232 | 53 |
| R#P4 | AY198221 | Abortive infection protein AbiGII from S. agalactiae 2603V/R | 178/190 | 93 |
Amino acids sharing significant homology with database matches for the total length of compared amino acids.
Percentage of amino acid identity shared between the clone and the respective region of the homologous protein.
Only two clones (R#23 and A#4) shared homology with the sequenced genome of S. suis strain P1/7 (http://www.sanger.ac.uk/Projects/S_suis/blast_server.shtml), which is a European strain with the phenotype MRP+ EF+ suilysin+. These results suggest that most clones obtained in this study may be specific to pathogenic Canadian strains 89-999 and 89-1591 that do not carry the above-mentioned phenotype. Clone R#23 showed homology with the open reading frame of a haloacid dehalogenase-like hydrolase found in the genome of strain P1/7. Interestingly, the gene encoding this protein is located upstream of the suilysin gene (sly) in S. suis strain P1/7. Recently, King et al. (15) showed that in strains lacking the sly gene, the genome arrangement of the region was conserved despite the presence of another gene of unknown function in the sly equivalent position.
The sequences of clones A#16 (AY198215) and E#67 (AY198225) did not share significant homology with the sequences of any proteins in the databases. It can be speculated that these fragments could potentially encode virulence factors or a new class of proteins involved in pathogenicity or virulence processes.
Bacteriophages in S. suis.
Sequence homologies to conserved hypothetical phage (clone A#38) or phage-associated proteins (clones R#32 and R#13) from Streptococcus pyogenes (6, 25) as well as phage resistance systems (clone R#P4) (18, 19) were found (Table 1). In S. pyogenes, phage-like elements account for the great majority of the variation in gene content relative to the genomes of S. pyogenes between the sequenced serotypes M3, M1, and M18 (6, 25). Recombination may have produced chimeric phages and strains with previously uncharacterized arrays of virulence factor genes. S. pyogenes strain MGAS315 has phage genes that encode proteins likely to contribute to pathogenesis, such as streptococcal pyrogenic exotoxin A (SpeA) and SpeK, streptococcal superantigen, and a previously uncharacterized phospholipase A (2) (designated Sla) (6). Phages in other pathogenic Streptococcus species have also been described (21, 27). Recently, Artiushin et al. (4) found that the genes encoding the pyrogenic mitogens in Streptococcus equi were acquired via a phage-mediated horizontal transfer. Interestingly, the genomic region that codes for a phage in S. equi showed extensive similarity to a defective phage sequence in S. pyogenes.
Since phage sequences were found in the selected clones, the presence of a prophage in the virulent strain 89-999 as well as in the avirulent strain 90-1330 was investigated by induction with mitomycin C. Both S. suis strains were grown in 500 ml of Todd-Hewitt broth supplemented with 0.2% maltose and 10 mM MgSO4 until an optical density at 600 nm of 0.2 was reached. The phage induction was attempted via overnight incubation at 37°C in the presence of mitomycin C (1 μg/ml). Complete cell lysis was observed with strain 89-999 but not with strain 90-1330.
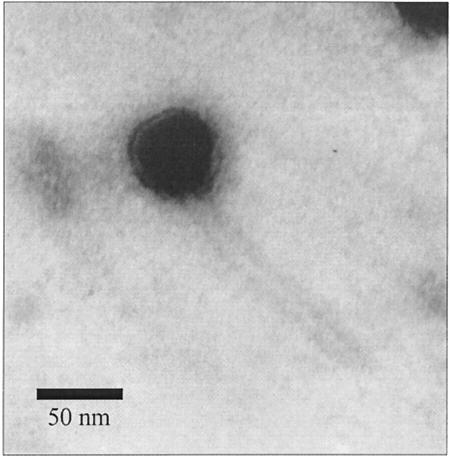
Bacterial lysates of strain 89-999 were then treated as described previously (17) and were observed with a JEOL 1200 electron microscope. Electron microscopy analysis of the lysate from S. suis strain 89-999 revealed the presence of phage particles (Fig. 2). The phage, named Ss1, has a long noncontractile tail of approximately 150 nm and is therefore a member of the Siphoviridae family (9). Phage Ss1 has a small isometric nucleocapsid approximately 50 nm in diameter and thus belongs to the morphotype B1. Phage genome was extracted using a Lambda Midi kit (Qiagen Inc.). Restriction fragment analysis of EcoRI- and HindIII-digested phage DNA indicated that the genome size of Ss1 is approximately 32 kb (data not shown).
FIG. 2.
Electron micrograph of mitomycin-induced temperate phage Ss1 from S. suis strain 89-999. Phosphotungstic acid staining was used. Magnification, ×100,000.
Phage sequences in the genomes of other S. suis strains.
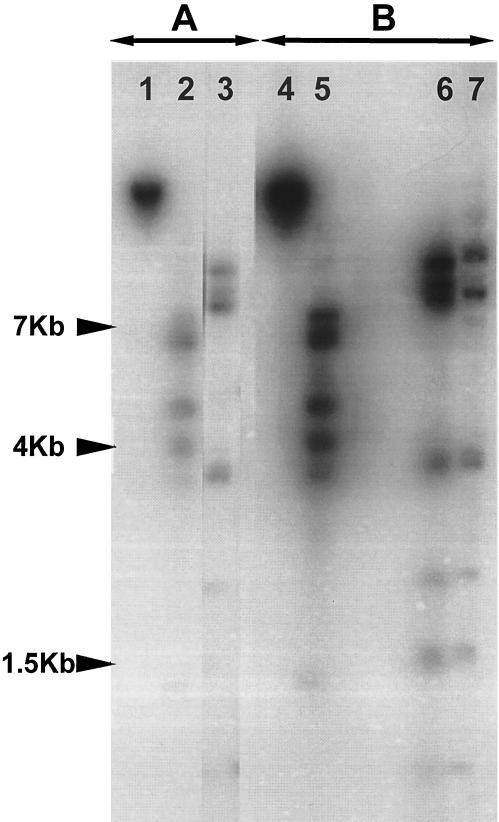
Bacterial and phage DNAs were then digested with EcoRI and HindIII, run on an agarose gel, and transferred onto a nylon membrane. The membrane was hybridized with radioactively labeled DNA from phage Ss1 or from S. suis strain 89-999. The hybridization results confirmed that S. suis 89-999 is a lysogenic strain (Fig. 3). Preliminary data indicated that the labeled phage Ss1 DNA also hybridized with most (9 of 12 strains were positive) of the Canadian S. suis strains isolated from diseased pigs or with proven virulence in mouse or pig models (data not shown). However, the Ss1 sequence was not detected in the genomes of five strains from Europe, including the reference strain of serotype 2 (S-735) and strain P1/7 (data not shown), and one strain from Mexico (data not shown).
FIG. 3.
Southern blots of digested phage and chromosomal DNAs from Ss1 and S. suis strain 89-999, respectively, hybridized with radioactively labeled Ss1 phage genomic DNA (A) or chromosomal DNA of S. suis strain 89-999 (B). Lanes: 1 and 4, uncut Ss1 phage DNA; 2 and 5, EcoRI-treated Ss1 phage DNA; 3 and 6, HindIII-treated Ss1 phage DNA; 7, HindIII-treated chromosomal DNA.
The presence of Ss1 suggests that phage could be involved in the acquisition of virulence genes by strain 89-999 and might have contributed to the genetic diversity observed for S. suis (7). The dissemination of genes encoding virulence factors by such horizontal transfer of DNA is critical in the emergence of new pathogenic organisms. Recent findings suggest that genetic exchange is a significant process involved in the evolution of the population of S. suis strains, which contains various combinations of virulence markers such as suilysin (22, 28). Furthermore, the capsular genes may be moving horizontally through the S. suis population, as has been suggested by a multilocus sequence typing study (16).
Conclusion.
PCR-based SSH was adapted to isolate unique DNA sequences which were specific to virulent strains of S. suis isolated in Canada. In addition, we have demonstrated for the first time the presence of an inducible phage in a virulent S. suis serotype 2 strain from Canada.
Nucleotide sequence accession numbers.
The GenBank accession numbers for the clones from S. suis strain 89-999 are as follows: AY198216 (A#3), AY198222 (A#4), AY198217 (A#15), AY198215 (A#16), AY198218 (A#38), AY198225 (E#67), AY198220 (R#13), AY198223 (R#14), AY198224 (R#23), AY198219 (R#32), and AY198221 (R#P4).
Acknowledgments
This research was supported in part by a grant from Conseil des Recherches en Pêche et Agroalimentaire du Québec (4501) and by the Canadian Research Network on Bacterial Pathogens of Swine from the National Sciences and Engineering Research Council of Canada (225155-00).
Editor: V. J. DiRita
REFERENCES
- 1.Allen, A. G., S. Bolitho, H. Lindsay, S. Khan, C. Bryant, P. Norton, P. Ward, J. Leigh, J. Morgan, H. Riches, S. Eastty, and D. Maskell. 2001. Generation and characterization of a defined mutant of Streptococcus suis lacking suilysin. Infect. Immun. 69:2732-2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allgaier, A., R. Goethe, H. J. Wisselink, H. E. Smith, and P. Valentin-Weigand. 2001. Relatedness of Streptococcus suis isolates of various serotypes and clinical background as evaluated by macrorestriction analysis and expression of potential virulence traits. J. Clin. Microbiol. 39:445-453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arends, J. P., and H. C. Zanen. 1988. Meningitis caused by Streptococcus suis in humans. J. Infect. Dis. 10:131-137. [DOI] [PubMed] [Google Scholar]
- 4.Artiushin, S. C., J. F. Timoney, A. S. Sheoran, and S. K. Muthupalani. 2002. Characterization and immunogenicity of pyrogenic mitogens SePE-H and SePE-I of Streptococcus equi. Microb. Pathog. 32:71-85. [DOI] [PubMed] [Google Scholar]
- 5.Beaudoin, M., J. Harel, R. Higgins, M. Gottschalk, M. Frenette, and J. I. MacInnes. 1992. Molecular analysis of isolates of Streptococcus suis capsular type 2 by restriction-endonuclease-digested DNA separated on SDS-PAGE and by hybridization with an rDNA probe. J. Gen. Microbiol. 138:2639-2645. [DOI] [PubMed] [Google Scholar]
- 6.Beres, S. B., G. L. Sylva, K. D. Barbian, B. Lei, J. S. Hoff, N. D. Mammarella, M. Y. Liu, J. C. Smoot, S. F. Porcella, L. D. Parkins, D. S. Campbell, T. M. Smith, J. K. McCormick, D. Y. Leung, P. M. Schlievert, and J. M. Musser. 2002. Genome sequence of a serotype M3 strain of group A Streptococcus: phage-encoded toxins, the high-virulence phenotype, and clone emergence. Proc. Natl. Acad. Sci. USA 99:10078-10083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chatellier, S., M. Gottschalk, R. Higgins, R. Brousseau, and J. Harel. 1999. Relatedness of Streptococcus suis serotype 2 isolates from different geographic origins as evaluated by molecular fingerprinting and phenotyping. J. Clin. Microbiol. 37:362-366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Greeff, A., L. van Alphen, and H. E. Smith. 2000. Selection of recombinant antibodies specific for pathogenic Streptococcus suis by subtractive phage display. Infect. Immun. 68:3949-3955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Francki, R. I. B., C. M. Fauquet, D. L. Knudson, and F. Brown. 1991. Classification and nomenclature of viruses. Fifth report of the International Committee on Taxonomy of Viruses. Arch. Virol. 2:1-450. [Google Scholar]
- 10.Gottschalk, M., A. Lebrun, H. Wisselink, J. D. Dubreuil, H. Smith, and U. Vecht. 1998. Production of virulence-related proteins by Canadian strains of Streptococcus suis capsular type 2. Can. J. Vet. Res. 62:75-79. [PMC free article] [PubMed] [Google Scholar]
- 11.Gottschalk, M. G., S. Lacouture, and J. D. Dubreuil. 1995. Characterisation of Streptococcus suis capsular type 2 haemolysin. Microbiology 141:189-195. [DOI] [PubMed] [Google Scholar]
- 12.Higgins, R., and M. Gottschalk. 2001. Distribution of Streptococcus suis capsular types in 2000. Can. Vet. J. 42:223. [PMC free article] [PubMed] [Google Scholar]
- 13.Higgins, R., and M. Gottschalk. 1999. Streptococcal disease, p. 563-578. In B. Straw, S. D'Allaire, W. Mengeling, and D. Taylor (ed.), Diseases of swine, 8th ed. Iowa State University Press, Ames.
- 14.Jacobs, A. A., P. L. Loeffen, A. J. van den Berg, and P. K. Storm. 1994. Identification, purification, and characterization of a thiol-activated hemolysin (suilysin) of Streptococcus suis. Infect. Immun. 62:1742-1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.King, S. J., P. J. Heath, I. Luque, C. Tarradas, C. G. Dowson, and A. M. Whatmore. 2001. Distribution and genetic diversity of suilysin in Streptococcus suis isolated from different diseases of pigs and characterization of the genetic basis of suilysin absence. Infect. Immun. 69:7572-7582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.King, S. J., J. A. Leigh, P. J. Heath, I. Luque, C. Tarradas, C. G. Dowson, and A. M. Whatmore. 2002. Development of a multilocus sequence typing scheme for the pig pathogen Streptococcus suis: identification of virulent clones and potential capsular serotype exchange. J. Clin. Microbiol. 40:3671-3680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moineau, S., S. Pandian, and T. R. Klaenhammer. 1994. Evolution of a lytic bacteriophage via DNA acquisition from the Lactococcus lactis chromosome. Appl. Environ. Microbiol. 60:1832-1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.O'Connor, L., A. Coffey, C. Daly, and G. F. Fitzgerald. 1996. AbiG, a genotypically novel abortive infection mechanism encoded by plasmid pCI750 of Lactococcus lactis subsp. cremoris UC653. Appl. Environ. Microbiol. 62:3075-3082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.O'Connor, L., M. Tangney, and G. F. Fitzgerald. 1999. Expression, regulation, and mode of action of the AbiG abortive infection system of Lactococcus lactis subsp. cremoris UC653. Appl. Environ. Microbiol. 65:330-335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Quessy, S., J. D. Dubreuil, M. Caya, and R. Higgins. 1995. Discrimination of virulent and avirulent Streptococcus suis capsular type 2 isolates from different geographical origins. Infect. Immun. 63:1975-1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ramirez, M., E. Severina, and A. Tomasz. 1999. A high incidence of prophage carriage among natural isolates of Streptococcus pneumoniae. J. Bacteriol. 181:3618-3625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sekizaki, T., Y. Otani, M. Osaki, D. Takamatsu, and Y. Shimoji. 2001. Evidence for horizontal transfer of SsuDAT1I restriction-modification genes to the Streptococcus suis genome. J. Bacteriol. 183:500-511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Smith, H. E., H. Buijs, H. J. Wisselink, N. Stockhofe-Zurwieden, and M. A. Smits. 2001. Selection of virulence-associated determinants of Streptococcus suis serotype 2 by in vivo complementation. Infect. Immun. 69:1961-1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Smith, H. E., U. Vecht, H. J. Wisselink, N. Stockhofe-Zurwieden, Y. Biermann, and M. A. Smits. 1996. Mutants of Streptococcus suis types 1 and 2 impaired in expression of muramidase-released protein and extracellular protein induce disease in newborn germfree pigs. Infect. Immun. 64:4409-4412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Smoot, J. C., K. D. Barbian, J. J. Van Gompel, L. M. Smoot, M. S. Chaussee, G. L. Sylva, D. E. Sturdevant, S. M. Ricklefs, S. F. Porcella, L. D. Parkins, S. B. Beres, D. S. Campbell, T. M. Smith, Q. Zhang, V. Kapur, J. A. Daly, L. G. Veasy, and J. M. Musser. 2002. Genome sequence and comparative microarray analysis of serotype M18 group A Streptococcus strains associated with acute rheumatic fever outbreaks. Proc. Natl. Acad. Sci. USA 99:4668-4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Staats, J. J., L. Brandon, B. L. Plattner, J. Nietfeld, S. Dritz, and M. M. Chengappa. 1998. Use of ribotyping and hemolysin activity to identify highly virulent Streptococcus suis type 2 isolates. J. Clin. Microbiol. 36:15-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Styriak, I., P. Pristas, and P. Javorsky. 2000. Lack of GATC sites in the genome of Streptococcus bovis bacteriophage F4. Res. Microbiol. 151:285-289. [DOI] [PubMed] [Google Scholar]
- 28.Takamatsu, D., M. Osaki, and T. Sekizaki. 2002. Evidence for lateral transfer of the suilysin gene region of Streptococcus suis. J. Bacteriol. 184:2050-2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Takamatsu, D., M. Osaki, and T. Sekizaki. 2000. Sequence analysis of a small cryptic plasmid isolated from Streptococcus suis serotype 2. Curr. Microbiol. 40:61-66. [DOI] [PubMed] [Google Scholar]
- 30.Turgeon, N., and S. Moineau. 2001. Isolation and characterization of a Streptococcus thermophilus plasmid closely related to the pMV158 family. Plasmid 45:171-183. [DOI] [PubMed] [Google Scholar]
- 31.Vecht, U., H. J. Wisselink, M. L. Jellema, and H. E. Smith. 1991. Identification of two proteins associated with virulence of Streptococcus suis type 2. Infect. Immun. 59:3156-3162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wisselink, H. J., H. E. Smith, N. Stockhofe-Zurwieden, K. Peperkamp, and U. Vecht. 2000. Distribution of capsular types and production of muramidase-released protein (MRP) and extracellular factor (EF) of Streptococcus suis strains isolated from diseased pigs in seven European countries. Vet. Microbiol. 74:237-248. [DOI] [PubMed] [Google Scholar]