Abstract
Invasion of nonphagocytic cells by bacteria provides a favorable niche for persistence and evasion of host defenses and antibiotics. M protein is a major virulence factor because it promotes high-frequency invasion of epithelial cells by group A Streptococcus (GAS) and also renders the bacterium resistant to phagocytosis. In this study, we investigated the role of M1 protein from serotype M1 strain 90-226 in regulating mammalian signal transduction and cytoskeletal rearrangement for bacterial entry. LY294002 and wortmannin, which are inhibitors of phosphatidylinositol 3-kinase (PI 3-K) blocked invasion of epithelial cells by GAS by 75 and 80%, respectively, but failed to inhibit invasion by Salmonella enterica serovar Typhimurium. Also, epithelial cells transiently transfected with dominant negative p85 and p110 genes, the regulatory and catalytic subunits of PI 3-K, respectively, were less able to be invaded by GAS. To separate the influence of other streptococcal virulence factors from M protein, Lactococcus lactis was engineered to express M1 protein on its surface. L. lactis(pLM1) invaded epithelial cells efficiently in vitro, and PI 3-K inhibitors blocked 90% of this invasion. Purified soluble M1 protein stimulated the formation of stress fibers and actin tuffs on epithelial cells. LY294002 and wortmannin inhibited these cellular changes. A phosphoinositide analogue also inhibited the invasion of epithelial cells by GAS. Therefore, M1 protein, either directly or via bound fibronectin, initiates signals that depend on the lipid kinase PI 3-K pathway, which paves the way for cytoskeletal rearrangement that internalize the bacterium.
Group A Streptococcus (GAS) is a gram-positive coccus that causes infection associated with diverse pathological changes, ranging from pharyngitis to skin infections, life-threatening toxic shock syndrome, acute glomerulonephritis, and rheumatic fever (2, 5). Pathogenic bacteria have evolved different mechanisms to evade the immune defenses and to access deeper tissues. The ability to invade epithelial cells has become an index of virulence for several pathogenic microorganisms. GAS, once considered to be an exclusively extracellular bacterium, has evolved mechanisms that lead to an intracellular state. GAS enters epithelial cells by a zipper like-mechanism (10), a feature it shares with Listeria and Yersinia (1, 6).
GAS produces two virulence factors that contribute to cellular invasion; PrtF (allelic variant SfbI) and M protein (8, 22). Expression of multiple invasins may broaden tissue specificity and/or enhanced virulence. Cue et al. (8, 9) reported that M1 protein is a primary invasin for strain 90-226, because it contributes to ∼90% of the measured bacterial uptake by immortalized epithelial cell lines and by primary tonsillar keratinized epithelial cells (9). Entry of bacteria into nonphagocytic cells is a complex process, involving interactions of several cellular processes with bacterial factors. These interactions result in rearrangement of the cytoskeleton at the site of bacterial penetration (12). Pathogens utilize specific cellular signaling pathways to initiate ingestion by epithelial cells. Although M1 protein (8, 9, 10) and PrtF (26) are known to utilize fibronectin, which subsequently interacts with integrins to promote invasion, the host cellular pathways triggered by these invasin molecules are poorly understood and may be different.
Phosphatidylinositol 3-kinase (PI 3-K) comprises a family of agonist-stimulated, heterodimeric lipid kinases composed of an 85-kDa regulatory subunit and a 110-kDa catalytic subunit that generates D-3-phosphorylated lipid products in the cell membrane on stimulation (3, 29). These phosphoinositides function as secondary messengers which recruit and activate various signaling proteins, which contain pleckstrin homology (PH) domains. Binding of these phosphoinositides to effector molecules result in a signaling cascade that leads to actin polymerization and subsequently to invasion.
The epithelial cell invasion process can be mimicked in vitro by infecting tissue-cultured cells of the pharynx (HEp-2 cells) or lung (A549 cells). Here we report that M1 protein activates a lipid kinase signaling pathway that is required for GAS entry into epithelial cells.
MATERIALS AND METHODS
Bacterial strains.
The serotype M1 GAS strain 90-226 was grown to stationary phase at 37°C in Todd-Hewitt broth supplemented with 2% neopeptone (Difco Laboratories). The solid medium for growth of streptococci was Todd-Hewitt or sheep blood agar. Lactococcus lactis VELL122 was cultured in M17 medium containing 0.5% dextrose. Erythromycin was added to media at a concentration of 5 μg/ml. Plasmid pLM1, encoding full-length M1 protein, was expressed in L. lactis(pLM1), as described elsewhere (9). Strain HB101(pVM101), an Escherichia coli strain expressing the Yersinia enterocolitica invasin gene, and Salmonella enterica serovar Typhimurium strain SL1344 were obtained from Brett Finlay, University of British Columbia, and were grown in Luria-Bertini broth at 37°C with or without 100 μg of ampicillin per ml.
Cell culture and invasion assay.
HEp-2 (ATCC CCL23) and A549 (ATCC CCL185) cells were cultured in minimal essential medium (MEM) or RPMI medium supplemented with 10% fetal bovine serum (Life Technologies) and antibiotics (5 μg of penicillin per ml and 100 μg of streptomycin per ml) (Sigma Chemical Co). Invasion assays with streptococci and L. lactis(pLM1) were performed as previously described (9). The cells were infected with bacteria at a multiplicity of infection of 1:5. After incubation for 2 h at 37°C to allow internalization, unbound bacteria were washed away with Hanks' balanced salt solution and the bound extracellular bacteria were killed by incubation with 100 μg of gentamicin per ml and 5 μg of penicillin per ml. Percent internalization was calculated as the percentage of CFU in the inoculum that survived antibiotic treatment. The toxicity of inhibitors for epithelial cells and bacteria was evaluated. Bacteria were incubated with inhibitors for 4 h, and the bacterial viability was determined. Epithelial cells were incubated with inhibitors for 6 h to determine their effect on viability by trypan blue exclusion.
Inhibitors.
Cytochalasin, staurosporine, 12-O-tetradecanoylphorbol-13-acetate (TPA), calphostin C, and bis-indolylmaleiimide were from Sigma Chemicals; the Akt inhibitor, genistein, LY294002, and wortmannin were from Calbiochem. Cells were preincubated for 30 min with inhibitors before the invasion assays were performed. The inhibitor was present throughout the invasion assay. For inhibition of protein kinase B (Akt), cells were starved of serum for 18 h and preincubated with the inhibitor for 3 h before being infected with streptococci.
Transient transfection.
Plasmid DNAs were purified with the Endo free plasmid purification kit from Qiagen. A549 cells (4 × 104) were plated in 24-well plates containing RPMI with 10% fetal bovine serum. Plasmids pSG5 (vector control), pSG5p85, pSG5p110, and p110CAAX, which express mutant forms of PI 3-K subunits, have been described previously (31). Plasmids pEGFPC2 and pEGFPp85 (vector control) encode a green fluorescent protein (GFP) fusion with the p85 subunit of PI 3-K. Plasmid DNA (1 μg) was mixed with 8 μl of Trans LT1 polyamine transfection reagent (Mirus) and incubated at room temperature for 20 min. DNA-LT1 vesicles were then added dropwise to cells in Opti-MEM and incubated for 8 h at 37°C. Finally, the medium was replaced with RPMI plus fetal bovine serum and the cells were incubated for 18 h. Invasion assays were performed as described above.
Protein techniques.
M1 protein was expressed and purified by using the chitin-intein expression system (9). Protein concentrations were determined by the bicinchoninic acid method (Pierce Chemical Co., Rockford, Ill.) using bovine serum albumin as the standard.
Immunofluorescence.
HEp-2 cells were seeded onto 13-mm coverslips and grown for 24 h. The cells were pretreated for 30 min with dimethyl sulfoxide or 50 nM wortmannin and then infected with bacteria. Streptococci were centrifuged onto the monolayer. The cells were then incubated at 37°C for 2 h to permit bacterial attachment and invasion. After incubation, the cells were washed with Hanks' balanced salt solution, fixed with 3.8% paraformaldehyde, and double stained for extracellular and intracellular bacteria (10). The extracellular bacteria were stained green by incubation with rabbit polyclonal antibodies against GAS (Fitzgerald) followed by secondary goat anti-rabbit antibodies conjugated to fluorescein isothiocyanate (FITC). The cells were permeabilized with 0.1% Triton X-100 for 5 min at room temperature and then stained red with anti-GAS antibody followed by donkey anti-rabbit Cy3. The coverslips were viewed by immunofluorescence microscopy. The extracellular bacteria appear yellow, and the intracellular bacteria appear red (10).
To observe F-actin stress fibers, HEp-2 cells were grown on coverslips and then incubated with M1 protein (400 ng/well) in MEM with 10 μg of fibronectin (Fn) per ml for 2.5 or 10 min, fixed, stained with phalloidin-FITC, and examined for stress fibers using a conventional fluorescence microscope. Pretreatment with 50 nM wortmannin prior to M protein treatment was used to block PI 3-K activation. Cells with one or more folds were considered positive for ruffling and stress fibers.
RESULTS
Entry of GAS into epithelial cells requires a protein kinase but is independent of PKC and tyrosine kinase activity.
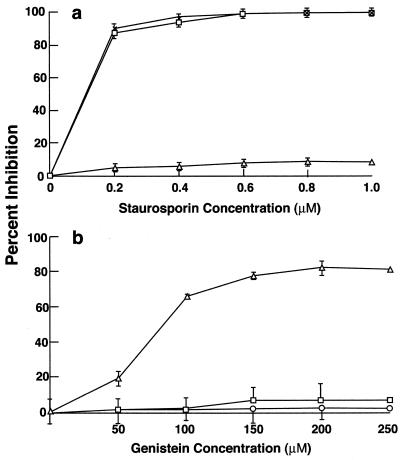
Various inhibitors were used to study the cell signaling pathways that are required for GAS entry into HEp-2 epithelial cells. Staurosporine, a broad-spectrum protein kinase inhibitor (but a poor tyrosine kinase inhibitor), inhibited GAS entry by 80% at a concentration of 0.2 μM. Invasion was completely abolished at higher concentrations (Fig. 1a). As expected, staurosporine inhibited invasion by E. coli that expresses the Yersinia invasin and failed to inhibit internalization of S. enterica serovar Typhimurium, even at a high concentration of 1 μM.
FIG. 1.
Effect of different inhibitors on GAS, S. enterica serovar Typhimurium, and E. coli HB101(pVM101) invasion of HEp-2 cells. Confluent monolayers were preincubated with the inhibitors for 30 min, and the standard invasion assay was performed in the presence of the inhibitor. Values are mean and standard deviation for at least three experiments. The results are expressed as percent inhibition, which is calculated as follows: [(CFUno inhibitor − CFUinhibitor)/CFUno inhibitor] × 100. Percent inhibition by staurosporine (a) and genistein (b) is shown. In the absence of inhibitor, 25% of the inoculum (1 × 108 to 5 × 108 CFU per well) was ingested by epithelial cells.
Inhibition of invasion by staurosporine suggested that a protein kinase pathway was required for internalization of GAS. Protein kinase C (PKC) constitutes a major subpopulation of the protein kinase family. Therefore, PKC inhibitors such as calphostin C and bisindolylmaleimide were tested for their inhibitory effect on GAS invasion of HEp-2 cells. Both inhibitors failed to reduce invasion of these cells by GAS, even at maximum concentrations (Table 1). PKC can be either upregulated by a pulsed exposure to TPA or downregulated by long-term exposure to TPA (29). To further rule out a requirement for PKC, the enzyme was activated or inhibited by incubation of the cells with TPA for 30 min or 24 h, respectively. Internalization of GAS by HEp-2 cells was unaltered by either condition (Table 1).
TABLE 1.
Effect of different inhibitors on the invasion of HEp-2 cells by GASa
| Inhibitor (concn) | % Inhibition |
|---|---|
| None | 0.0 |
| Calphostin C (250 nM) | 1.6 |
| Bisindolylmaleimide (50 nM) | 3.0 |
| TPA (100 nM for 30 min) | 3.8 |
| TPA (100 nM for 24 h) | 2.0 |
| Rapamycin | 3.6 |
The cells were preincubated with the inhibitor for 30 min, and invasion was performed in the presence of inhibitor for 2 h. Percent invasion is calculated as follows: [(CFUno inhibitor − CFUinhibitor)/CFUno inhibitor] × 100.
Genistein, another broad-spectrum inhibitor of tyrosine kinases, was reported to inhibit invasion of epithelial cells by a serotype M6 GAS that is mediated by fibronectin binding to protein PrtF (24, 27). As shown in Fig. 1b, genistein did not inhibit ingestion of the serotype M1 strain 90-226 or, as expected, S. enterica serovar Typhimurium when used at a concentration of 250 μM. Higher concentrations reduced the viability of GAS. Genistein at the same concentration did inhibit the entry of strain HB101(pVM101) into epithelial cells by 80%. Strain HB101(pVM101) is an E. coli recombinant that expresses invasin from Yersinia. Invasin-promoted internalization of Yersinia is blocked by genistein and thus served as a control (1, 28). These experiments differ from those reported by Ozeri et al. and demonstrate that internalization of GAS strain 90-226 requires the activation of a protein kinase (27) but eliminates the possible involvement of PKC or other genistein-sensitive tyrosine kinases, which are required for PrtE-mediated ingestion of streptococci.
Invasion by GAS requires activation of a lipid kinase.
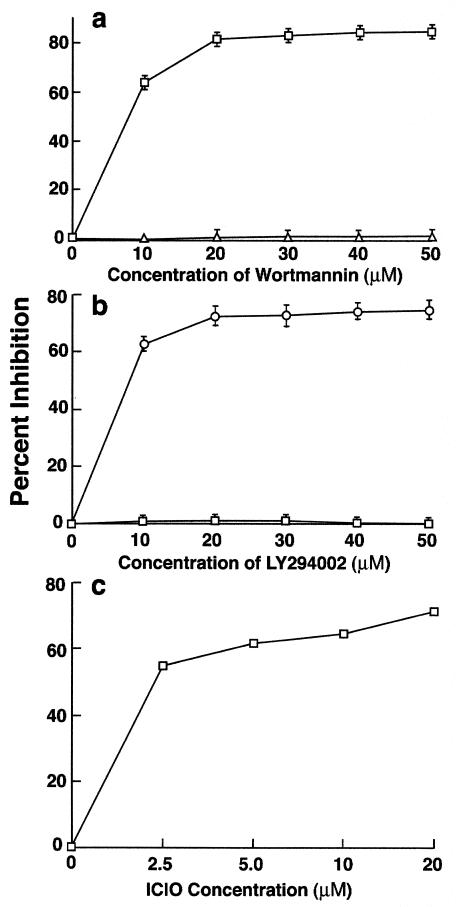
PI 3-K is a lipid kinase, which phosphorylates membrane-associated inositol molecules to generate phosphatidylinositol-3,4-bisphosphate (PI3,4P2) and phosphatidylinositol-3,4,5-trisphosphate (PI3,4,5P3) lipid phosphates (29). These secondary messengers regulate several cellular processes, including cytoskeletal reorganization. Two structurally unrelated compounds, wortmannin and LY294002, are known to inhibit PI 3-K by different mechanisms (16). Both inhibitors blocked the ingestion of GAS in a dose-dependent manner (Fig. 2). Wortmannin inhibited invasion by 66% at a concentration of 10 nM and 80% at a concentration of 20 nM (Fig. 2a), and LY294002 inhibited invasion by 61% at a concentration of 10 μM and 77% at a concentration of 50 μM (Fig. 2b). Inhibition was specific, since both wortmannin and LY294002 failed to inhibit the entry of S. enterica serovar Typhimurium into HEp-2 cells, which is independent of PI 3-K (12).
FIG. 2.
Effect of different inhibitors on GAS and S. enterica serovar Typhimurium invasion of HEp-2 cells. Monolayers were preincubated with inhibitors for 30 min, and the invasion was carried out in the presence of the inhibitor. Percent inhibition was calculated as described in the legend to fig. 1. Values are mean and standard deviation for at least three experiments. (a) Wortmannin, (b) LY294002, (c) Akt inhibitor ICIO. In the absence of inhibitor, 25% of the inoculum (1 × 108 to 5 × 108 CFU per well) was ingested by epithelial cells.
Kinase inhibitors do not prevent adherence or influence the survival of intracellular streptococci.
To rule out the possibility that the above chemical inhibitors were antibacterial or had cytotoxic effects on target cells, various control experiments were done. Chemical inhibitors or their solvent, such as dimethyl sulfoxide, were incubated for 4 h with bacteria, and their effect on viability was determined. Monolayers were incubated with the same inhibitors or solvents for 6 h, and cell viability was determined by trypan blue exclusion. None of the inhibitors or solvents affected bacterial or cell viability at the concentrations used (data not shown).
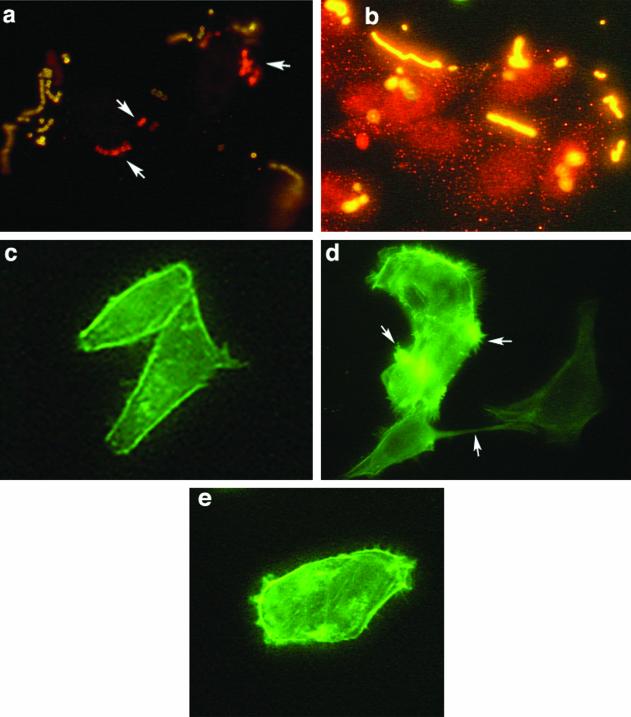
Chemical inactivation of PI 3-K confirmed a requirement for this lipid kinase for ingestion of strain 90-226 by A549 cells. Immunofluorescent staining of GAS associated with epithelial cells was performed to test whether wortmannin and LY294002 blocked adherence rather than internalization of bacteria by epithelial cells. Cells were infected with strain 90-226 in the presence or absence of 50 nm wortmannin or 100 μM LY294002. After a 2-h incubation, the cells were fixed with paraformaldehyde and extracellular bacteria were stained with rabbit anti-GAS antibody followed by anti-rabbit-FITC conjugate (green). Following detergent permeabilization, the slides were incubated again with rabbit anti-GAS antibody followed by anti-rabbit-Cy3 conjugate. Extracellular streptococci should stain both green and red, whereas intracellular streptococci should stain only red. As expected, when confocal immunofluorescent images were overlaid, extracellular streptococci appeared yellow and intracellular bacteria appeared red. Control cells without inhibitor contained numerous red intracellular bacteria (see Fig. 4a). In contrast, cells treated with wortmannin or LY294002 (data not shown) were associated only with yellow extracellular bacteria (see Fig. 4b).
FIG. 4.
M1 protein induction of localized cytoskeleton rearrangement and stress fibers in HEp-2 cells is dependent on the PI 3-K pathway. (a and b) Wortmannin inhibited the ingestion of streptococci but did not impede adherence to HEp-2 cells. Infected cells were fixed, and the extracellular bacteria were stained with rabbit anti-GAS antibody followed by anti-rabbit-FITC conjugate. The cells were permeabilized, and intracellular bacteria were again exposed to rabbit anti-GAS antibody and labeled with anti-rabbit Cy3. Arrows indicate the intracellular bacteria, which appear red. Extracellular bacteria are yellow. (a) Cells infected in the absence of wortmannin. (b) Cells infected in the presence of 50 nM wortmannin. (c to d) Cells preincubated with purified M protein. (c) Control untreated cells. (d) Cells treated with 400 ng of recombinant M1 protein per ml for 10 min. (e) Cells treated with wortmannin plus 400 ng of purified M1 protein per ml in MEM with Fn. Arrows indicate actin tufts of lamellipodia and stress fibers.
To confirm that PI 3-K inhibitors block the uptake of streptococci rather than reducing intracellular viability by inducing antibacterial activity, the impact of PI 3-K inhibitors on intracellular viability was analyzed. HEp-2 cells were incubated with bacteria for 2 h to permit ingestion, washed, and incubated for 2 h in medium containing antibiotics with or without staurosporine (500 nM), wortmannin (50 nM), or LY294002 (100 μM). No significant difference was observed in the viability of residual intracellular bacteria in the presence of inhibitors (data not shown). Therefore, inhibitors do not induce cellular antibacterial activity, nor does PI 3-K activation generate signals which protect streptococci from intracellular lysosomal antibacterial processes.
Genetic inactivation of PI 3-K prevents internalization of GAS by epithelial cells.
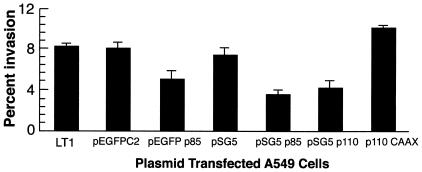
The above pharmacological inhibition studies suggested that activation of PI 3-K is required for GAS internalization by HEp-2 cells. To reaffirm this conclusion, PI 3-K was genetically inhibited by transient expression of dominant negative mutant forms of p85 and p110 subunits, the regulatory and catalytic subunits of PI 3-K, respectively. Cells that were transfected with plasmid pSG5 (vector only) served as a negative control. Invasion of cells that express the dominant negative p85 subunit was reduced by 58%, whereas invasion of cells that express the mutant p110 subunit was reduced by 50% (Fig. 3). In contrast, cells transfected with a constitutively active form of p110, plasmid p110 CAAX, showed enhanced uptake of streptococci; internalization was increased by 37%. Plasmid DNA and transfection conditions did not alter cellular viability. The pSG5 vector constructs did not allow us to assess the frequency of transfection; therefore, experiments were also performed using a GFP-p85 fusion protein (20). The control plasmid pEGFPC2 did not alter the ingestion of streptococci by A549 cells (Fig. 3). However, transfection with plasmid pEGFPp85, which expressed the dominant negative form of protein p85, reduced invasion by 37%. The degree of inhibition by dominant negative forms of PI 3-K was not equivalent to that by pharmacological inhibitors. This is presumably because only 25 to 30% of cells expressed transfected genes. These experiments confirmed that the lipid kinase pathway is required for successful entry of GAS into epithelial cells.
FIG. 3.
Inhibition of invasion of A549 cells by dominate negative and constitutive forms of PI 3-K. Data are expressed as the percent invasion of transiently transfected A549 cells relative to untransfected A549 cells with the LT1 transfection reagent. LT1 cells were exposed to the LT1 polyamine transfection reagent without plasmid DNA. The cells were transfected with pSG5 (vector only), pSG5-p85 (dominant negative p85), pSG5-p110 (dominant negative p110), or p110CAAX (constitutively active form of p110) (30). They were also transfected with a GFP fusion (pEGFP-p85) or vector only (pEGFPC2) (20).
M1 protein directly or indirectly activates the PI 3-K pathway.
Fn binding and ingestion of strain 90-226 is dependent primarily on M1 protein (8). We postulated that M1 protein interacts either directly or indirectly with epithelial cells to generate signals that result in invasion. To test this hypothesis, full-length M1 protein was cloned and expressed on the surface of L. lactis(pLM1) (9). Expression of M protein conferred invasive properties on this otherwise noninvasive culture. As previously shown (9), L. lactis(pLM1) invaded epithelial cells efficiently; 16% of the initial inoculum was ingested by HEp-2 cells in the presence of Fn (Table 2). Inhibitors of PI 3-K, i.e., LY294002 and wortmannin, blocked the invasion of epithelial cells by strain pLM1 by 84 and 92%, respectively. As expected, cytochalasin D and staurosporine inhibited invasion by 100 and 98.8%, respectively. Calphostin C (11), bisinolylmaleimide, and genistein failed to inhibit the invasion of epithelial cells by M+ L. lactis(pLM1), replicating our previous results with streptococci (Table 2; Fig. 1b). These experiments confirmed that M1 protein-dependent ingestion of streptococci is triggered by activation of the lipid-signaling pathway and are consistent with the possibility that M1 protein alone is sufficient to activate the appropriate signaling pathway.
TABLE 2.
Effect of different inhibitors on invasion of HEp-2 cells by L. lactis(pLM1) that expresses M1 protein on its surfacea
| Inhibitor (concn) | % Inhibition |
|---|---|
| None | 0.0 |
| Cytochalasin D (500 nM) | 100.0 |
| Staurosporine (1 μM) | 98.8 |
| Genistein (250 μM) | 1.3 |
| CalphostinC (250 nM) | 3.0 |
| Bisindolylmaleimide (50 nM) | 1.6 |
| Wortmannin (100 nM) | 92.0 |
| LY294002 (50 μM) | 84.8 |
Inhibitors were preincubated with cells for 30 min, and invasion was performed in the presence of inhibitors for 2 h. Results are the average of three individual experiments. Percent inhibition is calculated as described in Table 1, footnote a.
Induction of cytoskeletal changes by purified M1 protein is dependent on PI 3-K.
The above experiments suggested that M1 protein was sufficient to activate PI 3-K for efficient uptake of streptococci by epithelial cells. To determine whether M1 protein in association with Fn was an agonist for PI 3-K activation, we investigated whether purified M1 protein in solution could trigger actin rearrangement and formation of membrane stress fibers. HEp-2 cells were incubated with 400 ng of M1 protein per ml in MEM with Fn (10 μg/ml) for 2, 5, and 10 min. The cells were washed and stained for F-actin with phalloidin-FITC. Other cells were preincubated with 50 nM wortmannin for 30 min before incubation with purified M1 protein. Incubation of HEp-2 cells with M1 protein resulted in the formation of stress fibers and actin tufts within 5 min (Fig. 4d). These changes were abolished by treatment of cells with 50 nM wortmannin, suggesting a requirement for PI 3-K activation (Fig. 4e). The difference between treated and untreated cells was quantified by counting 15 random fields of semiconfluent monolayers. In M1 protein-treated cells without exposure to wortmannin, 84 of every 100 cells had actin tufts and lamellipodia. In contrast, among those treated with wortmannin and M1 protein and in control untreated cells, 3 of every 100 cells showed only mild membrane changes and none had actin tufts or lamellipodia. Thus, formation of lamellipodia and extensive cytoskeletal rearrangements, induced by M1 protein, were abolished on inhibition of the PI 3-K pathway. This supports our suggestion that M1 protein is either directly or indirectly an agonist of PI 3-K.
Invasion by GAS can be inhibited by PI analogues:
PI3,4P2 and PI3,4,5P3 function as secondary messengers for several downstream effectors such as p70 ribosomal S6 kinase (p70s6k), PKC, and Akt. p70s6k participates in translational control of mRNA transcripts and is sensitive to the drug rapamycin (15). Ingestion of strain 90-226 by HEp-2 cells was not inhibited by rapamycin, suggesting that p70s6k activation is not necessary for invasion (Table 1). Because PKC was not found to be required for ingestion of GAS by epithelial cells, a role for PKB/Akt was considered. IL-6-hydroxymethyl-chiro-inositol-2-[(R)-2-O-methyl-3-O-octadecylcarbonate] (ICIO), a relatively new Akt inhibitor (16), was tested for its potential to inhibit invasion (Fig. 2C). ICIO is an inactive PI analogue that competes with active phosphoinositide products produced by PI 3-K. This analogue has a 50% inhibitory concentration of 5 μM for Akt, which is well below 83 μM, the concentration required to inhibit PI 3-K. Ingestion of GAS was inhibited by 55 and 65% at 2.5 and 10 μM ICIO, respectively. This suggests that Akt phosphorylation is probably required for GAS internalization.
Requirement for Ras in GAS invasion of HEp-2 cells.
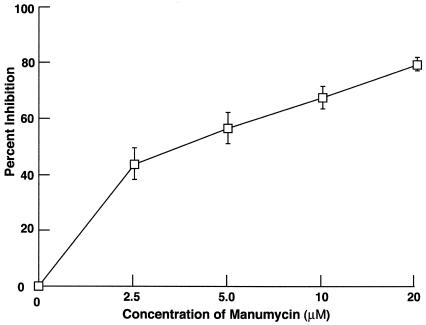
The small G protein, Ras, is an upstream signaling molecule of PI 3-K and is known to function as an alternative mechanism for recruitment of PI 3-K (4). Therefore, we investigated whether ingestion of streptococci by HEp-2 cells required the activation of Ras by testing the sensitivity of invasion to manumycin A (Fig. 5). Ras is activated by posttranslational isoprenylation of a C-terminal residual, and manumycin A, a farnesyl inhibitor, blocks isoprenylation. Manumycin A was found to block the internalization of 90-226 streptococci by HEp-2 cells in a dose-dependent manner. Invasion was inhibited by 56% at 5 μM manumycin A and by 80% at 20 μM, suggesting that Ras is a component of the signaling pathway that leads to ingestion of streptococci by epithelial cells.
FIG. 5.
Effect of manumycin A, a farnesyl transferase I inhibitor, on GAS invasion of HEp-2 cells. Values are mean and standard deviation for least three experiments. The results are expressed as percent inhibition, which is calculated as described in the legend to Fig. 1.
DISCUSSION
Intracellular life is ideally suited for bacteria to overcome unfavorable host conditions. Internalization requires a major, varied alteration of the underlying cytoskeleton. This change is initiated by activation of complex host cell-signaling pathways (12, 13). An “uptake signal” promotes the relocalization of bacteria from outside to inside a cell. For some bacterial pathogens, the mechanisms of internalization are well understood. For example, Listeria monocytogenes, a gram-positive bacterium, activates host tyrosine kinases and PI 3-K, leading to actin rearrangement and internalization of the bacterium (17, 18). Invasin from Yersinia binds to integrins, leading to their clustering and signaling through focal adhesion kinase (FAK) and the small G protein Rac (1).
GAS are efficiently internalized by epithelial cells in vitro, and some strains multiply intracellularly (21, 23). Österlund et al. suggested that the intracellular state is important for human disease by showing that tonsils from children with recurrent tonsillitis contain intracellular streptococci (25). The major invasins of GAS are PrtF and M1 protein (8, 9, 10, 23, 26). For the many GAS serotypes that fail to express PrtF, such as the M1 strain 90-226 used in this study, M protein is the primary invasin. For this reason and the fact that serotypes differ in their potential to induce more serious infections, we considered the possibility that M1 protein-activated uptake of streptococci may require different or additional signaling events with respect to those that express protein PrtF. M1 protein and PrtF bind to Fn, which bridges streptococci to integrin receptors (8, 23, 26). However, the intracellular signals which are activated by receptor ligation and which promote internalization, appear to differ for these invasins. PrtF-Fn complexes induce integrin clustering, which results in the recruitment of phosphorylated FAK (p125FAK) and activation of small G proteins, Rac/CdC42 (see Fig. 6) (27). Invasion of epithelial cells by strain JRS145 (PrtF+ M6−) was inhibited by genistein, a pharmacological inhibitor of tyrosine kinases. PrtF recruits FAK for GAS internalization, and this signaling protein is genistein sensitive. Ozeri et al. suggested that these two pathways act independently to promote actin rearrangement, which leads to invasion (27). We replicated this result using strain JRS4 (PrtF+ M6+) (data not shown). Genistein reduced the invasion of HEp-2 cells by 45%. The remaining activity, which is genistein resistant, is probably promoted by M protein, independent of PrtF.
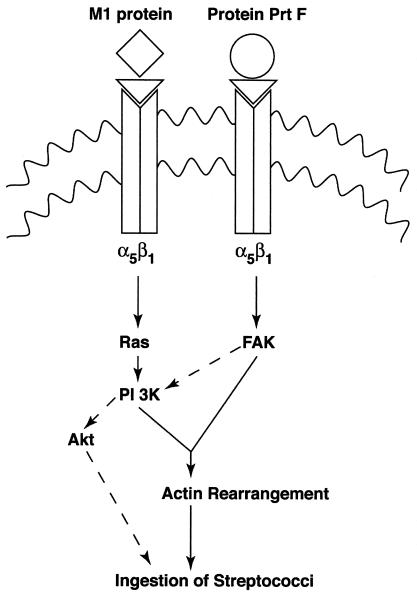
FIG. 6.
Schematic illustration of signaling pathways postulated to be activated by M1 protein and PrtF of GAS. ◊, M protein; ○, PrtF; ▿, Fn.
In comparison, genistein did not block M1 protein-mediated internalization of strain 90-226 by epithelial cells. Nor did it inhibit invasion of cells promoted by M1 protein expressed on the surface of L. lactis. This brings into question the role of FAK in uptake of PrtF− streptococci and suggests that although both invasins depend on Fn to interact with α5β1-integrins, different signaling pathways are used to induce required cytoskeletal changes. Although genistein sensitivity can be variable and dependent on the cell line, this does not explain our results because Yersinia invasin-promoted ingestion of E. coli was genistein sensitive, as expected under the conditions used here (1, 19, 28). Differences in the sensitivity of Listeria and M+ streptococci ingestion to genistein could depend on the receptors they engage on the cell surface. M1 protein is presumed to initiate the signaling cascade via fibronectin binding to α5β1-integrins, whereas InlB engages both C1qR and Met tyrosine receptors (6). It is less clear why PrtF and M1 protein use different pathways, since both initiate signals at α5β1-integrins. PrtF, however, binds with greater affinity and to a different domain of Fn. Such differences could influence the way in which Fn interacts with integrins, which, in turn, may affect the signals generated from engaged receptors.
Phosphoinositides are normally absent in quiescent cells, but their levels rapidly increase on agonist stimulation. Many Ser/Thr kinases are activated by the phosphoinositides generated by PI 3-K (29). PI 3-K is involved in various cellular processes such as phagocytosis, pseudopod formation, and membrane ruffling (7). A crucial requirement for PI 3-K activation in M1 protein-mediated GAS invasion was indicated by pharmacological and genetic inhibition studies. The p110 catalytic subunit of PI 3-K must be recruited to the plasma membrane in order to be active. This is dependent on the p85 regulatory subunit and adapter molecules. Artificial membrane docking of p110 catalytic subunits can be accomplished by genetic manipulation of the protein. The constitutively active p110 mutant, expressed from the p110CAAX plasmid, is an example. As expected, cells that express p110CAAX showed enhanced invasion by strain 90-226, further confirming that activation of the lipid kinase promotes the internalization of streptococci.
Immunofluorescence studies demonstrated that wortmannin blocks only internalization and does not inhibit bacterial adherence to epithelial cells; therefore, PI 3-K controls signaling events that are specific for internalization and follow the adherence of streptococci to cells. Uptake of lactococci that express M1 protein and soluble, purified M1 protein promote actin polymerization by PI 3-K-dependent mechanisms. Thus, PI 3-K is central to signaling events required for ingestion of some strains of GAS. InlB protein, an important surface invasin of L. monocytogenes, also activates PI 3-K; however, unlike for M1 protein, this signaling event is sensitive to genistein (17).
PI 3-K activation results in the generation of phosphorylated lipid products in the cell membrane and recruits proteins containing the PH domain. A number of signaling pathways are known to be present downstream of PI 3-K, including Rac, Ser/Thr kinases such as p70s6k, Akt, and PKC (3). p70s6k and PKC are not essential for M1 protein-mediated GAS invasion, because specific inhibitors of their activities failed to block internalization of GAS. Phosphorylation of Akt and activation can occur only at the plasma membrane by interaction with phosphoinositides generated by PI 3-K. The Akt inhibitor ICIO, an inactive analogue of PI3,4P2 can compete for the PH domain of Akt and thus hinders its recruitment to the cell membrane (16). ICIO inhibits invasion by GAS, suggesting that the downstream effector molecule from PI 3-K is Akt. Further studies with dominant negative and constitutively active Akt genes will be carried out to confirm its role in GAS invasion.
Recruitment of PI 3-K to the cell membrane is accomplished by several mechanisms. Phosphorylated tyrosines bind to the SH2 domain of p85, which translocates the catalytic subunit to the cell membrane (3). An alternate mechanism by which PI 3-K can be recruited to the membrane is through the small G protein Ras. PI 3-K can bind to activated Ras and thus can be recruited to the plasma membrane (3, 4). Activation of Ras requires posttranslational isoprenylation by farnesyl transferase I, which is specifically inhibit by manumycin A. This compound inhibited the invasion of epithelial cells by GAS in a dose-dependent fashion, suggesting a requirement for Ras activation. Inactivation of Ras probably inhibits the transport of PI 3-K to the plasma membrane. Mansell et al. (21) reported that Listeria InlB promotes PI 3-K translocation by two different mechanisms; one involves the phosphotyrosine adapter molecules Gab and Cbl, and the other uses Ras. Therefore, we suggest that the interaction of M1 protein with the cell surface activates Ras, either directly or indirectly via Fn, leading to activation and recruitment of PI 3-K and to the cytoskeletal changes required for ingestion of GAS by epithelial cells.
Figure 6 is a summary and comparison of signaling events, predicted to be generated by M1 protein and PrtF, that promote GAS internalization by epithelial cells. PrtF-mediated invasion requires activation of FAK, which is sensitive to genistein. In contrast, M1 protein-mediated invasion is predicted to require the activation of Ras and PI 3-K, which are insensitive to genistein. On the other hand, phosphoinositide products of PI 3-K have been reported to activate Rac, which is known to coordinate actin polymerization (3). Although PrtF can signal the ingestion of streptococci through Rac activation (27), we have not yet investigated whether M1 protein can act in a similar manner. Instead, preliminary pharmacological inhibition studies suggest that activation of the Ser/Thr kinase, Akt, may act downstream of PI 3-K when M1 protein initiates internalization signals. It is unclear which pathway dominates when streptococci express both M1 and PrtF proteins on their surface or whether both operate at the same time. The sensitivity of ingestion of an M6+ PrtF strain to genistein suggests that the PrtF pathway overrides that induced by M protein-activated signals (unpublished data).
Acknowledgments
We thank Tim Leonard for help in figure preparation and Yogi Shimizu for the pEGFPp85 fusion construct.
This work was supported by Public Health Service grant AI34503.
Editor: J. D. Clements
REFERENCES
- 1.Alrutz, M. A., and R. R. Isberg. 1998. Involvement of focal adhesion kinase in invasin-mediated uptake. Proc. Natl. Acad. Sci. USA 95:13658-13663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bisno, A. L., and D. L. Stevens. 1996. Streptococcal infection of skin and soft tissues. N. Engl. J. Med. 334:240-245. [DOI] [PubMed] [Google Scholar]
- 3.Cantrell, D. A. 2001. Phosphoinositide 3-kinase signaling pathways. J. Cell Sci. 114:1439-1445. [DOI] [PubMed] [Google Scholar]
- 4.Chan, T. O., U. Rodeck, A. M. Chan, A. Kimmelman, S. E. Rittenhouse, G. Panayotou, and N. Tischlis. 2002. Small GTPase and tyrosine kinases coregulate a molecular switch in the phosphoinositide 3-kinase regulatory subunit. Cancer Cell 1:181-191. [DOI] [PubMed] [Google Scholar]
- 5.Cleary, P. P., E. L. Kaplan, J. P. Handley, A. Wlazlo, M. H. Kim, A. R. Hauser, and P. M. Schlievert. 1992. Clonal basis for resurgence of serious Streptococcus pyogenes disease in the 1980s. Lancet 339:518-521. [DOI] [PubMed] [Google Scholar]
- 6.Cossart, P., J. Pizzaro-Cerda, and M. Leucit. 2003. Invasion of maamalian cells by Listeria monocytogenes: functional mimicry to subvert cellular functions. Trends Cell Biol. 13:23-31. [DOI] [PubMed] [Google Scholar]
- 7.Cox, D., C. C. Tseng, G. Bjekic, and S. Greenberg. 1999. A requirement for phosphatidylinositol 3-kinase in pseudopod extension. J. Biol. Chem. 274:1240-1247. [DOI] [PubMed] [Google Scholar]
- 8.Cue, D., P. E. Dombek, H. Lam, and P. P. Cleary. 1998. Streptococcus pyogenes serotype M1 encodes multiple pathways for entry into human epithelial cells. Infect. Immun. 66:4593-4601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cue, D., H. Lam, and P. P. Cleary. 2001. Genetic dissection of Streptococcus pyogenes M1 protein: regions involved in fibronectin binding and intracellular invasion. Micob. Pathog. 31:231-242. [DOI] [PubMed] [Google Scholar]
- 10.Dombek, P. E., D. Cue, J. Sedgewick, H. Lam, S. Ruschowski, B. B. Finlay, and P. P. Cleary. 1999. High-frequency intracellular invasion of epithelial cells by serotype M1 streptococci: M1 protein mediated invasion and cytoskeletal rearrangement. Mol. Microbiol. 31:859-870. [DOI] [PubMed] [Google Scholar]
- 11.Dubyak, G. R., and S. B. Kertesy. 1997. Inhibition of GTP gamma S-dependent phospholipase D and Rho membrane association by calphostin is independent of protein kinase C catalytic activity. Arch. Biochem. Biophys. 341:129-139. [DOI] [PubMed] [Google Scholar]
- 12.Finlay, B. B., and P. Cossart. 1997. Exploitation of mammalian host cell functions by bacterial pathogens. Science 276:718-725. [DOI] [PubMed] [Google Scholar]
- 13.Galan, J. E., and J. B. Bliska. 1996. Cross talk between bacterial pathogens and their host cells. Annu. Rev. Cell Dev. Biol. 12:221-255. [DOI] [PubMed] [Google Scholar]
- 14.Hanski, E., and M. Caparon. 1992. Protein F, a fibronectin-binding protein, is an adhesin of the group A streptococcus, Streptococcus pyogenes. Proc. Natl. Acad. Sci. USA 89:6172-6176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hidalgo, M., and E. K. Rowinsky. 2000. The rapamycin-sensitive signal transduction pathway as a target for cancer therapy. Oncogene 19:6680-6688. [DOI] [PubMed] [Google Scholar]
- 16.Hu, Y., L. Qiao, S. Wang, S.-B. Rong, E. J. Meuillet, M. Berggren, A. Gallegos, G. Powis, and A. P. Kozikowski. 2000. 3-(Hydroxymethyl)-bearing phosphatidylinositol ether lipid analogues and carbonate surrogates block PI3-k, Akt and cancer cell growth. J. Med. Chem. 43:3045-3051. [DOI] [PubMed] [Google Scholar]
- 17.Ireton, K., B. Payrastre, H. Chap, W. Ogawa, H. Sakaue, M. Kasuga, and P. Cossart. 1996. A role for phosphoinositide 3-kinase in bacterial invasion. Science 274:780-782. [DOI] [PubMed] [Google Scholar]
- 18.Ireton, K., B. Payrastre, and P. Cossart. 1999. The Listeria monocytogenes protein InlB is an agonist of mammalian PI 3-kinase. J. Biol. Chem. 274:17025-17032. [DOI] [PubMed] [Google Scholar]
- 19.Isberg, R. R. 1991. Discrimination between intracellular uptake and surface adhesion of bacterial pathogens. Science 252:934-938. [DOI] [PubMed] [Google Scholar]
- 20.Kivens, W. J., S. W. Hunt III, J. L. Mobley, T. Zell, C. L. Dell, B. E. Bierer, and Y. Shimizu. 1998. Identification of a proline-rich sequence in the CD2 cytoplasmic domain critical for regulation of integrin-mediated adhesion and activation of phosphoinositide 3-kinase. Mol. Cell. Biol. 18:5291-5307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mansell, A., N. Khelef, P. Cossart, and L. A. O'Neill. 2001. Internalin B activates nuclear factor-κB via ras, phosphoinositide 3-kinase and Akt. J. Biol. Chem. 276:43597-43603. [DOI] [PubMed] [Google Scholar]
- 22.Medina, E., O. Goldmann, A. W. Toppel, and G. S. Chhatwal. 2003. Survival of Streptococcus pyogenes within host phagocytic cells: a pathogenic mechanism for persistence and systemic invasion. J. Infect. Dis. 187:597-603. [DOI] [PubMed] [Google Scholar]
- 23.Molinari, G., S. R. Talay, P. Valentine-Weigand, M. Rhode, and G. S. Chhatwal. 1997. The fibronectin-binding protein of Streptococcus pyogenes, SfbI, is involved in the internalization of group A Streptococcus by epithelial cells. Infect. Immun. 65:1357-1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Molinari, G., M. Rohde, S. R. Talay, G. S. Chhatwal, S. Beckert, and A. Podbielski. 2001. The role played by the group A streptococcal negative regulator Nra on bacterial interactions with epithelial cells. Mol. Microbiol. 40:99-114. [DOI] [PubMed] [Google Scholar]
- 25.Österlund, A., R. Popa, T. Nikkila, A. Scheynius, and L. Engstrand. 1997. Intracellular reservoir of Streptococcus pyogenes in vivo: a possible explanation for recurrent pharyngotonsillitis. Laryngoscope. 107:640-647. [DOI] [PubMed] [Google Scholar]
- 26.Ozeri, V., I. Rosenshine, D. F. Mosher, R. Fassler, and E. Hanski. 1998. Roles of integrins and fibronectin in the entry of Streptococcus pyogenes into cells via protein F1. Mol. Microbiol. 30:625-637. [DOI] [PubMed] [Google Scholar]
- 27.Ozeri, V., I. Roseshine, A. Ben-Ze'ev, G. M. Bokoch, T.-S. Jou, and E. Hanski. 2001. De novo formation of focal complex-like structures in host cells by invading streptococci. Mol. Microbiol. 41:561-573. [DOI] [PubMed] [Google Scholar]
- 28.Rosenshine, I., S. Ruschkowski, V. Foubister, and B. B. Finlay. 1994. Salmonella typhimurium invasion of epithelial cells: role of induced host cell tyrosine protein phosphorylation. Infect. Immun. 62:4969-4974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Toker, A. 2000. Protein kinase as mediators of phosphoinositide 3-kinase signaling. Mol. Pharmacol. 57:652-658. [PubMed] [Google Scholar]
- 30.Velge, P., E. Bottreau, B. Kaeffer, N. Yurdusev, P. Pardon, and N. Van Lagendonck. 1994. Protein tyrosine kinase inhibitors block the entries of Listeria monocytogenes and Listeria ivanovii into epithelial cells. Microb. Pathog. 17:37-50. [DOI] [PubMed] [Google Scholar]
- 31.Wang, B., D. J. Lim, J. Han, Y. S. Kim, C. B. Basbaum, and J. D. Li. 2002. Novel cytoplasmic proteins of nontypeable Haemophilus influenzae up-regulate human MUC5AC mucin transcription via a positive p38 mitogen-activated protein kinase pathway and a negative phosphoinositide 3-kinase-Akt pathway. J. Biol. Chem. 277:949-957. [DOI] [PubMed] [Google Scholar]