Abstract
There is increasing evidence that by facilitating translocation of Shiga toxin (Stx) across the intestinal epithelium and by transporting bound toxin to remote sites such as the renal endothelium, polymorphonuclear leukocytes (PMNs) play a key role in the pathogenesis of Shiga-toxigenic Escherichia coli (STEC) disease. Plasma levels of PMN-attracting CXC chemokines such as interleukin-8 (IL-8) also appear to correlate in humans with the severity of disease. Thus, the capacity of STEC strains to elicit CXC chemokine responses in intestinal epithelial cells may be a crucial step in pathogenesis. Accordingly, we attempted to determine which STEC factors are responsible for CXC chemokine induction in human colonic epithelial cells. Infection of Hct-8 cells with locus for enterocyte effacement (LEE)-negative STEC strains isolated from patients with severe STEC disease resulted in up-regulation of IL-8, macrophage inflammatory protein 2α (MIP-2α), MIP-2β, and ENA-78 mRNA significantly higher and earlier than that elicited by several LEE-positive STEC strains, including the O157:H7 strain EDL933. Similarly, levels of IL-8 protein in LEE-negative STEC-infected Hct-8 culture supernatants were significantly higher than in LEE-positive STEC-infected culture supernatants. The difference in responses could not be attributed to the expression or nonexpression of LEE genes, the presence or absence of an STEC megaplasmid, or differences in O serogroups or in the type or amount of Stx produced. Interestingly, however, several of the LEE-negative STEC strains eliciting the strongest chemokine responses belonged to flagellar serotype H21. Incubation of Hct-8 cells with isolated H21 flagellin elicited IL-8 and MIP-2α responses similar to those seen in the presence of the most potent LEE-negative STEC strains. Deletion of the fliC gene, but not the stx2 gene, largely abolished the capacity of O113:H21 LEE-negative STEC strain 98NK2 to elicit IL-8 and MIP-2α responses in Hct-8 cells. Taken together, these data suggest that although Stx is capable of inducing CXC chemokine responses, the elevated responses seen in cells infected with certain STEC strains are largely attributable to the production of flagellin.
Shiga-toxigenic Escherichia coli (STEC) strains are a major cause of severe gastrointestinal disease in humans. STEC patients typically experience abdominal cramps and/or watery diarrhea, which may be followed within 1 to 2 days by severe bloody diarrhea and hemorrhagic colitis (22, 29). Some cases progress to the hemolytic uremic syndrome (HUS), a life-threatening sequela characterized by a triad of acute renal failure, microangiopathic hemolytic anemia, and thrombocytopenia (22, 29). STEC strains are noninvasive pathogens; after ingestion and establishment of intestinal colonization, they release Shiga toxin (Stx) into the gut lumen. The toxin is then absorbed across the gut epithelium into the circulation, where it targets host cells expressing the specific glycolipid receptor globotriaosylceramide (Gb3). In humans, Gb3 receptors are present at high levels in renal tubular epithelium; significant levels are also expressed in microvascular endothelial cells of the kidney, intestine, pancreas, and brain, particularly after exposure to inflammatory cytokines, and Stx-mediated damage at these sites accounts for the pathological features of STEC disease and HUS (29).
Although E. coli strains belonging to over 200 O:H serotypes produce Stx, not all of these have been associated with human disease, indicating that other STEC factors contribute to pathogenesis. Many STEC strains associated with serious disease in humans (e.g., those belonging to serogroups such as O157, O111, and O26) form attaching and effacing (A/E) lesions on enterocytes, a property carried on a chromosomal pathogenicity island termed the locus for enterocyte effacement (LEE). Nearly all LEE-positive as well as many LEE-negative STEC strains also carry large plasmids which encode putative accessory virulence factors such as the enterohemolysin EhxA, the catalase-peroxidase KatP, and an autotransporting extracellular serine protease, EspP; some LEE-negative STEC plasmids also encode an autoagglutinating adhesin (Saa) (26) and a type IV pilus (32).
Since STEC strains are noninvasive, the penetration of Stx into underlying tissues is a crucial step in pathogenesis; polymorphonuclear leukocytes (PMNs) are now thought to be important in this process. Recently it was observed that due to an increase in paracellular permeability and the breakdown of the tight junction barrier, the amount of Stx1 and Stx2 crossing polarized T84 cell monolayers in an apical-to-basolateral direction was proportional to the number of PMNs migrating in the opposite direction (15). PMNs have also been shown to directly bind Stx2 in vitro and in vivo via a receptor with a 100-fold lower affinity than Gb3 (34). Bound toxin was subsequently released on contact with target cells expressing Gb3 (34). Thus, PMNs may function in the pathogenesis of STEC disease by contributing to the breakdown of the intestinal epithelial barrier and by transporting Stx to target tissues.
Intestinal epithelial cells play an important role in mediating inflammatory responses, since they are the first cells to contact intestinal pathogens. Pathogenic bacteria, including Salmonella spp. (6, 16, 42), Shigella dysenteriae, Yersinia enterocolitica, Listeria monocytogenes, and enteroinvasive E. coli, have long been associated with up-regulation of a number of proinflammatory cytokines and chemokines (16, 42). Bacterial components such as flagella (13, 33) and lipopolysaccharide (LPS) (3, 30) are important mediators of such responses in these pathogens. In STEC strains, Stx1 is capable of inducing interleukin-8 (IL-8), melanoma growth stimulatory activity (MGSA) (Gro-α), macrophage inflammatory protein 2α (MIP-2α) (Gro-β), MIP-2β (Gro-γ), and epithelium-derived neutrophil-activating peptide 78 (ENA-78) mRNA (as well as IL-8 and MGSA protein expression) via a ribotoxic stress response pathway in a dose-dependent manner (37). This would be expected to lead to recruitment of inflammatory cells (including PMNs), damage to the intestinal barrier, and, therefore, increased Stx absorption. The migration of PMNs and the production of IL-8 by polarized T84 cells were induced by the apical application of STEC strains, but the level of this effect was higher in LEE-negative than in LEE-positive STEC strains (15) and may therefore involve factors other than Stx production. In cases of human STEC disease, elevated IL-8 levels have been shown to correlate with both leukocytosis and poor prognosis (10, 17, 18, 20, 40). IL-8 is a member of the CXC chemokine family and is a potent neutrophil chemoattractant, as are other members of this family, including ENA-78, granulocyte chemotactic protein 2 (GCP-2), MGSA, MIP-2α, and MIP-2β (11). Thus, CXC chemokines may be important in the recruitment of PMNs in serious STEC infection and may play a pivotal role in the pathogenesis of HUS. In the present study, we compared the capacities of various STEC strains to elicit CXC chemokine responses in human colonic epithelial (Hct-8) cells and investigated the relative contributions of Stx and other STEC factors, including the LEE pathogenicity island, the large virulence plasmid, and flagellin.
MATERIALS AND METHODS
Bacterial strains.
Properties of E. coli strains used in this study are listed in Table 1. O113:H21 STEC strain 98NK2 was isolated from a patient with HUS at the Women's and Children's Hospital, South Australia, as previously described (28). Its megaplasmid-cured derivative 98NK2-Cu has also been described previously (26). Except for O157:H7 strain EDL933 and its megaplasmid-cured derivative EDL933-Cu (38) (provided by R. Robins-Browne) and O91:H21 strain B2F1 (provided by A. Melton-Celsa), all of the STEC strains, as well as O111 enteropathogenic E. coli (EPEC) strain 87A, used in the study were also isolated at the Women's and Children's Hospital and have been described previously (27). E. coli strains were routinely cultured in Luria-Bertani (LB) broth with or without appropriate antibiotics.
TABLE 1.
E. coli strains used in this study
| Strain | Serotype | Sourcea | Characteristicb
|
|||
|---|---|---|---|---|---|---|
| eae | stx1 | stx2 | ehxA | |||
| 94CR | O48:H21 | HUS | − | + | + | + |
| 95HE4 | O91:H7 | Diarrhea | − | + | − | + |
| 95NR1 | O111:H− | HUS | + | + | + | + |
| 95SF2 | O157:H− | HUS | + | − | + | + |
| 95ZG1 | O26:H? | Diarrhea | + | + | − | + |
| 97MW1 | O113:H21 | Bloody diarrhea, MHA-T | − | − | + | + |
| 98NK2 | O113:H21 | HUS | − | − | + | + |
| 98NK2-Cu | O113:H21 | Plasmid-cured 98NK2 | − | − | + | − |
| B2F1 | O91:H21 | HUS | − | − | + | + |
| EDL933 | O157:H7 | Bloody diarrhea | + | + | + | + |
| EDL933-Cu | O157:H7 | Plasmid-cured EDL933 | + | + | + | − |
| EPEC 87A | O111:H? | Diarrhea | + | − | − | − |
STEC strains were originally isolated from the feces of patients with diarrhea, bloody diarrhea, HUS, or microangiopathic haemolytic anemia and thrombocytopenia (MHA-T).
Determined by multiplex PCR (24); eae and ehxA are markers for the LEE pathogenicity island and the large STEC virulence plasmid, respectively.
Stx assay.
Toxin titers were measured using a Vero cell cytotoxicity assay. Unless otherwise indicated, Vero cells were grown at 37°C in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% heat-inactivated fetal calf serum (FCS), 50 IU of penicillin, and 50 μg of streptomycin per ml. Vero cells were seeded into 96-well flat-bottom trays and incubated overnight at 37°C until confluent. Confluent monolayers were washed twice with phosphate-buffered saline (PBS) and then treated with 50 μl of filter-sterilized 4-h-culture supernatants which had been serially diluted in DMEM (without FCS) and incubated at 37°C for 30 min. After incubation, 150 μl of DMEM supplemented with 2% FCS was added per well. Cytotoxicity was assessed after 3 days of incubation at 37°C. The toxin titer was defined as the reciprocal of the maximum dilution producing a cytopathic effect with at least 50% of the cells in each well. Purified Stx1 and Stx2 were obtained from Toxin Technologies Inc., Sarasota, Fla.
Isolation of flagellin.
Flagellin was isolated from STEC strains as described previously (33). Briefly, bacteria were pelleted at 8,000 × g for 10 min, resuspended in 30 ml of 500 mM Tris-HCl (pH 8.0), and then sheared in an Omnimixer (Sorvall Products, Newtown, Conn.) at maximum speed for 1 min. Bacterial cells and debris were removed by centrifugation at 8,000 × g for 15 min. The supernatant was clarified by filtration (0.8-μm pore size), and flagella were then pelleted by centrifugation at 100,000 × g for 60 to 90 min and resuspended in PBS. Purity was assessed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and protein concentrations were assayed using a protein assay kit (Bio-Rad Laboratories, Richmond, Calif.) according to the method of Bradford (4). H21 and H7 antiserum was obtained from the Salmonella Reference Laboratory, Institute of Medical and Veterinary Science, Adelaide, Australia.
Infection of Hct-8 cells with E. coli.
All tissue culture media and reagents were obtained from Gibco BRL-Life Technologies (Grand Island, N.Y.). Unless otherwise indicated, Hct-8 cells were grown at 37°C in 5% CO2 in RPMI 1640 medium supplemented with 10 mM HEPES, 1 mM sodium pyruvate, 10% heat-inactivated FCS, and 50 IU of penicillin and 50 μg of streptomycin per ml. For chemokine assays, Hct-8 cells were seeded in 6-well tissue culture trays and allowed to attach overnight. Cells were used at 90 to 100% confluence. Cells were washed twice with PBS, 1 ml of RPMI medium (without antibiotics or FCS) was added to each well, and cells were left at 37°C in 5% CO2 for at least 2 h. A total of 1 ml an overnight culture of E. coli in LB broth was pelleted at 4,000 × g for 10 min, resuspended in 10 ml of FCS-, antibiotic-, and phenol red-free RPMI medium, and incubated for approximately 1 h at 37°C until the culture reached an absorbance at 600 nm (A600) of 0.21 to 0.22. E. coli strains were then centrifuged at 4,000 × g for 10 min and resuspended in an equal quantity of FCS-, antibiotic-, and phenol red-free RPMI medium. Hct-8 monolayers were then infected with 100 μl of the E. coli suspension (approximately 3 × 107 CFU/ml) or medium (for controls) and incubated at 37°C in 5% CO2 for 1 or 4 h, at which time the supernatant was collected and stored at −20°C for protein analysis and the monolayer was lysed in 1 ml of TRIzol reagent for RNA extraction. Samples were also collected at 0 h to determine baseline chemokine levels in Hct-8 cells before stimulation with E. coli.
RNA extraction.
RNA was extracted (using TRIzol reagent [Life Technologies] according to the manufacturer's instructions) from Hct-8 cells. RNA was precipitated in 1/10 volume of sodium acetate (pH 4.8)-2 volumes of 100% ethanol at −80°C overnight. RNA was then pelleted by centrifugation at 12,000 × g for 30 min at 4°C, washed in 70% ethanol, and resuspended in nuclease-free water. RNasin RNase inhibitor (Promega, Madison, Wis.) was then added to the samples. Contaminating DNA was digested (according to the instructions of the manufacturer [Promega]) with RQ1 RNase-free DNase followed by DNase stop solution. The absence of DNA contamination in all RNA preparations was confirmed by reverse transcription-PCR (RT-PCR) analysis using primers specific for the gene encoding the housekeeping enzyme glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (Table 1). The gene encoding GAPDH contains an intron such that the mRNA template directs amplification of a 239-bp product, whereas the chromosomal DNA template directs amplification of a 341-bp product.
Real-time RT-PCR.
The comparative levels of chemokine mRNA produced by Hct-8 cells after stimulation with various E. coli strains were determined using quantitative real-time RT-PCR. The oligonucleotide primer pairs used are specified in Table 2. RT-PCR was performed using a one-step Access RT-PCR system (Promega) according to the manufacturer's instructions. Each reaction was performed in a final volume of 20 μl containing 20 nmol of each oligonucleotide and a 1/20,000 dilution of SYBR Green I nucleic acid stain (Molecular Probes). The quantitative RT-PCR was performed on a Rotorgene RG-2000 cycler (Corbett Research, Mortlake, New South Wales, Australia) and included the following steps: 45 min of RT at 48°C followed by 2 min denaturation at 94°C and then 40 cycles of amplification at 94°C for 30 s, 56°C for 30 s, and 72°C for 45 s. Each RNA sample was assayed in triplicate using primers specific for the various chemokine mRNAs (or mRNA for GAPDH, which was used as an internal control). Results were calculated using the comparative cycle threshold (2ΔΔCt) method (user bulletin no. 2 [http://docs.appliedbiosystems.com/pebiodocs/04303859.pdf]; Applied Biosystems), in which the amount of target mRNA is normalized to a reference (0 h control) relative to an internal control (GAPDH mRNA). Results are expressed as relative changes in chemokine mRNA levels compared to 0-h control levels. Standard deviations (SD) were initially determined according to the formula 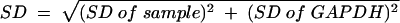 , and the result was then applied to the formulas
, and the result was then applied to the formulas 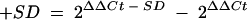 and
and 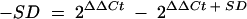 .
.
TABLE 2.
Oligonucleotides used in this study
| Primer | Sequence (5′-3′) | Amplicon size (bp) |
|---|---|---|
| IL-8 F | GAAGGAACCATTCTCACTGTGTGTA | 327 |
| IL-8 R | TTATGAATTCTCAGCCCTCTTCAAAAAC | |
| ENA-78 F | GAACCCGCGACCGCTCGC | 191 |
| ENA-78 R | AGAAAAGGGGCTTCTGGATCAA | |
| GCP-2 F | CTCCACCCAGCTCAGGAACC | 397 |
| GCP-2 R | GAAAAGGGGCTTCCGGGTCCA | |
| MGSA F | AGCCACACTCAAGAATGGGCG | 473 |
| MGSA R | TGGCATGTTGCAGGCTCCTC | |
| MIP-2α F | ATTTGTTAATATTTCTTCGTGATGACATATCA | 323 |
| MIP-2α R | TCGAAACCTCTCTGCTCTAACAC | |
| MIP-2β F | AGAACATCCAAAGTGTGAATGTAAGG | 281 |
| MIP-2β R | TCCTTTCCAGCTGTCCCTAGAA | |
| GAPDH F | TCCTTGGAGGCCATGTGGGCCAT | 239 |
| GAPDH R | TGATGACATCAAGAAGGTGGTGAAG |
IL-8 ELISA.
The levels of IL-8 in culture supernatants at 0, 1, and 4 h were assayed in duplicate using a commercial sandwich ELISA (R&D Systems, Minneapolis, Minn.). ELISAs were performed (using monoclonal mouse anti-human IL-8 antibody at 2 μg/ml for capture and a biotinylated polyclonal anti-human IL-8 antibody at 20 ng/ml for detection in accordance with the instructions of the manufacturer [R&D Systems]) in 96-well trays (Maxisorp Nunc-Immuno plates; Nunc, Roskilde, Denmark). The assay was calibrated using recombinant human IL-8 (R&D Systems). The sensitivity limit of the ELISA was 31.25 pg/ml.
Construction of stx2 and fliC deletion derivatives of STEC strain 98NK2.
Nonpolar stx2 and fliC deletion mutants of O113:H21 STEC 98NK2 were constructed using a lambda red recombinase system (8). This involved PCR amplification (using primers incorporating the direct repeated FLP recognition target common priming site and sequences derived from the 5′ and 3′ ends of the stx2 and fliC genes, respectively) of the kanamycin resistance cartridge in pKD4. The resultant linear fragments were electroporated into strain 98NK2 carrying the temperature-sensitive plasmid pKD46, which carries a gene for the lambda recombinase. Allelic replacement mutants were selected on LB broth-kanamycin plates at 37°C. Replacement of nucleotides 89 to 834 of stx2 and nucleotides 64 to 1281 of fliC with the kanamycin resistance cartridge was confirmed by PCR and sequence analysis of the mutant strains, which were designated 98NK2Δstx2 and 98NK2ΔfliC, respectively.
Statistical analysis.
Statistical analysis was performed using Prism 3.03 software (GraphPad Software, San Diego, Calif.). Differences in chemokine responses between treatment groups were analyzed by ANOVA with logarithmically transformed data; P < 0.05 was considered significant. When differences were significant, a Tukey test was performed.
RESULTS
Chemokine mRNA induction by STEC strains.
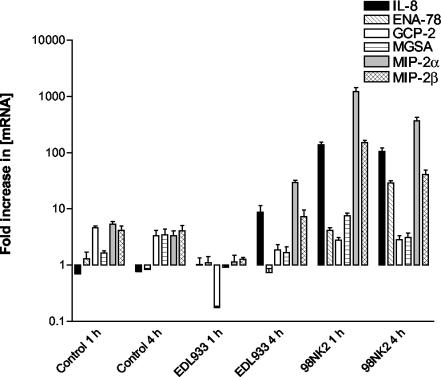
Initial experiments involved the identification of principal chemokines expressed by Hct-8 cells in response to the presence of two well-characterized LEE-positive and LEE-negative STEC strains (EDL933 and 98NK2, respectively) (Table 1). Hct-8 monolayers were incubated with approximately 3 × 107 STEC bacteria for either 1 or 4 h, after which total cellular RNA was extracted and analyzed for the presence of mRNA specific for IL-8, MIP-2α, MIP-2β, ENA-78, GCP-2, and MGSA by real-time RT-PCR, as described in Materials and Methods (Fig. 1). At 1 h, there were no clear differences in the levels of mRNA between unstimulated (control) cells and those infected with strain EDL933 for any of the six chemokines. However, cells infected with the same dose of strain 98NK2 exhibited marked up-regulation of mRNA for IL-8, MIP-2α, and MIP-2β but not for ENA-78, GCP-2, or MGSA (Fig. 1). The greatest responses were observed for MIP-2α and IL-8, the mRNA levels of which were up-regulated 199- and 277-fold, respectively, relative to those of the control cells. At 4 h, similar responses were observed in cells infected with 98NK2 except that ENA-78 mRNA was also up-regulated at that time. However, at 4 h, up-regulation of mRNA for IL-8, MIP-2α, and MIP-2β was also observed in Hct-8 cells infected with EDL933, although the degrees of up-regulation for IL-8 and MIP-2α (11- and 9-fold, respectively) remained lower than that seen in cells infected with strain 98NK2 (Fig. 1). Dose-response experiments indicated that infecting Hct-8 cells with twofold-higher or twofold-lower doses of either strain 98NK2 or strain EDL933 did not affect the degree of chemokine mRNA up-regulation (data not shown), but diminished responses were observed at doses of <1.5 × 107 CFU.
FIG. 1.
Induction of CXC chemokine mRNA in Hct-8 cells infected with strains EDL933 and 98NK2. Chemokine mRNA was measured at 1 and 4 h by real-time RT-PCR, as described in Materials and Methods. Results are expressed as the fold increase in [mRNA] relative to levels at 0 h, and data are shown as the means ± SD for triplicate assays.
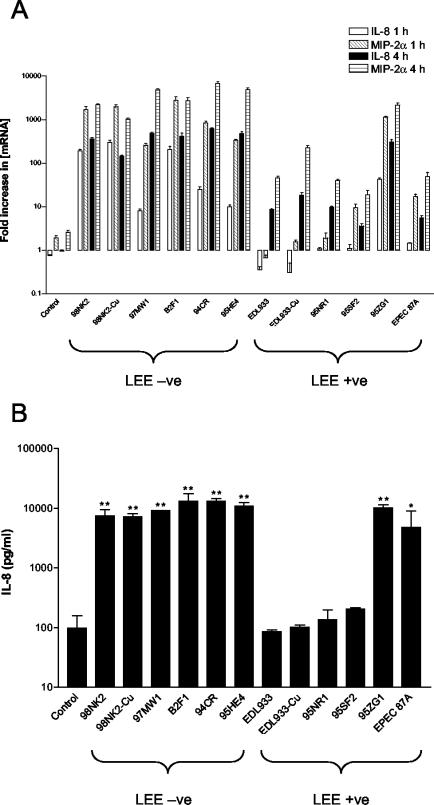
MIP-2α and IL-8 responses were then examined in Hct-8 cells infected with a range of LEE-positive and LEE-negative STEC strains (listed in Table 1). Results of these studies are expressed as the fold increase in IL-8 or MIP-2α [mRNA] at 1 or 4 h compared to results for a 0-h control (Fig. 2A). Significant differences (P < 0.0001) were seen between responses elicited by the various strains. In particular, all of the LEE-negative STEC strains tested (98NK2, 97MW1, B2F1, 94CR, and 95HE4) induced higher levels of both IL-8 and MIP-2α mRNA than any of the LEE-positive strains, with the exception of strain 95ZG1. All of the LEE-negative STEC strains tested induced significant up-regulation of IL-8 and MIP-2α mRNA at both 1 and 4 h after infection. 95ZG1 was the only LEE-positive strain tested that was capable of eliciting significant IL-8 and MIP-2α responses at 1 h. At 4 h after infection, significant IL-8 and MIP-2α responses were seen for all the LEE-positive strains relative to those observed for uninfected control cells but these remained inferior to those observed for LEE-negative strains. Taken collectively, IL-8 and MIP-2α responses seen at 1 h for cells infected with LEE-negative strains were on average 14- and 6-fold higher, respectively, than those elicited by infection with LEE-positive strains (P < 0.05 and 0.01, respectively); at 4 h, the responses were 6- and 8-fold higher, respectively (P < 0.01 in both cases).
FIG. 2.
Chemokine induction in Hct-8 cells infected with LEE-negative and LEE-positive STEC strains. Hct-8 cells were infected with the indicated STEC strains (Table 1). (A) At 1 or 4 h, total RNA was extracted from cells and IL-8 and MIP-2α mRNA was quantitated by real-time RT-PCR. Results are expressed as the fold increase in [mRNA] relative to levels at 0 h, and data are shown as the means ± SD for triplicate assays. (B) At 4 h, culture supernatants were collected and assayed for IL-8 protein by ELISA as described in Materials and Methods. Data are shown as the means ± standard errors of the means (SEM) from two experiments. The significance of differences between IL-8 secretion by infected versus uninfected cells is indicated as follows: **, P < 0.001; *, P < 0.05.
Derivatives of strains EDL933 and 98NK2 which had been cured of their megaplasmids (EDL933-Cu and 98NK2-Cu, respectively) elicited IL-8 and MIP-2α responses that were indistinguishable from those of their respective parent strains, indicating that genes carried on these elements do not contribute to chemokine induction (Fig. 2A). Furthermore, infection of Hct-8 cells with the nontoxigenic LEE-positive EPEC 87A strain induced up-regulation of IL-8 and MIP-2α mRNA similar to that seen with the LEE-positive STEC strains, indicating that production of Stx is not essential for these responses (Fig. 2A).
STEC infection induces IL-8 secretion by Hct-8 cells.
To confirm that up-regulation of IL-8 mRNA resulted in increased secretion of the chemokine itself, levels of IL-8 in STEC-infected Hct-8 culture supernatants were measured by ELISA, as described in Materials and Methods. At 1 h there was no detectable increase in IL-8 secretion for any of the strains tested compared to that observed with the uninfected control cultures (limit of detection, 31.25 pg of IL-8 per ml) (data not shown). However, at 4 h the levels of IL-8 protein in culture supernatants of cells infected with the LEE-negative STEC strains were significantly (approximately 100-fold) higher than that in uninfected Hct-8 culture supernatants or those from cells infected with the LEE-positive STEC strains EDL933, 95NR1, and 95SF2 (Fig. 2B). However, strain 95ZG1 induced levels of IL-8 secretion similar to those seen with the LEE-negative STEC strains and EPEC strain 87A induced an intermediate response (Fig. 2B). Furthermore, the megaplasmid-cured derivatives 98NK2-Cu and EDL933-Cu elicited levels of IL-8 indistinguishable from those elicited by their respective parental strains (Fig. 2B). All these findings are in accordance with those predicted from the mRNA induction studies described above.
LEE-encoded STEC factors do not influence chemokine induction.
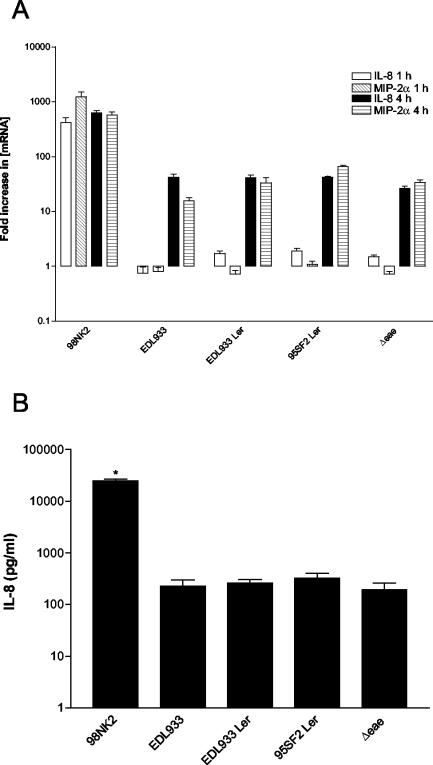
One possible explanation for the difference in CXC chemokine induction between LEE-positive and LEE-negative STEC-infected Hct-8 cells might be that host cell perturbations (such as cytoskeletal rearrangements) caused by LEE-encoded products can interfere with cellular signaling pathways. To examine this, chemokine induction was investigated in cells infected with strain EDL933, an otherwise isogenic derivative with an in-frame deletion mutation in the intimin gene (EDL933Δeae) which cannot form A/E lesions on enterocytes (23). We also tested derivatives of strain EDL933 carrying either normal or defective copies of the LEE-encoded regulatory gene (ler) on a multicopy plasmid. Overexpression of the defective ler gene (derived from STEC 95SF2) represses production of multiple LEE-encoded proteins and abolishes A/E lesion formation in strain EDL933. Overexpression of wild-type ler (derived from strain EDL933) significantly up-regulates production of LEE proteins and confers a hyper-adherent phenotype on strain EDL933 (23). There was no significant difference at either 1 or 4 h between the levels of IL-8 and MIP-2α mRNA in Hct-8 cells infected with either strain EDL933 or any of the derivatives described above. Infection with strain 98NK2 elicited a 288- and 17-fold increase in IL-8 mRNA and a 1,490- and 15-fold increase in MIP-2α mRNA at 1 and 4 h, respectively, compared to infection with strain EDL933 and its various derivatives (Fig. 3A). Similarly, there was no difference between levels of IL-8 protein in culture supernatants of cells infected for 4 h with strain EDL933 and those infected any of the EDL933 derivatives whereas IL-8 levels were approximately 100-fold higher in supernatants of cells infected with strain 98NK2 (P < 0.001) (Fig. 3B). These data indicate that the massive differences in chemokine induction observed between cells infected with LEE-positive STEC strains and those infected with LEE-negative STEC strains cannot be attributed to factors encoded by the LEE locus.
FIG. 3.
Stimulation of Hct-8 cells by strain EDL933 derivatives. Cells were infected with strains 98NK2, EDL933, EDL933 expressing wild-type or defective copies of the ler gene carried on a multicopy plasmid (designated EDL933 Ler and 95SF2 Ler, respectively), and EDL933 with a deletion mutation in eae (Δeae) (all described previously [23]). (A) At 1 or 4 h, total RNA was extracted from cells and IL-8 and MIP-2α mRNA was quantitated by real-time RT-PCR. Results are expressed as the fold increase in [mRNA] relative to levels at 0 h, and data are shown as the means ± SD for triplicate assays. (B) At 4 h, supernatants were collected and assayed for IL-8 by ELISA. Data are shown as the means ± SEM from two experiments. *, significant difference relative to control cells (P < 0.001).
Effect of Stx1 and Stx2 on chemokine production by Hct-8 cells.
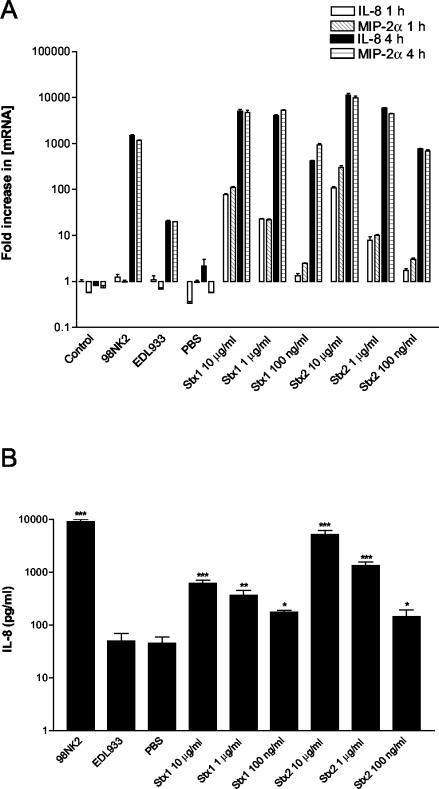
Thorpe et al. (37) have previously demonstrated that purified Stx1 stimulates production of IL-8 and other CXC chemokines by intestinal epithelial cells. Stx2 was not tested in that study (except for being tested for MGSA induction), and so it was conceivable that differences in chemokine induction elicited by LEE-positive and LEE-negative strains might be attributable to differences in the type or amount of Stx secreted during the 4-h assay. To examine this, we first compared chemokine induction in Hct-8 cells treated with strain 98NK2, strain EDL933, purified Stx1, or purified Stx2 at doses ranging from 100 ng to 10 μg per ml (Fig. 4). Both toxins elicited IL-8 and MIP-2α mRNA responses (in a dose-dependent fashion) which at 4 h were comparable to those seen for cells infected with strain 98NK2 and markedly more elevated than those seen for cells infected with strain EDL933 (Fig. 4A). Dose-dependent stimulation of IL-8 secretion by Stx1 and Stx2 was also demonstrated by ELISA, and at the highest dose (10 μg per ml), the response elicited by Stx2 was significantly more elevated than that elicited by Stx1 (P < 0.01) (Fig. 4B). The concentration of Stx produced by the various STEC strains used in this study during the 4-h assay was estimated by assaying the culture supernatants for Vero cell cytotoxicity, using the purified toxins as standards (see Materials and Methods). Total levels of Stx ranged from 25 to 150 ng/ml (results not shown). However, there was no correlation between total Stx activity in culture supernatants for a given STEC strain and the level of chemokine mRNA or IL-8 protein induced. Moreover, the total amount of Stx in culture supernatants of LEE-negative STEC strains appeared insufficient to account for the level of chemokine induction observed. For example, the 4-h strain 98NK2 culture supernatant contained approximately 25 ng of Stx2/ml but infection of Hct-8 cells with strain 98NK2 elicited 100 times more IL-8 secretion than treatment with 100 ng of Stx2/ml (Fig. 4B). In another experiment, infection of Hct-8 cells with E. coli K-12 clones expressing either Stx1 or Stx2 did not elicit levels of IL-8 or MIP-2α mRNA or of IL-8 protein that were significantly different from those elicited by treatment with the host E. coli strain (data not shown). Thus, STEC factors other than Stx appear to be responsible for a major portion of the chemokine induction observed in infected Hct-8 cultures.
FIG. 4.
Induction of IL-8 and MIP-2α mRNA and IL-8 protein in Hct-8 cells treated with purified Stx1 and Stx2. Hct-8 cells were treated with purified Stx1 or Stx2 at the indicated concentrations or with STEC strain 98NK2 or EDL933. (A) At 1 or 4 h, total RNA was extracted from cells and IL-8 and MIP-2α mRNA was quantitated by real-time RT-PCR. Results are expressed as the fold increase in [mRNA] relative to levels at 0 h, and data are shown as the means ± SD for triplicate assays. (B) At 4 h, supernatants were collected and assayed for IL-8 by ELISA. Data are shown as the means ± SEM from two experiments. Significant differences relative to untreated control cells are indicated as follows: *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Stimulation of Hct-8 cells by H21 flagellin induces chemokine production.
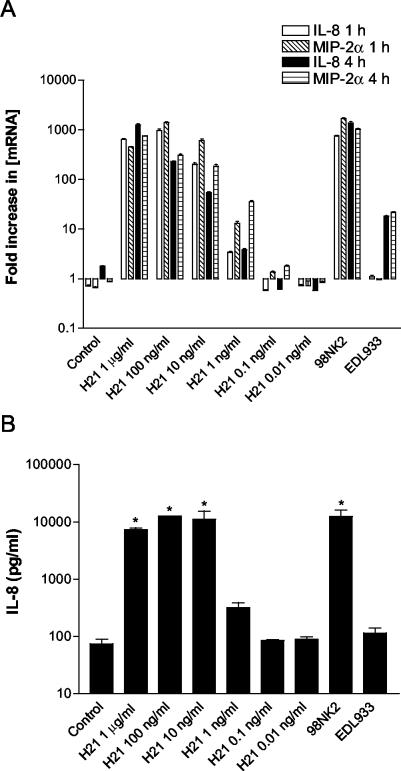
Interestingly, Paton and Paton have previously observed that many LEE-negative STEC strains associated with serious human disease (including four of the STEC strains tested in this study) belong to flagellar type H21 (24). Purified flagellin (the major structural component of flagella) from several enteric pathogens has been shown to elicit inflammatory responses in epithelial cells (9, 12, 13, 33). This raised the possibility that flagellin was a major contributor to chemokine induction in STEC-infected cells and that serotype H21 flagellin may be particularly potent. To examine this, H21 flagellin was isolated from strain 98NK2, as described in Materials and Methods. The capacity of this preparation to elicit chemokine responses in Hct-8 cells was then investigated using flagellin concentrations ranging from 0.01 ng to 1 μg per ml. Western blot analysis using anti-H21 serum, with purified flagellin as a standard, indicated that the total amounts of H21 flagellin present in strain 98NK2-infected Hct-8 cultures were approximately 8 and 60 ng/ml at 1 and 4 h, respectively. As shown in Fig. 5A, cells stimulated with H21 flagellin at 1 μg/ml, 100 ng/ml, and 10 ng/ml produce IL-8 and MIP-2α mRNA levels at 1 and 4 h comparable to those elicited by infection with 98NK2. Even at H21 flagellin concentrations as low as 1 ng/ml, the levels of IL-8 and MIP-2α mRNA were similar to those observed in cell cultures infected with strain EDL933. However, significant chemokine induction was not observed at 0.1 and 0.01 ng of H21 flagellin per ml. Similar results were obtained when IL-8 levels in culture supernatants were measured by ELISA (Fig. 5B). Stimulation of cells with H21 flagellin at 1 μg/ml, 100 ng/ml, and 10 ng/ml yielded 7,300 ± 848, 12,600 ± 141, and 11,100 ± 6,222 pg of IL-8/ml, respectively, and these levels were significantly different from those seen with unstimulated control cell supernatants (<100 pg/ml; P < 0.001). At these H21 doses, the levels of IL-8 were similar to that elicited by infection with strain 98NK2 (12,500 ± 4,950 pg/ml). Even at 1 ng/ml, H21 flagellin was capable of inducing 320 ± 99 pg of IL-8/ml, a level comparable to that elicited by strain EDL933.
FIG. 5.
Induction of IL-8 and MIP-2α mRNA and IL-8 protein in Hct-8 cells treated with H21 flagellin. Hct-8 cells were stimulated with H21 flagellin at the indicated concentrations or with STEC strains 98NK2 and EDL933. (A) At 1 or 4 h, total RNA was extracted from cells and IL-8 and MIP-2α mRNA was quantitated by real-time RT-PCR. Results are expressed as the fold increase in [mRNA] relative to levels at 0 h, and data are shown as the means ± SD for triplicate assays. (B) At 4 h, supernatants were collected and assayed for IL-8 by ELISA. Data are shown as the means ± SEM from two experiments. Significant differences relative to untreated control cells are indicated as follows: *, P < 0.001.
Effect of mutagenesis of stx2 and fliC genes in strain 98NK2.
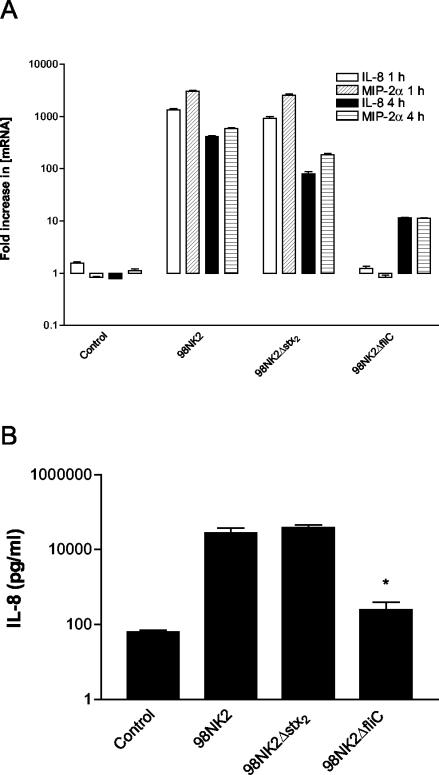
To determine the relative contribution of H21 flagellin and Stx2 to chemokine induction in strain 98NK2-infected Hct-8 cells, 98NK2 derivatives with deletion mutations in the single stx2 gene or the fliC gene (which encodes flagellin) were constructed as described in Materials and Methods. The capacity of the mutant and wild-type strains to elicit CXC chemokine responses in Hct-8 cells was then examined. IL-8 and MIP-2α mRNA responses in Hct-8 cells infected with strain 98NK2 or with the 98NK2 stx2 deletion mutant (98NK2Δstx2) were similar. In contrast, chemokine mRNA responses of cells infected with the fliC deletion mutant (98NK2ΔfliC) were markedly depressed (Fig. 6A). Similarly, IL-8 levels in infected Hct-8 culture supernatants at 4 h were indistinguishable for strains 98NK2 and 98NK2Δstx2, whereas for cells infected with 98NK2ΔfliC, IL-8 levels were more than 100-fold lower (P < 0.01) (Fig. 6B).
FIG. 6.
Induction of IL-8 and MIP-2α mRNA and IL-8 protein in Hct-8 cells infected with strain 98NK2 stx2 and fliC deletion mutants. Hct-8 cells were stimulated with strain 98NK2, 98NK2Δstx2, or 98NK2ΔfliC. (A) At 1 or 4 h, total RNA was extracted from cells and IL-8 and MIP-2α mRNA was quantitated by real-time RT-PCR. Results are expressed as the fold increase in [mRNA] relative to levels at 0 h, and data are shown as the means ± SD for triplicate assays. (B) At 4 h, supernatants were collected and assayed for IL-8 by ELISA. Data are shown as the means ± SEM from two experiments. *, significant difference relative to strain 98NK2-treated cells (P < 0.01).
Chemokine responses to other flagellin types.
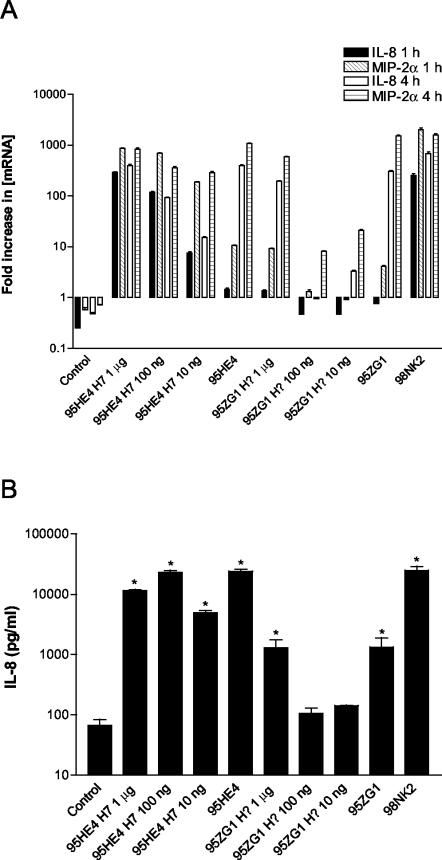
Two other STEC strains that were tested in the present study and elicited strong chemokine responses in Hct-8 cells were the LEE-negative O91:H7 strain 95HE4 and the LEE-positive O26 strain 95ZG1 (this strain has not been H typed, although most STEC strains belonging to the O26 serogroup are type H11). Flagellin was also isolated from these strains (as described in Materials and Methods) and used to stimulate Hct-8 cells (Fig. 7A). At 1 μg/ml, 100 ng/ml, and 10 ng/ml, H7 flagellin isolated from strain 95HE4 induced high IL-8 and MIP-2α mRNA levels comparable to that observed with 95HE4-infected Hct-8 cultures. However, the same concentrations of flagellin from strain 95ZG1 were less active, inducing mRNA levels similar to those seen with strain 95ZG1 at 1 μg/ml, while at 100 and 10 ng/ml, the degree of induction was only slightly higher than that seen in control cells. Measurement of IL-8 in the supernatants corroborated these findings (Fig. 7B). At 1 μg/ml, 100 ng/ml, and 10 ng/ml, 95HE4 flagellin produced 11,500 ± 707, 23,000 ± 2,828, and 4,900 ± 707 pg of IL-8/ml compared with the 24,000 ± 2828 pg/ml elicited by infection of Hct-8 cells with 95HE4 STEC strains. IL-8 levels induced by strain 95HE4 bacteria, as well as those induced by all three dilutions of strain 95HE4 H7 flagellin, were significantly different from that in control supernatants (P < 0.001). However, stimulation of Hct-8 cells with strain 95ZG1 flagellin at 1 μg/ml, 100 ng/ml, and 10 ng/ml produced 1,290 ± 650, 106 ± 34, and 142 ± 4 pg of IL-8/ml, respectively, compared to 1,330 ± 806 pg/ml for STEC strain 95ZG1. Only treatment with STEC strain 95ZG1 and the highest concentration of 95ZG1 flagellin tested (1 μg/ml) resulted in significantly increased IL-8 secretion relative to that seen with control cells (P < 0.001).
FIG. 7.
Induction of IL-8 and MIP-2α mRNA and IL-8 protein in Hct-8 cells treated with flagellin from strains 95HE4 and 95ZG1. Hct-8 cells were stimulated with strain 95HE4 (H7) or 95ZG1 flagellin at the indicated concentrations or with STEC strain 95HE4, 95ZG1, or 98NK2. (A) At 1 or 4 h, total RNA was extracted from cells and IL-8 and MIP-2α mRNA was quantitated by real-time RT-PCR. Results are expressed as the fold increase in [mRNA] relative to levels at 0 h, and data are shown as the means ± SD for triplicate assays. (B) At 4 h, supernatants were collected and assayed for IL-8 by ELISA. Data are shown as the means ± SEM from two experiments. Significant differences relative to untreated control cells are indicated as follows: *, P < 0.001.
The fact that the LEE-positive STEC strains 95NR1 (O111:H−) and 95SF2 (O157:H−) were poor inducers of CXC chemokine responses in Hct-8 cells is compatible with the fact that they are nonmotile and do not produce flagellin. On the other hand, the poor responses elicited by strain EDL933 (O157:H7) were unexpected, particularly since H7 flagellin isolated from strain 95HE4 was a strong inducer. However, examination of flagellin preparations from strain EDL933 by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and Western blot analysis using H7-specific antiserum indicated that little intact flagellin was present; stimulation of Hct-8 cells with this material induced minimal CXC chemokine responses similar to those elicited by infection with strain EDL933 (result not shown).
DISCUSSION
Much of the intestinal pathology associated with STEC disease, as well as its life-threatening complications, results from microvascular angiopathy due to systemic absorption and dissemination of Stx. Unlike many enteric pathogens, STEC strains do not invade the gut mucosa and their capacity to cause severe disease is heavily dependent upon translocation of Stx across the epithelial barrier. Recent evidence strongly implicates PMNs in both penetration of Stx through the epithelium into underlying tissues (15) and transport to remote target tissues (34, 35). Thus, recruitment of PMNs to the site of STEC colonization may be a watershed event in the disease process. In this study we examined the capacity of various STEC strains to elicit CXC chemokine responses in a human colonic epithelial cell line (Hct-8), because these chemokines are potent PMN chemoattractants.
In general, infection of Hct-8 cells with LEE-negative STEC strains produced much higher and earlier induction of IL-8 and MIP-2α mRNA and IL-8 protein than that seen with the LEE-positive STEC strains examined in this study. This is consistent with previous reports that LEE-negative STEC strains induce higher transmigration of PMNs across polarized T84 cells than LEE-positive strains (15). However, our analysis of an in-frame eae deletion mutant of strain EDL933 and of EDL933 derivatives overexpressing wild-type or defective copies of the LEE regulatory gene ler demonstrated that the marked difference in chemokine induction levels elicited by LEE-positive versus LEE-negative STEC strains was not directly related to their capacity to form A/E lesions or to the level of expression of intimin and other genes carried by the LEE. Differences in LPS O-antigen serogroups also do not account for the observed difference, as E. coli K-12 clones expressing either O111 (1) or O113 (25) LPS (derived from strains exhibiting low or high chemokine responses, respectively) elicited responses similar to those of the E. coli K-12 host strain (results not presented). Experiments with plasmid-cured derivatives of strains EDL933 and 98NK2 also demonstrated that genes carried on the large virulence plasmids of these STEC strains have no impact on chemokine induction.
Stx1 and Stx2 have previously been shown to induce CXC chemokine production in intestinal epithelial cells as well as in endothelial cells (36, 37, 41, 44). However, the differences in responses elicited by the various STEC strains examined in this study were unrelated to the type or amount of Stx produced. Moreover, IL-8 and MIP-2α mRNA levels and IL-8 protein levels elicited by infection of Hct-8 cells with strain 98NK2 were significantly higher than those induced by treatment of cells with purified Stx2 at a concentration comparable to that present in 98NK2-infected cultures (strain 98NK2 produces Stx2 only). Deletion mutagenesis of the single stx2 gene in strain 98NK2 also had a negligible impact on IL-8 and MIP-2α responses in Hct-8 cells infected with the mutant compared with those infected with the wild-type strain. A recent study has also shown that an O157:H7 STEC strain and an otherwise isogenic stx-negative derivative induced similar levels of IL-8 secretion by CaCo-2 cells (2).
Flagellin from a variety of pathogens, including Salmonella (12, 13), enteroaggregative E. coli (9, 33), and EPEC (43), is known to induce inflammatory responses in epithelial cells, and Berin et al. (2) demonstrated that much of the IL-8 response elicited by the O157:H7 STEC strain studied was attributable to the presence of H7 flagellin. Recognition of flagellin is part of the innate immune response for pathogen detection and is mediated by Toll-like receptor 5 (14, 39). Flagellin induces intracellular signaling via Toll-like receptor 5 that results in the activation of transcription factors such as NF-κB and AP-1, which are known to be produced in response to STEC strains (2, 5, 7) and Stx (44) as well as to EPEC strains (31). Activation of these transcription factors produces a wide variety of effects, such as the up-regulation of cytokines (including the CXC chemokines [42]) and the up-regulation of ICAM and VCAM on endothelial cells (19), which are important for the recruitment and migration of PMNs (21).
Interestingly, four of the LEE-negative strains used in this study, all of which were isolated from patients with serious disease, belonged to flagellar type H21. Furthermore, treatment of Hct-8 cells with low doses of H21 flagellin elicited CXC chemokine responses comparable to those achieved by infection with the STEC strains themselves. Semiquantitative Western blot analysis (data not shown) confirmed that the concentrations of purified H21 flagellin used in these experiments were comparable to the total amounts of flagellin present in the STEC-infected cell cultures. Deletion mutagenesis of the fliC gene in strain 98NK2 also resulted in a massive reduction in CXC chemokine responses of infected cells. Thus, our findings unequivocally demonstrate that the bulk of the CXC chemokine responses elicited by infection of intestinal epithelial cells with strain 98NK2 can be attributed to its H21 flagella. Interestingly, two of the non-H21 STEC strains examined in this study (the LEE-negative O91:H7 strain 95HE4 and the LEE-positive O26 strain 95ZG1) elicited significant CXC chemokine responses. Flagellin isolated from these strains also elicited similar responses from Hct-8 cells, and the H7 flagellin preparation from 95HE4 was as potent as H21 flagellin. However, flagellin prepared from strain 95ZG1 was a weaker chemokine inducer. The N- and C-terminal portions of flagellin are highly conserved, while the central domain is hypervariable. Thus, comparison of deduced amino acid sequences of fliC genes from these and other STEC strains may enable localization of critical proinflammatory domains. Interestingly, a previous study also found that LEE-negative STEC strains are capable of inducing higher IL-8 responses in T84 cells than the LEE-positive STEC strains that were tested, but the LEE-negative strains studied were all reported to be H− (15). It is therefore possible that additional LEE-negative STEC factors are also capable of inducing CXC chemokine responses. However, an alternative explanation could be that a nontypeable flagellin was actually produced by these STEC strains under the conditions used in the assay.
Three of the four LEE-positive strains tested in this study elicited poor CXC chemokine responses, and this correlated with an apparent inability to produce flagellin. These STEC strains were the O111:H− strain 95NR1 and the O157:H− strain 95SF2, both of which were isolated from patients with HUS, as well as the prototype O157:H7 STEC strain EDL933. The poor capacity of strain EDL933 to elicit CXC chemokine responses appears to be due to a defect in H7 flagellin expression, in spite of its having an intact fliC gene (as determined from the EDL933 genome sequence; accession number NC_002655). This defect is unlikely to be due to a recently introduced mutation, as its plasmid-cured derivative strain EDL933-Cu, which was constructed in 1987 (38), also elicits similarly weak CXC chemokine responses. We subsequently tested two other O157:H7 clinical isolates in our collection, but these induced CXC chemokine responses in Hct-8 cells similar to those seen for strain 98NK2 (results not presented).
In summary, flagellin appears to be the most important determinant affecting the high and early chemokine production seen in Hct-8 cells infected with the STEC strains used in this study. H21 is a particularly potent type, as is H7, but the relative potency of flagellins produced by other common STEC serotypes remains to be determined. Given the important role postulated for PMNs in the translocation of Stx across the intestinal epithelium and in transport of Stx to remote tissues, chemokine induction by flagellin may be crucial to the pathogenesis of STEC disease. Moreover, the increased intestinal inflammation may cause significant local damage. These effects may be particularly important for LEE-negative STEC strains, as they might compensate in part for the inability of those strains to produce the A/E lesions characteristic of LEE-positive STEC strains.
Acknowledgments
This work was supported by a grant from the National Health and Medical Research Council of Australia.
Editor: A. D. O'Brien
REFERENCES
- 1.Bastin, D. A., L. K. Romana, and P. R. Reeves. 1991. Molecular cloning and expression in Escherichia coli K-12 of the rfb gene cluster determining the O antigen of an E. coli O111 strain. Mol. Microbiol. 5:2223-2231. [DOI] [PubMed] [Google Scholar]
- 2.Berin, M. C., A. Darfeuille-Michaud, L. J. Egan, Y. Miyamoto, and M. F. Kagnoff. 2002. Role of EHEC O157:H7 virulence factors in the activation of intestinal epithelial cells NF-κB and MAP kinase pathways and the upregulated expression of interleukin 8. Cell. Microbiol. 4:635-647. [DOI] [PubMed] [Google Scholar]
- 3.Bliss, C. M., Jr., D. T. Golenbock, S. Keates, J. K. Linevsky, and C. P. Kelly. 1998. Helicobacter pylori lipopolysaccharide binds to CD14 and stimulates release of interleukin-8, epithelial neutrophil-activating peptide 78, and monocyte chemotactic protein 1 by human monocytes. Infect. Immun. 66:5357-5363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bradford, M. M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248-254. [DOI] [PubMed] [Google Scholar]
- 5.Cameron, P., D. Bingham, A. Paul, M. Pavelka, S. Cameron, D. Rotondo, and R. Plevin. 2002. Essential role for verotoxin in sustained stress-activated protein kinase and nuclear factor kappa B signaling, stimulated by Escherichia coli O157:H7 in Vero cells. Infect. Immun. 70:5370-5380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Coates, N. J., and S. R. McColl. 2001. Production of chemokines in vivo in response to microbial stimulation. J. Immunol. 166:5176-5182. [DOI] [PubMed] [Google Scholar]
- 7.Dahan, S., V. Busuttil, V. Imbert, J.-F. Peyron, P. Rampal, and D. Czerucka. 2002. Enterohemorrhagic Escherichia coli infection induces interleukin-8 production via activation of mitogen-activated protein kinases and the transcription factors NF-κB and AP-1 in T84 cells. Infect. Immun. 70:2304-2310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Datsenko, K. A., and B. L. Wanner. 2000. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. USA 97:6640-6645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Donnelly, M. A., and T. S. Steiner. 2002. Two nonadjacent regions in enteroaggregative Escherichia coli flagellin are required for activation of toll-like receptor 5. J. Biol. Chem. 277:40456-40461. [DOI] [PubMed] [Google Scholar]
- 10.Fitzpatrick, M. M., V. Shah, R. S. Trompeter, M. J. Dillon, and T. M. Barratt. 1992. Interleukin-8 and polymorphoneutrophil leucocyte activation in hemolytic uremic syndrome of childhood. Kidney Int. 42:951-956. [DOI] [PubMed] [Google Scholar]
- 11.Gale, L. M., and S. R. McColl. 1999. Chemokines: extracellular messengers for all occasions? Bioessays 21:17-28. [DOI] [PubMed] [Google Scholar]
- 12.Gewirtz, A. T., T. A. Navas, S. Lyons, P. J. Godowski, and J. L. Madara. 2001. Cutting edge: bacterial flagellin activates basolaterally expressed TLR5 to induce epithelial proinflammatory gene expression. J. Immunol. 167:1882-1885. [DOI] [PubMed] [Google Scholar]
- 13.Gewirtz, A. T., P. O. Simon, C. K. Schmitt, L. J. Taylor, C. H. Hagedorn, A. D. O'Brien, A. S. Neish, and J. L. Madara. 2001. Salmonella typhimurium translocates flagellin across intestinal epithelia, inducing a proinflammatory response. J. Clin. Investig. 107:99-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hayashi, F., K. D. Smith, A. Ozinsky, T. R. Hawn, E. C. Yi, D. R. Goodlett, J. K. Eng, S. Akira, D. M. Underhill, and A. Aderem. 2001. The innate immune response to bacterial flagellin is mediated by Toll-like receptor 5. Nature 410:1099-1103. [DOI] [PubMed] [Google Scholar]
- 15.Hurley, B. P., C. M. Thorpe, and D. W. K. Acheson. 2001. Shiga toxin translocation across intestinal epithelial cells is enhanced by neutrophil transmigration. Infect. Immun. 69:6148-6155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jung, H. C., L. Eckmann, S.-K. Yang, A. Panja, J. Fierer, E. Morzycka-Wroblewska, and M. F. Kagnoff. 1995. A distinct array of proinflammatory cytokines is expressed in human colon epithelial cells in response to bacterial invasion. J. Clin. Investig. 95:55-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Karpman, D., A. Andreasson, H. Thysell, B. S. Kaplan, and C. Svanborg. 1995. Cytokines in childhood hemolytic uremic syndrome and thrombotic thrombocytopenic purpura. Pediatr. Nephrol. 9:694-699. [DOI] [PubMed] [Google Scholar]
- 18.Litalien, C., F. Proulx, M. M. Mariscalco, P. Robitaille, J. P. Turgeon, E. Orrbine, P. C. Rowe, P. N. McLaine, and E. Seidman. 1999. Circulating inflammatory cytokine levels in hemolytic uremic syndrome. Pediatr. Nephrol. 13:840-845. [DOI] [PubMed] [Google Scholar]
- 19.Maaser, C., S. Schoeppner, T. Kucharzik, M. Kraft, E. Schoenherr, W. Domschke, and N. Luegering. 2001. Colonic epithelial cells induce endothelial cell expression of ICAM-1 and VCAM-1 by a NF-κB-dependent mechanism. Clin. Exp. Immunol. 124:208-213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Murata, A., T. Shimazu, T. Yamamoto, N. Taenaka, K.-I. Nagayama, T. Honda, H. Sugimoto, M. Monden, N. Matsuura, and S. Okada. 1998. Profiles of circulating inflammatory and anti-inflammatory cytokines in patients with hemolytic uremic syndrome due to E. coli 0157 infection. Cytokine 10:544-548. [DOI] [PubMed] [Google Scholar]
- 21.Nasreen, N., K. A. Mohammed, J. Hardwick, R. D. Van Horn, K. L. Sanders, C. M. Doerschuck, J. W. Hott, and V. B. Antony. 2001. Polar production of interleukin-8 by mesothelial cells promotes the transmesothelial migration of neutrophils: role of intercellular adhesion molecule-1. J. Infect. Dis. 183:1638-1645. [DOI] [PubMed] [Google Scholar]
- 22.Nataro, J. P., and J. B. Kaper. 1998. Diarrheagenic Escherichia coli. Clin. Microbiol. Rev. 11:142-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ogierman, M. A., A. W. Paton, and J. C. Paton. 2000. Up-regulation of both intimin and eae-independent adherence of Shiga toxigenic Escherichia coli 0157 by ler and phenotypic impact of a naturally occurring ler mutation. Infect. Immun. 68:5344-5353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Paton, A. W., and J. C. Paton. 1998. Detection and characterization of Shiga toxigenic Escherichia coli by using multiplex PCR assays for stx1, stx2, eaeA, enterohemorrhagic E. coli hlyA, rfbO111, and rfbO157. J. Clin. Microbiol. 36:598-602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Paton, A. W., and J. C. Paton. 1999. Molecular characterization of the locus encoding biosynthesis of the lipopolysaccharide O antigen of Escherichia coli serotype O113. Infect. Immun. 67:5930-5937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Paton, A. W., P. Srimanote, M. C. Woodrow, and J. C. Paton. 2001. Characterization of Saa, a novel autoagglutinating adhesin produced by locus of enterocyte effacement-negative Shiga-toxigenic Escherichia coli strains that are virulent for humans. Infect. Immun. 69:6999-7009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Paton, A. W., E. Voss, P. A. Manning, and J. C. Paton. 1997. Shiga toxin-producing Escherichia coli isolates from cases of human disease show enhanced adherence to intestinal epithelial (Henle 407) cells. Infect. Immun. 65:3799-3805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Paton, A. W., M. C. Woodrow, R. M. Doyle, J. A. Lanser, and J. C. Paton. 1999. Molecular characterization of a Shiga toxigenic Escherichia coli 0113:H21 strain lacking eae responsible for a cluster of cases of hemolytic-uremic syndrome. J. Clin. Microbiol. 37:3357-3361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Paton, J. C., and A. W. Paton. 1998. Pathogenesis and diagnosis of Shiga toxin-producing Escherichia coli infections. Clin. Microbiol. Rev. 11:450-479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Philpott, D. J., S. Yamaoka, A. Israel, and P. J. Sansonetti. 2000. Invasive Shigella flexneri activates NF-κB through a lipopolysaccharide-dependent innate intracellular response and leads to IL-8 expression in epithelial cells. J. Immunol. 165:903-914. [DOI] [PubMed] [Google Scholar]
- 31.Savkovic, S. D., A. Koutsouris, and G. Hecht. 1997. Activation of NF-κB in intestinal epithelial cells by enteropathogenic Escherichia coli. Am. J. Physiol. Cell Physiol. 273:C1160-C1167. [DOI] [PubMed] [Google Scholar]
- 32.Srimanote, P., A. W. Paton, and J. C. Paton. 2002. Characterization of a novel type IV pilus locus encoded on the large plasmid of locus of enterocyte effacement-negative Shiga-toxigenic Escherichia coli strains that are virulent for humans. Infect. Immun. 70:3094-3100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Steiner, T. S., J. P. Nataro, C. E. Poteet-Smith, J. A. Smith, and R. L. Guerrant. 2000. Enteroaggregative Escherichia coli express a novel flagellin that causes IL-8 release from intestinal epithelial cells. J. Clin. Investig. 105:1769-1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.te Loo, D. M. W. M., L. A. H. Monnens, T. J. A. M. van der Velden, M. A. Vermeer, F. Preyers, P. N. M. Demacker, L. P. W. J. van den Heuvel, and V. W. M. van Hinsberg. 2000. Binding and transfer of verocytotoxin by polymorphonuclear leukocytes in hemolytic uremic syndrome. Blood 95:3396-3402. [PubMed] [Google Scholar]
- 35.te Loo, D. M. W. M., V. W. M. Van Hinsbergh, L. P. W. J. Van Den Heuvel, and L. A. H. Monnens. 2000. Detection of verocytotoxin bound to circulating polymorphonuclear leukocytes of patients with hemolytic uremic syndrome. J. Am. Soc. Nephrol. 12:800-806. [DOI] [PubMed] [Google Scholar]
- 36.Thorpe, C. M., B. P. Hurley, L. L. Lincicome, M. S. Jacewicz, G. T. Keusch, and D. W. K. Acheson. 1999. Shiga toxins stimulate secretion of interleukin-8 from intestinal epithelial cells. Infect. Immun. 67:5985-5993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Thorpe, C. M., W. E. Smith, B. P. Hurley, and D. W. K. Acheson. 2001. Shiga toxins induce, superinduce, and stabilize a variety of C-X-C chemokine mRNAs in intestinal epithelial cells, resulting in increased chemokine expression. Infect. Immun. 69:6140-6147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tzipori, S., H. Karch, I. K. Wachsmuth, R. M. Robins-Browne, A. D. O'Brien, H. Lior, M. L. Cohen, J. Smithers, and M. M. Levine. 1987. Role of a 60-megadalton plasmid and Shiga-like toxins in the pathogenesis of infection caused by enterohemorrhagic Escherichia coli O157:H7 in gnotobiotic piglets. Infect. Immun. 55:3117-3125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vasselon, T., and P. A. Detmers. 2002. Toll receptors: a central element in innate immune responses. Infect. Immun. 70:1033-1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Westerholt, S., T. Hartung, M. Tollens, A. Gustrau, M. Oberhoffer, H. Karch, B. Klare, K. Pfeffer, P. Emmrich, and R. Oberhoffer. 2000. Inflammatory and immunological parameters in children with haemolytic uremic syndrome (HUS) and gastroenteritis—pathophysiological and diagnostic clues. Cytokine 12:822-827. [DOI] [PubMed] [Google Scholar]
- 41.Yamasaki, C., Y. Natori, X.-T. Zeng, M. Ohmura, S. Yamasaki, Y. Takeda, and Y. Natori. 1999. Induction of cytokines in a human colon epithelial cell line by Shiga toxin 1 (Stx1) and Stx2 but not by non-toxic mutant Stx1 which lacks N-glycosidase activity. FEBS Lett. 442:231-234. [DOI] [PubMed] [Google Scholar]
- 42.Yang, S.-K., L. Eckmann, A. Panja, and M. F. Kagnoff. 1997. Differential and regulated expression of C-X-C, C-C, and C-chemokines by human colon epithelial cells. Gastroenterology 113:1214-1223. [DOI] [PubMed] [Google Scholar]
- 43.Zhou, X., J. A. Giron, A. G. Torres, J. A. Crawford, E. Negrete, S. N. Vogel, and J. B. Kaper. 2003. Flagellin of enteropathogenic Escherichia coli stimulates interleukin-8 production in T84 cells. Infect. Immun. 71:2120-2129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zoja, C., S. Angioletti, R. Donadelli, C. Zanchi, S. Tomasoni, E. Binda, B. Imberti, M. Te Loo, L. Monnens, G. Remuzzi, and M. Morigi. 2002. Shiga toxin-2 triggers endothelial leukocyte adhesion and transmigration via NF-κB dependent up-regulation of IL-8 and MCP-1. Kidney Int. 62:846-856. [DOI] [PubMed] [Google Scholar]