Abstract
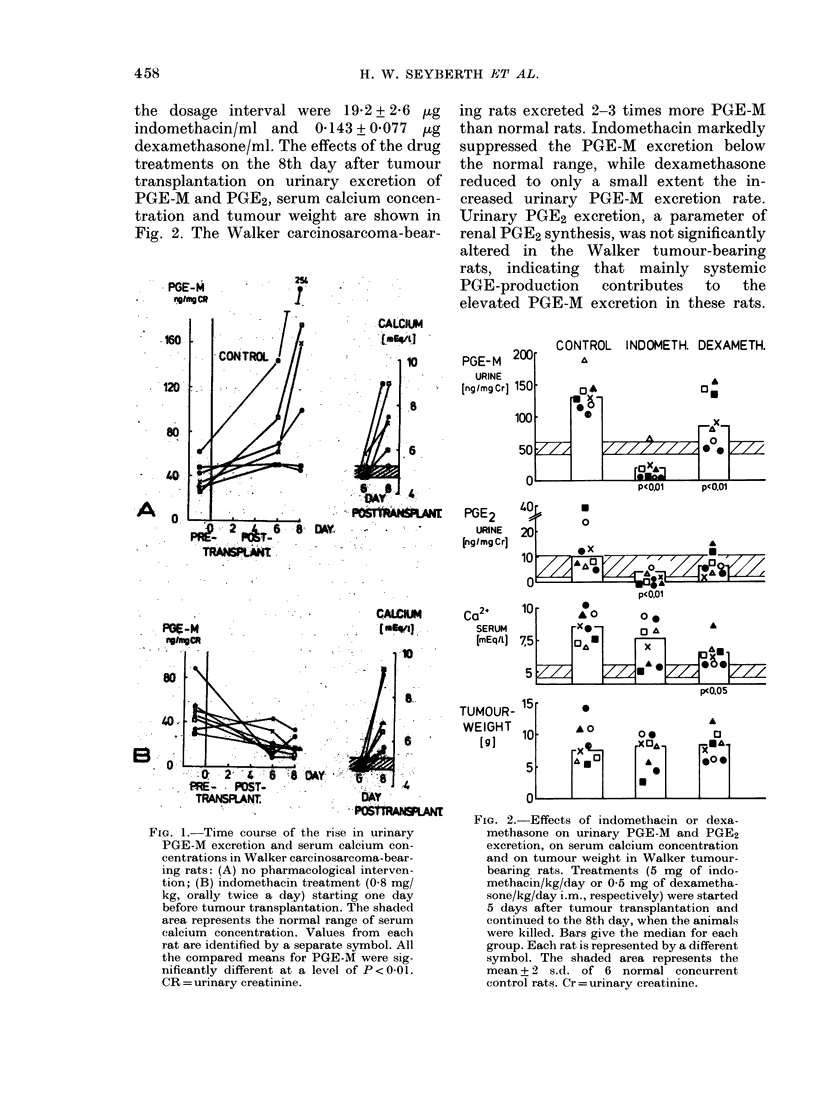
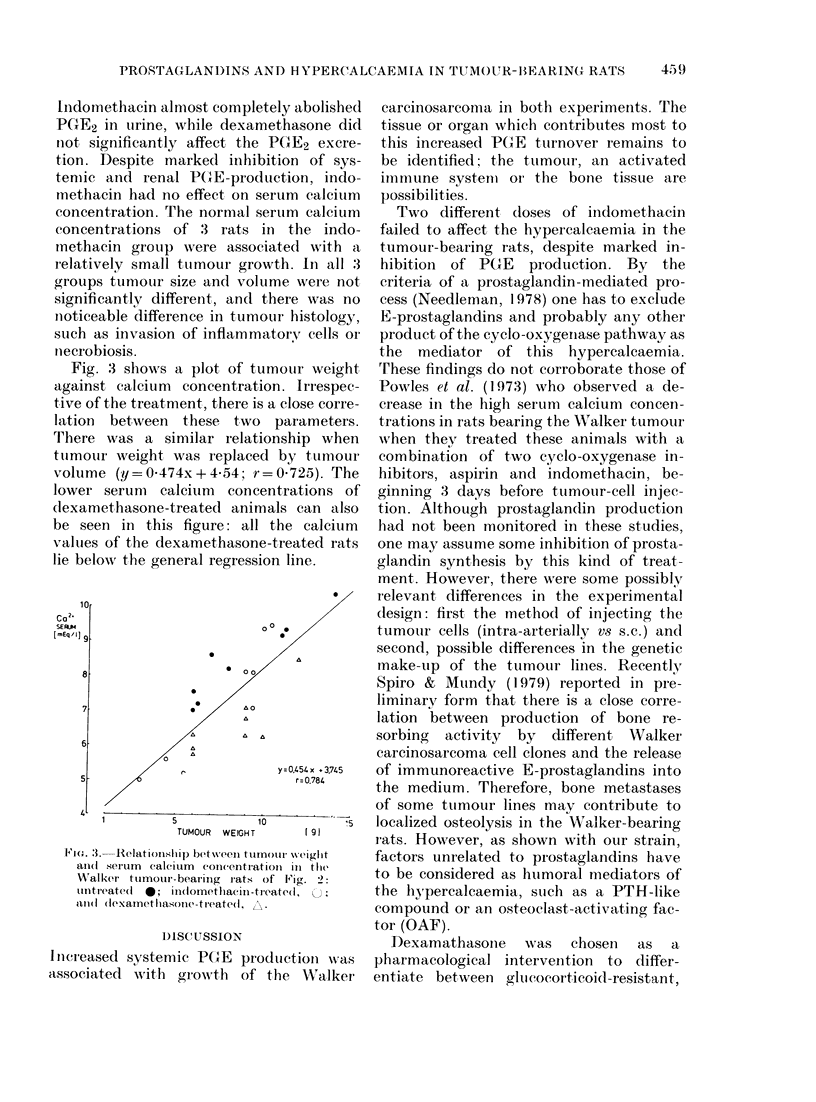
The hypothesis that there is prostaglandin-mediated hypercalcaemia associated with the Walker carcinosarcoma in the rat was tested by measuring PGE production during the development of the hypercalcaemia, and determining the effects of inhibition of prostaglandin synthesis on serum calcium concentration. Parathyroid hormone (PTH) activity was estimated by the determination of the serum concentration of immunoreactive PTH. There was a 3-fold increase in the urinary excretion of 7α-hydroxy-5,11-diketotetranor-prostane-1,16-dioic acid (PGE-M), a major urinary metabolite of the E prostaglandins from basal levels. Treatment with indomethacin, a potent inhibitor of prostaglandin synthesis, did not lower serum calcium concentrations with two different doses (1·6 mg/kg/day orally and 5 mg/kg/day i.m.); effective inhibition of prostaglandin synthesis was demonstrated by the suppression of PGE-M excretion rates below basal levels. Serum concentrations of immunoreactive PTH were not significantly altered by either tumour growth or indomethacin. Dexamethasone (0·5 mg/kg/day i.m.) attenuated both the increased urinary excretion of PGE-M and the rise in serum calcium concentration, suggesting that one or several lipoxygenase products might be the actual mediators of the hypercalcaemia. We conclude that the hypercalcaemia in the rat with Walker carcinosarcoma is probably not mediated by E-prostaglandins and probably not by any other product of the cyclo-oxygenase pathway. The increased PGE turnover may be considered as a biochemical marker of tumour load, but not as an indicator of a prostaglandin-mediated hypercalcaemia.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Borgeat P., Samuelsson B. Arachidonic acid metabolism in polymorphonuclear leukocytes: unstable intermediate in formation of dihydroxy acids. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3213–3217. doi: 10.1073/pnas.76.7.3213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caro J. F., Besarab A., Flynn J. T. Prostaglandin E and hypercalcemia in breast carcinoma: only a tumor marker? A need for perspective. Am J Med. 1979 Feb;66(2):337–341. doi: 10.1016/0002-9343(79)90561-8. [DOI] [PubMed] [Google Scholar]
- Chen T. L., Feldman D. Glucocorticoid receptors and actions in subpopulations of cultured rat bone cells. Mechanism of dexamethasone potentiation of parathyroid hormone-stimulated cyclic AMP production. J Clin Invest. 1979 Apr;63(4):750–758. doi: 10.1172/JCI109359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DENT C. E. Some problems of hyperparathyroidism. Br Med J. 1962 Dec 1;2(5317):1419–1425. doi: 10.1136/bmj.2.5317.1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erlenmaier T., Müller H., Seyberth H. W. Combined capillary column gas chromatography-mass spectrometric method for the quantitative analysis of urinary prostaglandins. J Chromatogr. 1979 Jul 21;163(3):289–293. doi: 10.1016/s0378-4347(00)81416-4. [DOI] [PubMed] [Google Scholar]
- Flower R. J. Drugs which inhibit prostaglandin biosynthesis. Pharmacol Rev. 1974 Mar;26(1):33–67. [PubMed] [Google Scholar]
- Frölich J. C., Wilson T. W., Sweetman B. J., Smigel M., Nies A. S., Carr K., Watson J. T., Oates J. A. Urinary prostaglandins. Identification and origin. J Clin Invest. 1975 Apr;55(4):763–770. doi: 10.1172/JCI107987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gréen K. Metabolism of prostaglandin E2 in the rat. Biochemistry. 1971 Mar 16;10(6):1072–1086. doi: 10.1021/bi00782a022. [DOI] [PubMed] [Google Scholar]
- Hamberg M. Inhibition of prostaglandin synthesis in man. Biochem Biophys Res Commun. 1972 Nov 1;49(3):720–726. doi: 10.1016/0006-291x(72)90470-6. [DOI] [PubMed] [Google Scholar]
- Hamberg M., Samuelsson B. Prostaglandin endoperoxides. Novel transformations of arachidonic acid in human platelets. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3400–3404. doi: 10.1073/pnas.71.9.3400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lands W. E. The biosynthesis and metabolism of prostaglandins. Annu Rev Physiol. 1979;41:633–652. doi: 10.1146/annurev.ph.41.030179.003221. [DOI] [PubMed] [Google Scholar]
- Minne H., Raue F., Bellwinkel S., Ziegler R. The hypercalcaemic syndrome in rats bearing the Walker carcinosarcoma 256. Acta Endocrinol (Copenh) 1975 Mar;78(3):613–624. doi: 10.1530/acta.0.0780613. [DOI] [PubMed] [Google Scholar]
- Mundy G. R. Calcium and cancer. Life Sci. 1978 Oct 30;23(17-18):1735–1744. doi: 10.1016/0024-3205(78)90101-7. [DOI] [PubMed] [Google Scholar]
- Mundy G. R., Rick M. E., Turcotte R., Kowalski M. A. Pathogenesis of hypercalcemia in lymphosarcoma cell leukemia. Role of an osteoclast activating factor-like substance and a mechanism of action for glucocorticoid therapy. Am J Med. 1978 Oct;65(4):600–606. doi: 10.1016/0002-9343(78)90847-1. [DOI] [PubMed] [Google Scholar]
- Murphy R. C., Hammarström S., Samuelsson B. Leukotriene C: a slow-reacting substance from murine mastocytoma cells. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4275–4279. doi: 10.1073/pnas.76.9.4275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Needleman P. Experimental criteria for evaluating prostaglandin biosynthesis and intrinsic function. Biochem Pharmacol. 1978;27(11):1515–1518. doi: 10.1016/0006-2952(78)90477-x. [DOI] [PubMed] [Google Scholar]
- Powles T. J., Clark S. A., Easty D. M., Easty G. C., Neville A. M. The inhibition by aspirin and indomethacin of osteolytic tumor deposits and hypercalcaemia in rats with Walker tumour, and its possible application to human breast cancer. Br J Cancer. 1973 Oct;28(4):316–321. doi: 10.1038/bjc.1973.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raisz L. G., Trummel C. L., Wener J. A., Simmons H. Effect of glucocorticoids on bone resorption in tissue culture. Endocrinology. 1972 Apr;90(4):961–967. doi: 10.1210/endo-90-4-961. [DOI] [PubMed] [Google Scholar]
- Seyberth H. W., Hubbard W. C., Oelz O., Sweetman B. J., Watson J. T., Oates J. A. Prostaglandin-mediated hypercalcemia in the VX2 carcinoma-bearing rabbit. Prostaglandins. 1977 Aug;14(2):319–331. doi: 10.1016/0090-6980(77)90177-0. [DOI] [PubMed] [Google Scholar]
- Seyberth H. W. Prostaglandin-mediated hypercalcemia: a paraneoplastic syndrome. Klin Wochenschr. 1978 Apr 15;56(8):373–387. doi: 10.1007/BF01477292. [DOI] [PubMed] [Google Scholar]
- Seyberth H. W., Raisz L. G., Oates J. A. Prostaglandins and hypercalcemic states. Annu Rev Med. 1978;29:23–29. doi: 10.1146/annurev.me.29.020178.000323. [DOI] [PubMed] [Google Scholar]
- Seyberth H. W., Segre G. V., Hamet P., Sweetman B. J., Potts J. T., Jr, Oates J. A. Characterization of the group of patients with the hypercalcemia of cancer who respond to treatment with prostaglandin synthesis inhibitors. Trans Assoc Am Physicians. 1976;89:92–104. [PubMed] [Google Scholar]
- Seyberth H. W., Sweetman B. J., Frolich J. C., Oates J. A. Quantifications of the major urinary metabolite of the E prostaglandins by mass spectrometry: evaluation of the method's application to clinical studies. Prostaglandins. 1976 Feb;11(2):381–397. doi: 10.1016/0090-6980(76)90160-x. [DOI] [PubMed] [Google Scholar]
- Skellern G. G., Salole E. G. A high-speed liquid chromatographic analysis of indomethacin in plasma. J Chromatogr. 1975 Nov 26;114(2):483–485. doi: 10.1016/s0021-9673(01)92018-7. [DOI] [PubMed] [Google Scholar]
- Streibl W., Minne H., Raue F., Ziegler R. Radioimmunoassay for human parathyroid hormone for differentiation between patients with hypoparathyroidism, hyperparathyroidism and normals. Horm Metab Res. 1979 May;11(5):375–376. doi: 10.1055/s-0028-1095781. [DOI] [PubMed] [Google Scholar]
- Sweetman B. J., Frölich J. C., Watson J. T. Quantitative determination of prostaglandins. Prostaglandins. 1973 Jan;3(1):75–87. doi: 10.1016/0090-6980(73)90139-1. [DOI] [PubMed] [Google Scholar]
- Tam S., Hong S. C., Levine L. Relationships, among the steroids, of anti-inflammatory properties and inhibition of prostaglandin production and arachidonic acid release by transformed mouse fibroblasts. J Pharmacol Exp Ther. 1977 Oct;203(1):162–168. [PubMed] [Google Scholar]
- Tashjian A. H., Jr Role of prostaglandins in the production of hypercalcemia by tumors. Cancer Res. 1978 Nov;38(11 Pt 2):4138–4141. [PubMed] [Google Scholar]
- Tashjian A. H., Jr, Voelkel E. F., Levine L. Effects of hydrocortisone on the hypercalcemia and plasma levels of 13,14-dihydro-15-keto-prostaglandin E2 in mice bearing the HSDM1 fibrosarcoma. Biochem Biophys Res Commun. 1977 Jan 10;74(1):199–207. doi: 10.1016/0006-291x(77)91394-8. [DOI] [PubMed] [Google Scholar]
- Tashjian A. H., Jr, Voelkel E. F., Levine L. Plasma concentrations of 13,14-dihydro-15-keto-prostaglandin E2 in rabbits bearing the VX2 carcinoma: effects of hydrocortisone and indomethacin. Prostaglandins. 1977 Aug;14(2):309–317. doi: 10.1016/0090-6980(77)90176-9. [DOI] [PubMed] [Google Scholar]
- Yoneda T., Mundy G. R. Monocytes regulate osteoclast-activating factor production by releasing prostaglandins. J Exp Med. 1979 Aug 1;150(2):338–350. doi: 10.1084/jem.150.2.338. [DOI] [PMC free article] [PubMed] [Google Scholar]



