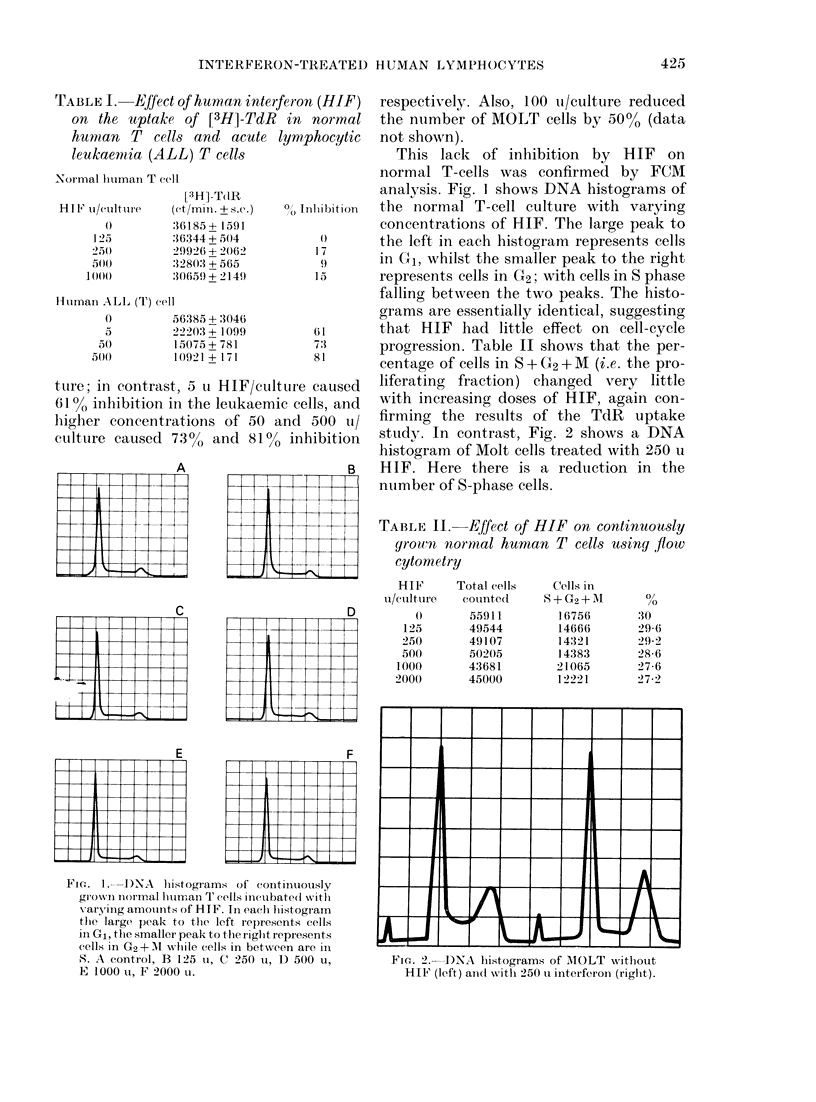
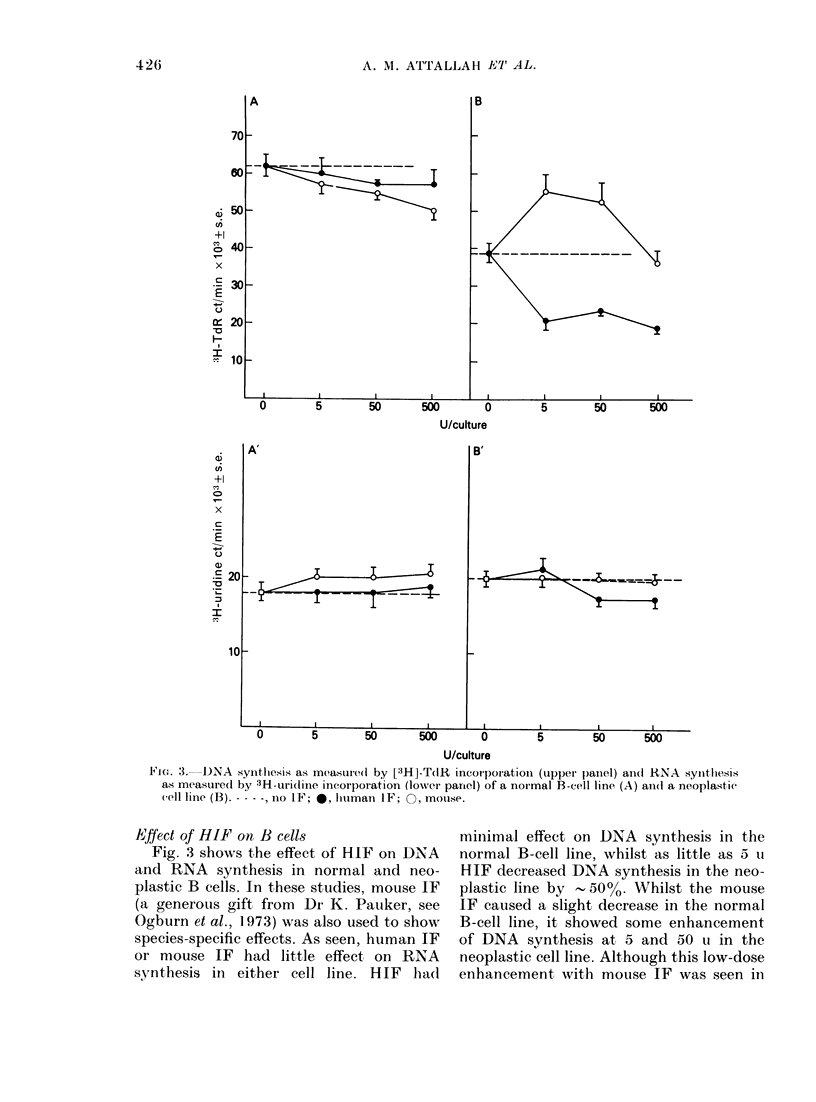
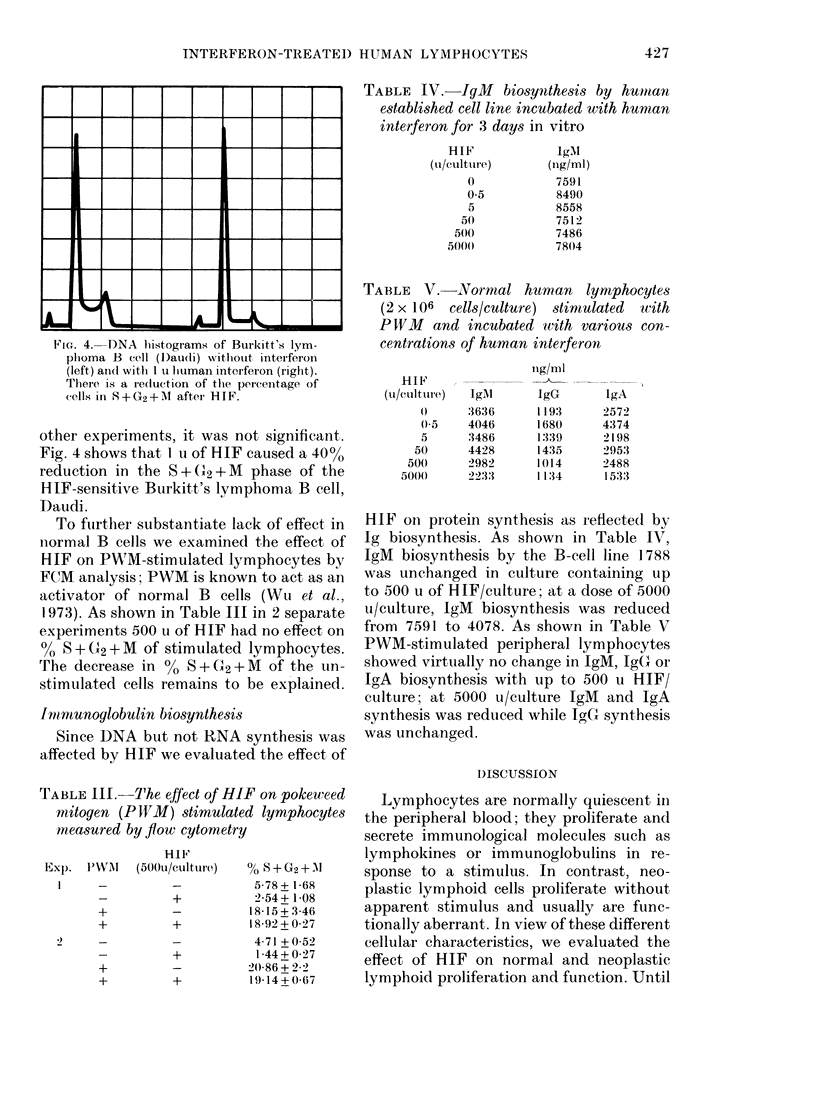
Abstract
Previous studies have shown that normal as well as neoplastic B-cell lines vary substantially in their response to the antiproliferative effects of human interferon (HIF). In this study we took advantage of a recent method to generate long-term continuous normal T-cell cultures (CTC) to investigate the effects of HIF on proliferating lymphoid cells. Normal CTC proved to be resistant to inhibition of proliferation; up to 1000 u HIF had little effect on [3H] TdR uptake, and up to 2000 u HIF had little effect on cell-cycle progression, measured by flow cytometry. Proliferating normal B cells were also resistant to the antiproliferative effect. Nor did up to 500 m HIF inhibit RNA synthesis or immunoglobulin biosynthesis of normal B cells. In contrast, a neoplastic myeloma B cell, a Burkitt's lymphoma cell and a neoplastic leukaemic T cell showed marked inhibition of [3H] TdR uptake and cell cycle progression with as little as 5 u HIF. These results suggest that amounts of HIF sufficient to inhibit proliferation of some neoplastic lymphoid cells have little effect on T- and B-cell proliferation and differentiation of normal B lymphocytes.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Attallah A. M., Folks T. Interferon enhanced human natural killer and antibody-dependent cell-mediated cytotoxic activity. Int Arch Allergy Appl Immunol. 1979;60(4):377–382. doi: 10.1159/000232367. [DOI] [PubMed] [Google Scholar]
- Attallah A. M., Needy C. F., Noguchi P. D., Elisberg B. L. Enhancement of carcinoembryonic antigen expression by interferon. Int J Cancer. 1979 Jul 15;24(1):49–52. doi: 10.1002/ijc.2910240109. [DOI] [PubMed] [Google Scholar]
- Attallah A. M., Strong D. M. Differential effects of interferon on the MHC expression of human lymphocytes. Enhanced expression of HLA without effect on Ia. Int Arch Allergy Appl Immunol. 1979;60(1):101–105. doi: 10.1159/000232328. [DOI] [PubMed] [Google Scholar]
- Attallah A. M., Yeatman T. J., Noguchi P. D., Petricciani J. C. Is DNA synthesis a requisite for the differentiation of B lymphocytes into immunoglobulin-secreting plasma cells? Int Arch Allergy Appl Immunol. 1979;60(2):132–139. doi: 10.1159/000232334. [DOI] [PubMed] [Google Scholar]
- Blomgren H., Cantell K., Johansson B., Lagergren C., Ringborg U., Strander H. Interferon therapy in Hodgkin's disease. A case report. Acta Med Scand. 1976;199(6):527–532. doi: 10.1111/j.0954-6820.1976.tb06776.x. [DOI] [PubMed] [Google Scholar]
- Brodeur B. R., Merigan T. C. Suppressive effect of interferon on the humoral immune response to sheep red blood cells in mice. J Immunol. 1974 Oct;113(4):1319–1325. [PubMed] [Google Scholar]
- Böyum A. A one-stage procedure for isolation of granulocytes and lymphocytes from human blood. General sedimentation properties of white blood cells in a 1g gravity field. Scand J Clin Lab Invest Suppl. 1968;97:51–76. [PubMed] [Google Scholar]
- Einhorn S., Strander H. Interferon therapy for neoplastic diseases in man in vitro and in vivo studies. Adv Exp Med Biol. 1978;110:159–174. doi: 10.1007/978-1-4615-9080-4_13. [DOI] [PubMed] [Google Scholar]
- Herberman R. R., Ortaldo J. R., Bonnard G. D. Augmentation by interferon of human natural and antibody-dependent cell-mediated cytotoxicity. Nature. 1979 Jan 18;277(5693):221–223. doi: 10.1038/277221a0. [DOI] [PubMed] [Google Scholar]
- Lindahl-Magnusson P., Leary P., Gresser I. Interferon inhibits DNA synthesis induced in mouse lymphocyte suspensions by phytohaemagglutinin or by allogeneic cells. Nat New Biol. 1972 May 24;237(73):120–121. doi: 10.1038/newbio237120a0. [DOI] [PubMed] [Google Scholar]
- Minowada J., Onuma T., Moore G. E. Rosette-forming human lymphoid cell lines. I. Establishment and evidence for origin of thymus-derived lymphocytes. J Natl Cancer Inst. 1972 Sep;49(3):891–895. [PubMed] [Google Scholar]
- Ogburn C. A., Berg K., Paucker K. Purification of mouse interferon by affinity chromatography on anti-interferon globulin-sepharose. J Immunol. 1973 Oct;111(4):1206–1218. [PubMed] [Google Scholar]
- Ruscetti F. W., Morgan D. A., Gallo R. C. Functional and morphologic characterization of human T cells continuously grown in vitro. J Immunol. 1977 Jul;119(1):131–138. [PubMed] [Google Scholar]
- Sokawa Y., Watanabe Y., Watanabe Y., Kawade Y. Interferon suppresses the transition of quiescent 3T3 cells to a growing state. Nature. 1977 Jul 21;268(5617):236–238. doi: 10.1038/268236a0. [DOI] [PubMed] [Google Scholar]
- Vindelov L. L. Flow microfluorometric analysis of nuclear DNA in cells from solid tumors and cell suspensions. A new method for rapid isolation and straining of nuclei. Virchows Arch B Cell Pathol. 1977 Aug 10;24(3):227–242. [PubMed] [Google Scholar]
- Waldmann T. A., Durm M., Broder S., Blackman M., Blaese R. M., Strober W. Role of suppressor T cells in pathogenesis of common variable hypogammaglobulinaemia. Lancet. 1974 Sep 14;2(7881):609–613. doi: 10.1016/s0140-6736(74)91940-0. [DOI] [PubMed] [Google Scholar]
- Waldmann T. A., Polmar S. H., Balestra S. T., Jost M. C., Bruce R. M., Terry W. D. Immunoglobulin E in immunologic deficiency diseases. II. Serum IgE concentration of patients with acquired hypogammaglobulinemia, thymoma and hypogammaglobulinemia, myotonic dystrophy, intestinal lymphangiectasia and Wiskott-Aldrich syndrome. J Immunol. 1972 Aug;109(2):304–310. [PubMed] [Google Scholar]
- Wu L. Y., Lawton A. R., Cooper M. D. Differentiation capacity of cultured B lymphocytes from immunodeficient patients. J Clin Invest. 1973 Dec;52(12):3180–3189. doi: 10.1172/JCI107518. [DOI] [PMC free article] [PubMed] [Google Scholar]



