Abstract
Pseudomonas aeruginosa has emerged as an important causative agent of bacterial keratitis, a rapidly progressive ocular condition that may result in blindness. Secretory mucin forms the main constituent of the precorneal tear film, a three-layer film on the ocular surface protecting the underlying corneal epithelium from potential pathogens. The purpose of the present study was to compare mucin degradation mechanisms between ocular P. aeruginosa strains. Mucin degradation was assessed by agarose electrophoresis, lectin blotting, and size exclusion chromatography. The results indicate that certain P. aeruginosa strains (Paer12, ATCC 15442, 6294, and Paer25) had depleted mucin from the culture supernatant and that this was contingent on the inherent ability of these isolates to produce proteases. Non-protease-producing strains (Paer1 and Paer3) did not appreciably degrade mucin. Further, galactosidase, N-acetylglucosaminidase, and N-acetylgalactosaminidase activities were detected in some strains, suggesting the operation of further mechanisms of mucin degradation by P. aeruginosa. Mucin degradation by P. aeruginosa also seemed to be for the acquisition of nutrients, as a growth advantage was observed in mucin-depleting strains over nondepleting strains in the long term. It is postulated that the degradation of mucin serves to collapse the mucin barrier and its associated network containing antibacterial tear components and to provide energy for sustained bacterial growth.
Microbial keratitis is a highly destructive and invasive infection that may result in corneal ulceration and loss of vision if prompt treatment is not administered (11). Pseudomonas aeruginosa has been isolated from most microbial keratitis patients, in some cases as the predominant bacterium (7, 56) and in others as the main gram-negative bacterium (44). It has also been associated with ocular disease resulting from contact lens wear, namely, contact lens acute red eye (CLARE) (24). This noninvasive ocular condition, characterized by a host inflammatory response, is presumably initiated by bacterial lipopolysaccharide (47). Contact lens wear seems to reduce tear exchange (39), where mucus is the predominant component of the tear film. Delayed tear clearance in certain contact lens wearers may prove opportune for the initiation of pathogenesis such as microbial keratitis.
In the eye, mucus is the predominant component of the tear film (8, 37, 43), a three-layered film on the surface of the eye forming the first barrier between the outside environment and the underlying corneal epithelial cells. It is constituted primarily of secretory MUC5AC mucin secreted from conjunctival goblet cells (28), while membrane-bound mucins, MUC1 and MUC4, are present on corneal and conjunctival epithelial cells (1), with recent evidence that a soluble form of MUC4 is present in human tears (41). Ocular mucin is polydisperse, consisting of species with different size, charge, and glycosylation patterns (3, 17, 23) that contribute to the structure and function of mucus.
In general, the mucin layer has a protective role in overlying the epithelial cells. In vitro studies have documented that preincubation of microbes with mucin decreased their subsequent adhesion to epithelial cells (9, 16, 46). Thus, pathogens have developed virulence mechanisms aimed at evasion of the mucin barrier. Helicobacter pylori is able to cleave the disulfide bonds of mucin with its thioredoxin system (55), microbes alter the secretory profile of host mucin on infection (5, 10, 52), while bacterial proteases (13, 16, 35) and glycosidases (2, 4, 20) degrade mucin for the dissolution of the mucin barrier or the uptake of nutrients.
A well-documented case of bacterial pathogenesis linked to alterations in mucin is in cystic fibrosis, an autosomal recessive genetic disorder caused by a mutation in the cystic fibrosis transmembrane conductance regulator, a chloride ion channel protein (31). The abnormal chloride secretion leads to a decrease in water transport to the lumen of the respiratory tract and dehydration of mucus. The resulting mucus has increased viscosity and adhesivity, which impairs the transport of mucus from the lungs by cilia (19), causing relatively stagnant mucus and colonization and pathogenesis by P. aeruginosa. This preponderance of P. aeruginosa infections in relatively stagnant mucus of immunocompetent patients suggests an important virulence mechanism initiated by the interaction of P. aeruginosa and mucin, which deserves further study.
The aim of the present study was to investigate whether P. aeruginosa strains isolated from patients with different clinical ocular outcomes varied in their ability to interact with mucin. Alterations to mucin after bacterial incubations were assessed relative to control mucin incubations. The information obtained was compared with bacterial protease and glycosidase activities to provide a detailed study of the differential interaction of diverse P. aeruginosa isolates with mucin.
The results consistently showed depletion of high-molecular-weight mucin from the supernatant of certain P. aeruginosa strains that coincided with their ability to produce proteases. This was assayed using two independent techniques (agarose electrophoresis and chromatography) along with supplementary studies to assay residual mucin in the bacterial pellet, comparative stains (wheat germ agglutin lectin (WGA), periodic acid-Schiff (PAS), and digoxigenin glycoprotein), and comparative membranes for blotting (nitrocellulose and polyvinylidere difluoride [PVDF]). This is the first time, to our knowledge, that a detailed comparison of mucin degradation by six P. aeruginosa strains has been conducted using biochemical techniques specific to mucin. There is an important interaction between P. aeruginosa and mucin, and our results point toward a protease-driven degradation of mucin by distinct P. aeruginosa strains.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
P. aeruginosa strains were obtained from the culture collection at the Cooperative Research Centre for Eye Research and Technology and were stored at −86°C in tryptone soya broth (TSB) (Oxoid, Basingstoke, England) supplemented with 30% (vol/vol) glycerol. Clinical P. aeruginosa strains were originally isolated from contact lens wearers. Paer1 and Paer25 were isolated from CLARE events, Paer3 was isolated from an asymptomatic subject, and Paer12 and 6294 were isolated from microbial keratitis patients. ATCC 15442 is a standard environmental strain.
Strains were identified as P. aeruginosa by standard biochemical methods and the Analytical Profile Index 20 strips (API 20; BioMerieux, Marcy l'Etoile, France). Serotyping of clinical strains using P. aeruginosa antisera identified Paer1 and Paer3 as serotype I (O:1), Paer12 and 6294 as serotype G (O:6), and Paer25 as serotype F (O:4) (51). Further, the invasive and cytotoxic phenotype for each strain had previously been determined using cultured corneal epithelial cells (57). Paer1 and ATCC 15442 are both noninvasive and noncytotoxic strains; Paer3 is cytotoxic; and Paer12, 6294, and Paer25 are invasive (57).
Bacteria were initially cultured on chocolate blood agar plates (Oxoid Australia, West Heidelberg, Australia) to decrease the aggregation of bacteria by mucin in subsequent solution-based assays (14) and incubated at 35°C for 18 h. Bacterial cells were harvested from plates by the addition of phosphate-buffered saline (PBS [pH 7.4]; 137 mM NaCl, 2.7 mM KCl, 6.5 mM Na2HPO4, 1.5 mM KH2PO4), and the bacterial suspension was washed three times by centrifugation at 2,000 × g for 10 min in PBS.
Mucin preparation.
Preliminary experiments (results not shown) had shown identical growth and degradation capabilities of P. aeruginosa strains in porcine gastric mucin (PGM) using an ocular defined medium (12), a medium based on artificial tear solution (6.8 g of NaCl per liter, 1.4g of KCl per liter, 0.147 g of CaCl2 · 2H2O per liter, 0.147 g of MgSO4 · 2H2O per liter, 4.18 g of morpholinepropanesulfonic acid [MOPS] per liter, 2.18 g of Na2CO3 [pH 7.0] per liter, 0.093 g of NaH2PO4 per liter, 5.55 mM glucose, 10 mM NH4Cl, vitamins, and trace elements), PBS, and PBS with the addition of divalent cations (0.0107 g of CaCl2 · 2H2O per liter and 0.0112 g of MgCl2 · 6H2O per liter). Therefore, PBS was used as the incubating medium for the comparison of multiple strains in commercial PGM.
Commercial PGM (type III; Sigma, St Louis, Mo.) was prepared at a final concentration of 1% (wt/vol) in 30 ml of PBS. Mucin solutions were sterilized by autoclaving for 15 min at 121°C. Mucin sterilized in this way did not affect the “biological activity of its constituents” (2). Further, sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) analyses confirmed that there was no change in the protein profile of PGM after this procedure (results not shown). Mucin control flasks contained 1% (wt/vol) PGM in PBS alone with no bacteria. As controls for the use of mucin as the sole nutrient source, bacteria were also grown in PBS alone and PBS containing 5.55 mM glucose and 10 mM ammonium chloride.
Bacteria were added at an initial concentration of approximately 108 cells/ml, and cultures were incubated in a 37°C water bath with 120 rpm orbital shaking for 72 h. Purity tests were performed on chocolate agar plates at 10, 24, 48, and 72 h to ensure no contamination of cultures. Aliquots were periodically collected and centrifuged at 12,000 × g for 5 min, and the supernatant was stored at −20°C until further analysis.
Viable-cell counts were performed in duplicate on nutrient agar (Oxoid) plates for incubation at 35°C overnight. Due to the colony growth characteristics of certain P. aeruginosa strains, the plates were tilted so that each drop was able to spread down the plate and give effective separation of colonies for counting purposes.
Analysis of mucin degradation. (i) Agarose gel electrophoresis.
A 1% (wt/vol) agarose (Agarose MP; Roche Diagnostics GmbH, Mannheim, Germany, and Roche Diagnostics Corp., Indianapolis, Ind.) gel was prepared in electrophoresis running buffer (40 mM Tris acetate, 1 mM EDTA [pH 8.0] 0.1% SDS) as previously described (50). Samples were diluted in 4× sample buffer (2.5 ml of Tris-acetate electrophoresis buffer, 4.0 ml of glycerol, 2.0 ml of 4% [wt/vol] bromophenol blue, 1.5 ml of Milli-Q water), while reduced samples also contained 4 mM dithiothreitol and were heated at 100°C for 5 min. Electrophoresis was conducted at 30 V constant voltage at room temperature until the bromophenol blue dye front was near the edge of the gel (approximately 24 h).
The samples were transferred to a nitrocellulose membrane (0.2-μm-pore-size pure nitrocellulose [Bio-Rad, Hercules, Calif.]) overnight by capillary blotting using 4× standard sodium citrate buffer (0.6 M sodium chloride, 0.06 M sodium citrate [pH 7]) as the transfer buffer. The membrane was allowed to dry for 30 min at room temperature and washed three times in PBS to remove excess salt.
(ii) Lectin staining of blots.
Mucins present in culture supernatants were probed with WGA (Sigma) by a modification of the method of Gravel and Golaz (21). In this case, the membrane was blocked with PBS-0.5% (vol/vol) Tween 20 (PBST) for 1 h and incubated in the presence of 1 μg of biotinylated WGA per ml in PBST for 2 h. After being washed six times (10 min each) with PBST, the membrane was incubated in a 1:2,000 dilution of ExtrAvidin-peroxidase (Sigma) in PBST for 1 h. A further three 10-min washes in PBST followed by three 10-min washes in PBS were conducted, after which peroxidase was developed by a solution consisting of 80 ml of PBS, two tablets of 3,3′-diaminobenzidine tetrahydrochloride (10 mg/tablet [Sigma]) dissolved in 10 ml of methanol, two tablets of 4-chloro-1-naphthol (30 mg/tablet [Sigma]) dissolved in 10 ml of methanol, and 40 μl of hydrogen peroxide solution (30% solution [BDH Laboratory Supplies, Poole, England]).
(iii) SDS-PAGE.
Culture supernatants were diluted with 4× SDS-PAGE sample buffer (0.2 g of SDS, 4.0 ml of glycerol, 4.0 ml of 4% [wt/vol] bromophenol blue, 2.5 ml of 1 M Tris-HCl [pH 6.8], Milli-Q water to 10 ml) under reducing and nonreducing conditions. Each sample (9 μl) was added to a 4 to 15% polyacrylamide ready-made gel (Bio-Rad), and electrophoresis commenced at 100 V constant voltage at room temperature with 0.025 M Tris-HCl-0.192 M glycine-1% (wt/vol) SDS (pH 8.3) as the running buffer. The gels were subsequently silver stained (36). As a reference for elastase production by strains, 200 μg of purified elastase (type I, porcine pancreas [Sigma]) per ml in 1% (wt/vol) PGM was also applied to 4 to 15% polyacrylamide gels under reducing and denaturing conditions.
(iv) ELLA.
To investigate whether bacterial adhesion to mucin was responsible for the mucin depletion observed from the culture supernatant in certain strains, the amount of residual mucin in the bacterial pellet was measured. For this, the bacterial pellet obtained after centrifugation of the culture was washed once by centrifugation in PBS and resuspended to its original volume with PBS. The control included the pellet from the mucin control flask, containing no bacteria, at the same time point.
Mucin control standards included serial 1:5 dilutions of 0.1% (wt/vol) PGM in PBS containing no bacteria. Standards for the mucin and bacterial pellet solution consisted of 1:5 serial dilutions of 0.1% (wt/vol) PGM containing a set amount of undiluted strain 6294.
The amount of mucin in each was quantified using the microtiter enzyme-linked lectin assay (ELLA) (34). To coat the wells, 100 μl of sample or standard was added to wells of a medium-binding 96-well microtiter plate (Microlan 200; Greiner Bio-One, Frickenhausen, Germany) and kept at 4°C overnight. Blank wells consisted of undiluted samples with no lectin added and also PBS alone. The wells were blocked with 120 μl of PBS-0.1% Tween 20 for 2 h at 35°C with shaking. After a further three washes in PBS, 100 μl of biotinylated WGA was added to the appropriate wells at 2 μg/ml in PBS-0.1% Tween 20 and incubated for 2 h at 35°C with shaking.
The wells were washed three times in PBS and incubated with 100 μl ExtrAvidin-peroxidase (1:1,000 dilution in PBS-0.1% Tween 20) for 60 min at 35°C with shaking. After three washes in PBS-0.1% Tween 20 and two further washes in PBS, 100 μl of o-phenylenediamine dihydrochloride (OPD) substrate was added to each well and the plate was incubated at room temperature for 6 min with shaking. The reaction was stopped by the addition of 100 μl 4 M sulfuric acid, and the absorbance was read at 490 nm with a microtiter plate reader (SpectraFluor Plus; TECAN, Salzburg, Austria). Residual mucin in the test pellet solutions was quantified from linear regression equations of respective standard curves.
(v) Size exclusion chromatography.
Gel filtration of culture supernatants was conducted to identify the formation of mucin subunits after bacterial incubation. A Sepharose CL-2B column (Amersham Pharmacia Biotech AB, Uppsala, Sweden) was equilibrated with 4 M guanidine hydrochloride (ultrapure grade) (ICN) in PBS. Culture supernatants were centrifuged at 5,000 × g for 15 min at 4°C, and 5-ml samples were applied to the column. Samples were eluted from the column with 4 M guanidine hydrochloride in PBS at a rate of 0.2 ml/min, and 3-ml fractions were collected using the GradiFrac system (Amersham Pharmacia Biotech AB).
PAS (49) and WGA lectin staining were performed on fractions immobilized on nitrocellulose by a slot blot apparatus apparatus (Hoefer Pharmacia Biotech Inc., San Francisco, Calif.). As high-molecular-weight mucin glycopeptides bind poorly to nitrocellulose (49), PAS and WGA staining of fractions was also repeated on prewetted PVDF (Immobilon-P; Millipore Corp., Bedford, Mass.). Densitometry was performed using a GS-710 scanning densitometer (Bio-Rad) in the reflectance mode. The Quantity One software-calculated peak density and the densities of samples were converted to micrograms per milliliter from a standard curve constructed from the density of standard PGM concentrations applied to each blot. The last three samples of each blot were repeated on the next blot, average differences were calculated, and the values for the second blot were adjusted.
(vi) Protease production.
Zymography was based on a previous method (29). Supernatants corresponding to 3 or 3.75 μg of protein per well in 4× nonreducing SDS-PAGE sample buffer were applied to a 7.5% acrylamide gel containing 0.1% (wt/vol) gelatin (type B bovine skin [Sigma]). SDS-PAGE was performed at 4°C at 100 V.
The gel was incubated on a rocking platform in 2.5% (vol/vol) Triton X-100 (Sigma) at room temperature for 1 h to remove SDS and incubated overnight at 35°C in development buffer (0.05 M Tris-HCl [pH 8.0], 0.01 M CaCl2, 1 mM ZnCl2, 0.15 M NaCl). The gel was stained for 2 h with Coomassie blue and subsequently destained. To examine the constitutive proteolytic profile due to general growth, protease activity by strain 6294 grown overnight in TBS was investigated.
(vii) Glycosidase production.
Glycosidase production was detected in 72-h culture supernatants by a modification of a previously established chromogenic method (26). The chromogenic substrates p-nitrophenol-linked (pNP)-β-galactoside, pNP-N-acetyl-β-galactoside and pNP-N-acetyl-β-glucoside were all purchased from Sigma. Substrates were prepared at 10 mM in 20% (vol/vol) dimethyl sulfoxide in Milli-Q water. Standard solutions included various concentrations of pNP alone (spectrophotometric grade; Sigma) in 20% (vol/vol) dimethyl sulfoxide. Samples were applied in duplicate to a 96-well medium-binding microtiter plate (Microlan 200) as follows: 35 μl of 0.2 M citric acid-trisodium citrate buffer (pH 6.0), 20 μl pNP-linked substrate, and 45 μl of culture supernatant. Standard samples included the application of 20 μl of pNP standard and 80 μl of 0.2 M citric acid buffer (pH 6.0). Blanks comprised 80 μl of citric acid buffer and 20 μl of pNp-linked substrate for P. aeruginosa culture supernatants. The wells were incubated at 35°C for 3 h with shaking, and the reaction was stopped by the addition of 100 μl of 0.5 M NaHCO3-Na2CO3 (pH 10.2).
Absorbance was read at 405 nm (SpectraFluor Plus), and the release of pNP was quantified by referring to the standard curve constructed from standard values of pNP. Enzyme activity was calculated as units per microgram of protein, where 1 unit was defined as the amount of enzyme catalyzing the release of 1 nmol of pNP. The enzyme activity of the mucin control incubation supernatant was subtracted from the enzyme activities of the bacterial culture supernatants to correct for “background” enzyme activity.
Statistics.
The exact nonparametric test was used to detect statistical differences in growth between P. aeruginosa strains within two time points. Since differences in growth were compared to Paer12, a one-tailed analysis was conducted. Due to the small sample size, significance was reported at the 10% level (P < 0.1).
RESULTS
Analysis of mucin degradation. (i) Agarose gel electrophoresis.
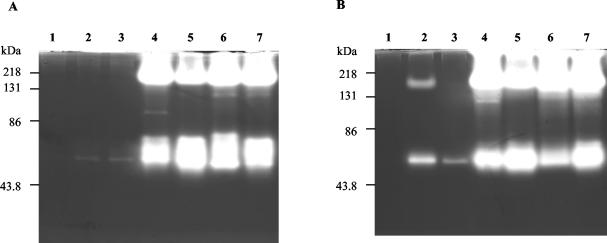
Agarose gel electrophoresis and probing with WGA lectin was performed on culture supernatants from P. aeruginosa incubations with PGM to observe alterations to high-molecular-weight mucin after bacterial incubation. Supernatants from the culture of strains Paer12, ATCC 15442, 6294, and Paer25 had a substantial reduction in mucin staining by WGA after 24 h compared with the mucin control (no bacteria), Paer1, and Paer3 (Fig. 1). The result was not specific for WGA lectin, since staining of the blots with the digoxigenin general glycoprotein stain (Roche Diagnostics) gave the same outcome (results not shown).
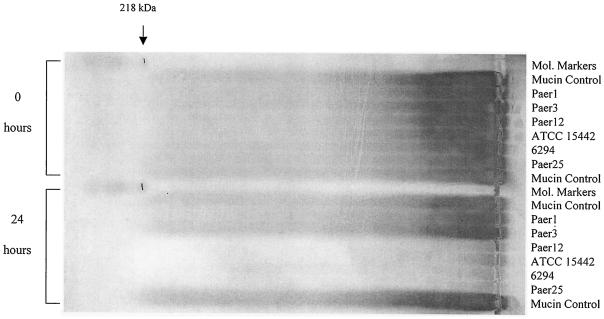
FIG. 1.
Comparison of mucin degradation between P. aeruginosa isolates at 0 and 24 h. Culture supernatants were loaded onto a 1% agarose gel and stained with biotin-labelled WGA lectin to detect mucin components on the blot. The mucin control included the incubation of PGM alone without bacteria.
Similar lectin profiles were observed at 72 h, although a slight reduction in staining was also evident with Paer1 compared with the control at 72 h, which was minor compared with the extensive depletion by the mucin-depleting strains mentioned above (results not shown). It is also noteworthy that two representative strains, Paer1 and 6294, had also shown the same differential mucin depletion pattern when bovine conjunctival mucin (purified by two sequential cesium chloride density gradient centrifugations was used in an ocular defined medium (12; results not shown).
Thus, the results showed that certain P. aeruginosa strains had appreciably depleted the mucin material from the culture supernatant. Depletion of mucin from the culture supernatant suggests that mucin degradation had occurred and that the P. aeruginosa strains tested have a differential ability to degrade mucin.
(ii) SDS-PAGE.
SDS-PAGE analyses of culture supernatants were conducted over time to investigate the possible degradation of mucin to lower-molecular-mass forms, not identifiable by agarose electrophoresis. Figure 2 shows that the culture supernatants contained no significant additional band corresponding to mucin degradation products. The notable band at approximately 70 kDa is a non-mucin protein found in porcine gastric mucin preparations (40) and was not degradable by P. aeruginosa isolates in the present study.
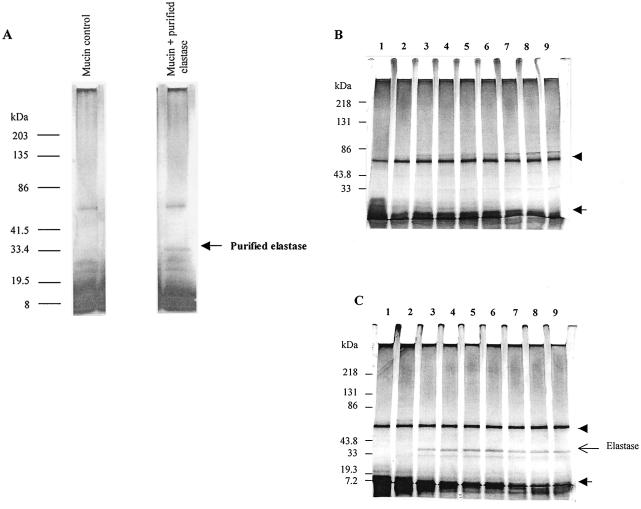
FIG. 2.
SDS-PAGE protein profile of culture supernatants after incubation of P. aeruginosa strains with mucin. (A) Reference protein profiles of mucin alone and purified elastase in mucin. (B and C) Culture supernatants of representative strains Paer1 (B) and 6294 (C) in mucin at various time points. Samples were applied to a 4 to 15% polyacrylamide gel under reducing and denaturing conditions and stained with silver. Lanes: 1, 0 h; 2, 2 h; 3, 4 h; 4, 6 h; 5, 8 h; 6, 10 h; 7, 24 h; 8, 48 h; 9, 72 h. The arrow indicates the position of low-molecular-weight material depleted by strains over time. The arrowhead indicates the position of the “link” protein.
Purified elastase in the presence of mucin migrated as a band of approximately 33 kDa under reducing SDS-PAGE conditions (Fig. 2A). This protein was present only in mucin culture supernatants of the mucin-degrading strains Paer12, 6294, ATCC 15442, and Paer25 and was absent in cultures of the non-mucin-degrading isolates Paer1 and Paer3 (results are shown for representative strains Paer1 and 6294 [Fig. 2B and C]. Thus, by reference to SDS-PAGE of purified elastase, mucin-degrading strains were able to secrete elastase during incubation with mucin while non-mucin-degrading strains were unable.
Interestingly, all P. aeruginosa isolates tested, as shown here for the representative strains Paer1 and 6294, were able to utilize low-molecular-mass components (smaller than 10 kDa) in the partially purified mucin preparation (Fig. 2B and C). As depletion of these proteins commenced at the beginning of log phase (2 to 4 h [see Fig. 6B]), it is likely that P. aeruginosa isolates use them as an initial nutrient source.
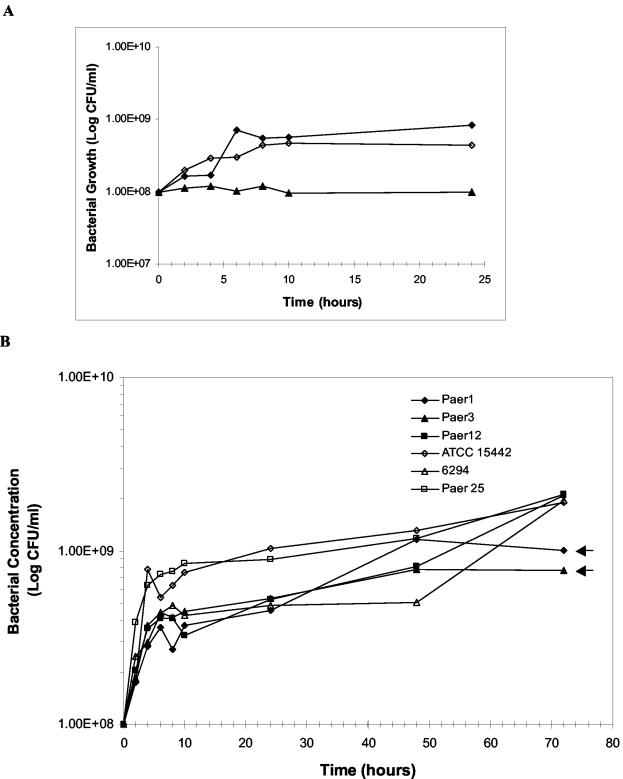
FIG. 6.
Use of mucin as the sole carbon and nitrogen source for growth of P. aeruginosa isolates. (A) Control incubations of representative strain 6294 relative to growth in mucin. ⧫, 6294 with mucin in PBS; ◊, 6294 in PBS containing ammonium and glucose; ▴, 6294 in PBS alone. (B) Growth of various P. aeruginosa isolates in mucin. Data are presented as mean values from at least two independent experiments. Arrows indicate two strains having no significant increase in growth between 48 and 72 h.
(iii) ELLA.
The amount of mucin in the remaining bacterial pellet after collection of the supernatant was measured to investigate the adhesion of bacteria to mucin as the cause of mucin depletion from the supernatant. Regression analysis of respective standard curves gave similar mucin concentrations of the mucin control pellet (no bacteria) and the mucin-6294 pellet at 24 h (5.45 μg/ml for the mucin control versus 6.59 μg/ml for mucin-bacteria). Given the extensive depletion of mucin from the mucin-bacterial culture supernatant at 24 h, the depletion of PGM by strain 6294 at 24 h was not due to the removal of mucin from the culture supernatant by adhesion to bacteria in the pellet.
In contrast, probing of the bacterial pellet of Paer1 with mucin at 72 h showed about a 10-fold increase in the amount of bound mucin compared with the mucin control pellet at 72 h (mucin control pellet, 12.47 μg of mucin per ml; mucin-Paer1 pellet, 108.65 μg of mucin per ml). This implies that the slight depletion observed with this strain after a prolonged time was probably due to the observed aggregation or adhesion of this strain in mucin, which facilitated mucin removal from the supernatant.
(iv) Size exclusion chromatography.
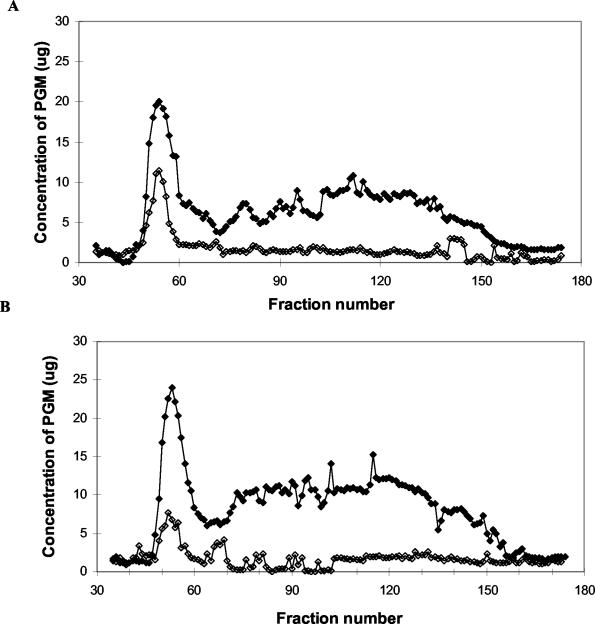
Gel filtration was used to more accurately determine the extent of mucin degradation and detect the presence of mucin subunits that cannot be properly resolved by agarose electrophoresis. Polymeric mucin usually elutes in the void volume, as observed in the mucin control supernatants (Fig. 3). After incubation with strain 6294, there was a progressive depletion of polymeric mucin in the void volume at both 24 and 72 h compared with the respective mucin controls (no bacteria), indicating mucin degradation by this strain (Fig. 3). This is consistent with the mucin depletion profile observed by electrophoretic analysis of this culture.
FIG. 3.
Gel filtration profile of spent culture supernatant after incubation of strain 6294 in mucin for 24 h (A) and 72 h (B). The control included the incubation of 1% (wt/vol) PGM in PBS without the inoculation of bacteria. ⧫, mucin control (no bacteria); ◊, mucin with strain 6294.
A subsequent shift of staining to the included volume would indicate the degradation of polymeric mucin to subunit forms. Interestingly, this was not observed in the present study despite the additional use of an alternative stain (PAS) and membrane (PVDF) for analysis of mucin in eluted fractions. It is possible that degradation of mucin to subunits had preceded the 24-h time point analyzed in the present study. Regardless, it is clear that strain 6294 had depleted polymeric mucin by 24 h. Moreover, glycoprotein material in the included volume was also depleted by 24 h; this may represent smaller mucins, mucin degradation products, or other glycoproteins in this partially purified preparation.
(v) Protease production.
Culture supernatants from the incubation of P. aeruginosa with PGM were also subjected to analysis of active protease activity by zymography. From a technical perspective, gelatin as a substrate gave a more complete proteolytic profile than did casein. In addition, bovine skin gelatin as a substrate seemed to display a more clear and distinct proteolytic banding pattern than did porcine skin gelatin, under the present experimental conditions.
Differential proteolytic activity was shown by various P. aeruginosa strains during incubation with mucin. At 24 h of incubation (Fig. 4A), the mucin-degrading isolates Paer12, ATCC 15442, 6294, and Paer25 exhibited clear zones at 56 kDa, which seems to be in agreement with cited values for alkaline protease of 53 kDa (33), 54 kDa (29), and 50 kDa (38). A band of approximately 160 kDa corresponded to known values of elastase of 163 kDa (38, 53), approximately 200 kDa (29), and about 125 kDa (33). The high-molecular-mass protease aggregate (greater than 200 kDa) present in the mucin culture supernatants may be the recently characterized protease IV from P. aeruginosa, along with the additional gelatinolytic band at about 120 kDa observable in the 6294 and Paer25 mucin cultures (38) (Fig. 4A). Furthermore, the culture supernatant of strain 6294 contained a band immediately above the 56-kDa band, corresponding to a molecular mass of approximately 76 kDa, while Paer12 exhibited a distinct 90-kDa proteolytic band that may correspond to the unidentified 97-kDa protease observed in a P. aeruginosa isolate (53) (Fig. 4A). At this 24-h time point, the mucin control and the strains not degrading mucin, Paer1 and Paer3, produced hardly any detectable proteolytic activity (Fig. 4A).
FIG. 4.
Zymograms of protease production by P. aeruginosa isolates after incubation with PGM for 24 h (A) and 72 h (B). Lanes: 1, mucin control; 2, Paer1; 3, Paer3; 4, Paer12; 5, ATCC 15442; 6, 6294; 7, Paer25.
After 72 h of incubation of P. aeruginosa strains with mucin, the proteolytic repertoire of P. aeruginosa strains was the same as that observed at 24 h (Fig. 4B). The only exceptions were that Paer1 and Paer3 had produced a small amount of alkaline protease activity while Paer1 also contained a minor amount of elastase (Fig. 4B). Strain 6294 grown in TSB produced a substantial amount of alkaline protease, suggesting that this enzyme was constitutively expressed during growth (results not shown). Elastase was also produced by TSB-grown strain 6294; however, the amount was considerably smaller than that observed in mucin, even though the level of growth of strain 6294 was higher in TSB (results not shown). This suggests that the increased expression of elastase was induced by the incubation of strain 6294 with mucin and not by general growth. Thus, the expression of active protease activity by P. aeruginosa isolates correlated with the ability to degrade mucin from the culture supernatant.
(vi) Glycosidase production.
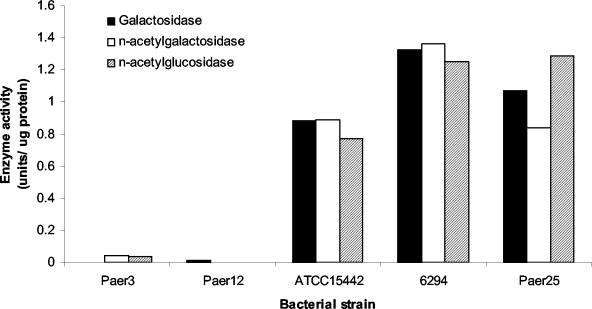
Culture supernatants of P. aeruginosa incubated with mucin were assessed for glycosidase production by p-nitrophenol-linked substrates for carbohydrate residues commonly found on mucin. ATCC 15442, 6294, and Paer25 produced similar high levels of galactosidase, N-acetylgalactosidase, and N-acetylglucosidase after 72 h of incubation with mucin (Fig. 5). Paer3 and Paer12 produced negligible amounts of any of the tested glycosidases (Fig. 5). The fact that Paer12, a mucin-depleting strain, produced a low level of glycosidase activity suggests that glycosidases play a relatively minor role in the depletion of mucin by P. aeruginosa isolates.
FIG. 5.
Glycosidase production by P. aeruginosa strains after incubation with PGM for 72 h. One unit of enzyme activity is defined as the amount of enzyme catalyzing the release of 1 nmol of pNP, and values were corrected for background levels of the mucin control culture supernatant containing no bacteria.
(vii) Growth of P. aeruginosa in mucin.
The growth of P. aeruginosa strains in the presence of PGM was investigated. Control cultures consisting of strain 6294, a representative strain, in PBS alone showed no growth, while the same strain in PBS containing ammonium and glucose instead of mucin showed a similar profile to that of mucin (Fig. 6A). All P. aeruginosa strains (Paer1, Paer3, Paer12, ATCC 15442, 6294, and Paer25) exhibited a fold increase in growth of about 1 log unit (Fig. 6B). Within 1 h of incubation, bacteria entered exponential phase with a short lag phase and mid-log phase at 4 h. At 6 to 8 h, growth had started to stabilize, although a significant increase in growth was observed by mucin-depleting strains (Paer12, ATCC 15442, 6294, and Paer25) between 48 and 72 h (P < 0.1).
At 24 h, all strains grew to the same level despite mucin depletion from selective strains. From 48 to 72 h, a significant increase in growth in mucin-depleting strains only was observed, suggesting that these bacteria may store energy from mucin degradation for growth on a long-term basis. This would be advantageous for persistent colonization and multiplication over nondepleting strains. Thus, the results suggest that mucin-depleting P. aeruginosa strains degrade mucin as a long-term nutrient source. Presumably, low-molecular-weight proteins found to be depleted at the start of log phase (2 h) were used as a nutrient source for short-term growth of all strains.
The two nondepleting strains, Paer1 and Paer3, formed tiny aggregates in the presence of mucin, while the other strains did not. In addition, P. aeruginosa isolates Paer12, 6294, and Paer25 (mucin-depleting strains) produced intense pigmentation in the culture medium on incubation with PGM (Table 1). This may consist of one or both of the main soluble pigments produced by P. aeruginosa: pyoverdin, a fluorescent pigment, and pyocyanin, a blue phenazine derivative, both of which function as siderophores for the bacterium. Paer1, Paer3 (nondepleting strains) and ATCC 15442 (mucin-depleting strain) did not produce any chromophore in their incubations with mucin (Table 1). Therefore, the production of chromaphores was not needed for mucin degradation.
TABLE 1.
Summary of mucin degradation and exoproducts secreted by P. aeruginosa isolates in relation to the bacterial source of isolation
| P. aeruginosa strain | Source of isolate | Mucin degradation | Protease production | Glycosidase production | Chromophore production |
|---|---|---|---|---|---|
| Paer1 | CLARE | No | Low | NDa | No |
| Paer3 | Asymptomatic person | No | Low | Low | No |
| Paer12 | Microbial keratitis | Yes | Yes | Low | Yes |
| ATCC 15442 | Environment | Yes | Yes | High | No |
| 6294 | Microbial keratitis | Yes | Yes | High | Yes |
| Paer25 | CLARE | Yes | Yes | High | Yes |
ND, not determined.
DISCUSSION
Infections caused by P. aeruginosa are potentially fatal, an outcome heightened by the increased resistance of this bacterium to antibiotics (22). There is a need for greater understanding of the infection process as a basis for the development of novel therapeutics. As the mucosa provides a protective coating of underlying epithelial cells and is the primary barrier against potential pathogens, the interaction of P. aeruginosa with mucin was evaluated. In the present approach, mucin was analyzed biochemically by electrophoresis, lectin blotting, and chromatography after bacterial incubation. The analyses spanned the interaction of mucin with six predominantly ocular P. aeruginosa isolates and their proteolytic and glycosidic profiles.
Agarose gel electrophoresis facilitates the migration of high-molecular-weight mucin into the gel for detection (50). The electrophoretic profile and staining with mucin probes of the culture supernatants had shown that microbial keratitis isolates (Paer12 and 6294), one CLARE strain (Paer25), and one standard strain (ATCC 15442) depleted mucin while one CLARE strain (Paer1) and an asymptomatic strain (Paer3) did not (Table 1). A lectin microtiter assay to probe for mucin in the bacterial pellet had confirmed that there was no adhesion of these strains to mucin for their removal from the supernatant. Similarly, separation of culture supernatant constituents by size exclusion chromatography and staining with PAS and WGA stains for mucin-containing fractions also showed mucin depletion by the same isolates. Both void-volume staining, where polymeric mucin usually elutes, and included-volume profiles were greatly reduced after incubation with P. aeruginosa compared to those of the control supernatant consisting of mucin with no bacteria (Fig. 3).
The depletion of mucin from the culture supernatant suggests that degradation of mucin by certain P. aeruginosa isolates took place. Mucin degradation was characteristic of strains isolated from patients with more virulent ocular conditions such as microbial keratitis. At least in P. aeruginosa-infected patients with respiratory disease, mucin degradation products were isolated from sputa (27), suggesting that this bacterium does degrade mucin in the infection process. Interestingly, the 70-kDa putative “link” protein associated with mucin (40), postulated to be part of a fibronectin fragment (48), was not degraded by P. aeruginosa (Fig. 2).
Mucin degradation by bacteria begins with the proteolytic attack on the nonglycosylated regions of the peptide core, yielding mucin glycopeptides (15). The bacteria then utilize their glycosidic enzymes for cleavage of carbohydrates from the mucin molecule (15). Protease activity was the prime mediator of mucin degradation by P. aeruginosa in the present study. Only strains producing appreciable levels of protease activity were able to deplete mucin, while clinical isolates producing low levels of protease were unable to degrade mucin (Table 1). Bands corresponding to P. aeruginosa elastase and protease IV rather than alkaline protease seemed to be the key proteases involved. Higher levels of activity of these proteases were induced after bacterial incubation with mucin than under general growth conditions as assayed by zymography. Further, only mucin-degrading strains (Paer12, ATCC 15442, 6294, and Paer25) were able to secrete the 33-kDa elastase protein, in contrast to non-mucin-degrading strains (Paer1 and Paer3), as shown by SDS-PAGE analyses (Fig. 2). This is consistent with a study by Poncz et al. in which rapid proteolysis of mucin by P. aeruginosa elastase was detected at a rate of 2 to 10 peptides after 5 min (42). Moreover, Paer1 and Paer3 were defined as producing no detectable levels of elastase in a previous study (57), which confirms the present results using mucin and relates to the inability of these strains to degrade mucin.
Mucin degradation by protease action weakens the protective mucosal coat and facilitates bacterial penetration of underlying epithelial cells. The pretreatment of mucin with Candida albicans secretory aspartyl proteinase partially resolved yeast adhesion to epithelial cells, indicative of a less obtrusive mucin barrier after protease treatment (16). Protease activity of Vibrio cholerae degraded mucin, causing a reduction in the viscosity of mucin (13), and Entamoeba histolytica cysteine protease activity degraded mucin and rendered it 40% less effective in inhibiting bacterial penetration of cultured cells (35). The proteolytic hydrolysis of mucin by certain P. aeruginosa isolates observed in the present study is set to be a factor that may influence the infectivity of that strain in the eye. Indeed, the removal of ocular mucus from the cornea in vitro resulted in increased adhesion of P. aeruginosa to the corneal surface (18).
Mucin degradation by bacterial proteases may also be necessary for bacteria to use mucin as a nutrient source. Increased growth of mucin-degrading strains was observed relative to nondegrading strains in the present study, although only toward the end of the incubation period between 48 and 72 h (P < 0.1 [Fig. 6B]). It is likely that all isolates utilized low-molecular-weight components in the partially purified mucin preparation as a nutrient source initially, since these were depleted during this time as indicated by SDS-PAGE (Fig. 2). We speculate that once these reserves had been exhausted, mucin-degrading strains were able to use energy from mucin degradation for prolonged growth. This would be advantageous in vivo as a long-term survival strategy.
The role of glycosidase activity in mucin degradation by P. aeruginosa was evident with some strains. The glycosidases tested included N-acetylglucosidase, N-acetylgalactosidase, and galactosidase, whose substrates are commonly found on mucins (54). Glycosidase secretion was not necessary for mucin depletion from the culture as not all mucin-degrading isolates secreted glycosidases (Table 1). Paer12, although able to degrade mucin, was unable to secrete active glycosidases. Oral bacteria depend on glycosidase activity and mucin carbohydrates for growth (2, 4, 20, 25, 26, 32). This does not seem to be the case with P. aeruginosa, since glycosidase-producing strains did not display pronounced growth compared with the remaining strains tested. Moreover, the majority of sugars present on mucins, such as galactose, N-acetylgalactosamine, mannose, sialic acid, and fucose, are able to maintain only low-level viability of P. aeruginosa (6). It is possible that glycosidase action on mucin may contribute to virulence by unmasking carbohydrate receptors on mucin in vivo and increasing bacterial adherence to facilitate pathogenesis. Various carbohydrate moieties have been identified as receptors for P. aeruginosa adhesion (45).
It was also of interest that siderophore production during the incubation of P. aeruginosa with mucin was not correlated with the ability of isolates to degrade mucin (Table 1). All mucin-degrading strains secreted siderophores, except one strain (ATCC 15442), which degraded mucin despite an inability to produce siderophores. Siderophore production by P. aeruginosa may contribute to infection at a later stage of pathogenesis, as recent evidence suggests that pyoverdin acts as a signaling molecule for the production of virulence factors such as exotoxin A and PrpL protease (30).
In conclusion, P. aeruginosa clinical isolates differed in their ability to degrade mucin, showing that the infection process may be dependent, in part, on the particular P. aeruginosa strain colonizing the site and its mucin degradation ability. Mucin depletion from the culture supernatant was evident with more virulent strains and was mediated primarily by proteolytic activity. Certain P. aeruginosa strains additionally secreted glycosidases, suggesting that further mucin degradation processes were also functional. Also, mucin-depleting strains exhibited a long-term growth advantage over nondepleting strains implying that mucin was used as a nutrient source. The repercussions of mucin degradation in vivo may include closer proximity of bacteria to corneal epithelial cells and the collapse of the ocular protection network, including immune and nonimmune cells, of which mucin is the scaffold. These findings may prove useful in the prevention or treatment of P. aeruginosa colonization in such environments of reduced mucin outflow.
Acknowledgments
This study was supported in part by grants from the Australian Commonwealth Government under the Cooperative Research Centers Programme and from the American Optometric Foundation.
Editor: J. D. Clements
REFERENCES
- 1.Argüeso, P., and I. K. Gipson. 2001. Epithelial mucins of the ocular surface: structure, biosynthesis and function. Exp. Eye Res. 73:281-289. [DOI] [PubMed] [Google Scholar]
- 2.Beighton, D., K. Smith, D. A. Glenister, K. Salamon, and C. W. Keevil. 1988. Increased degradative enzyme production by dental plaque bacteria in mucin-limited continuous culture. Microb. Ecol. Health Dis. 1:85-94. [Google Scholar]
- 3.Berry, M., R. B. Ellingham, and A. P. Corfield. 1996. Polydispersity of normal human conjunctival mucins. Investig. Ophthalmol. Visual Sci. 37:2559-2571. [PubMed] [Google Scholar]
- 4.Bradshaw, D. J., K. A. Homer, P. D. Marsh, and D. Beighton. 1994. Metabolic cooperation in oral microbial communities during growth on mucin. Microbiology 140:3407-3412. [DOI] [PubMed] [Google Scholar]
- 5.Byrd, J. C., P. Yan, L. Sternberg, C. K. Yunker, J. M. Scheiman, and R. S. Bresalier. 1997. Aberrant expression of gland-type gastric mucin in the surface epithelium of Helicobacter pylori-infected patients. Gastroenterology 113:455-464. [DOI] [PubMed] [Google Scholar]
- 6.Chance, D. L., and T. P. Mawhinney. 2000. Carbohydrate sulfation effects on growth of Pseudomonas aeruginosa. Microbiology 146:1717-1725. [DOI] [PubMed] [Google Scholar]
- 7.Chatterjee, A., J. Kwartz, A. E. A. Ridgway, and J. K. Storey. 1995. Disposable soft contact lens ulcers: a study of 43 cases seen at Manchester Eye Hospital. Cornea 14:138-141. [PubMed] [Google Scholar]
- 8.Chen, H.-B., S. Yamabayashi, B. Ou, Y. Tanaka, S. Ohno, and S. Tsukahara. 1997. Structure and composition of rat precorneal tear film. A study by an in vitro cryofixation. Investig. Ophthalmol. Visual Sci. 38:381-387. [PubMed] [Google Scholar]
- 9.Chen, X.M., and N. F. LaRusso. 2000. Mechanisms of attachment and internalisation of Cryptosporidium parvum to biliary and intestinal epithelial cells. Gastroenterology 118:368-379. [DOI] [PubMed] [Google Scholar]
- 10.Coconnier, M.-H., E. Dlissi, M. Robard, C. L. Laboisse, J. L. Gaillard, and A. L. Servin. 1998. Listeria monocytogenes stimulates mucus exocytosis in cultured human polarized mucosecreting intestinal cells through action of listeriolysin O. Infect. Immun. 66:3673-3681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cokingtin, C. D., and R. A. Hyndiuk. 1996. Bacterial keratitis, p. 323-347. In K. F. Tabbara and R. A. Hyndiuk (ed.), Infections of the eye, 2nd ed. Little, Brown and Co., Boston, Mass.
- 12.Cowell, B. A., M. D. P. Willcox, B. Herbert, and R. P. Schneider. 1999. Effect of nutrient limitation on adhesion characteristics of P. aeruginosa. J. Appl. Microbiol. 86:944-954. [DOI] [PubMed] [Google Scholar]
- 13.Crowther, R., N. Roomi, R. Fahim, and J. Forstner. 1987. Vibrio cholerae metalloproteinase degrades intestinal mucin and facilitates enterotoxin-induced secretion from rat intestine. Biochim. Biophys. Acta 924:393-402. [DOI] [PubMed] [Google Scholar]
- 14.Davies, J., I. Carlstedt, A.-K. Nilsson, A. Håkannson, H. Sabharwal, L. Van Alphen, M. Van Ham, and C. Svanborg. 1995. Binding of Haemophilus influenzae to purified mucins from the human respiratory tract. Infect. Immun. 63:2485-2492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Deplancke, B., and H. Gaskins. 2001. Microbial modulation of innate defense: goblet cells and the intestinal mucus layer. Am. J. Clin. Nutr. 73:1131S-1141S. [DOI] [PubMed] [Google Scholar]
- 16.de Repentigny, L., F. Aumont, K. Bernard, and P. Belhumeur. 2000. Characterization of binding of Candida albicans to small intestinal mucin and its role in adherence to mucosal epithelial cells. Infect. Immun. 68:3172-3179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ellingham, R., M. Berry, D. Stevenson, and A. Corfield. 1999. Secreted human conjunctival mucus contains MUC5AC glycoforms. Glycobiology 9:1181-1189. [DOI] [PubMed] [Google Scholar]
- 18.Fleiszig, S., T. Zaidi, R. Ramphal, and G. Pier. 1994. Modulation of Pseudomonas aeruginosa adherence to the corneal surface by mucus. Infect. Immun. 62:1799-1804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Girod, S., J.-M. Zahm, C. Plotkowski, G. Beck, and E. Puchelle. 1992. Role of physicochemical properties of mucus in the protection of the respiratory epithelium. Eur. Respir. J. 5:477-487. [PubMed] [Google Scholar]
- 20.Glenister, D. A., K. E. Salamon, K. Smith, D. Beighton, and C. W. Keevil. 1988. Enhanced growth of complex communities of dental plaque bacteria in mucin-limited continuous culture. Microb. Ecol. Health Dis. 1:31-38. [Google Scholar]
- 21.Gravel, P., and O. Golaz. 1996. Identification of glycoproteins on nitrocellulose membranes using lectin blotting, p. 603-617. In J. M. Walker (ed.), The protein protocols handbook. Humana Press Inc., Totowa, N.J.
- 22.Hanberger, H., D. Diekema, A. Fluit, R. Jones, M. Struelens, R. Spencer, and M. Wolff. 2001. Surveillance of antibiotic resistance in European ICUs. J. Hosp. Infect. 48:161-176. [DOI] [PubMed] [Google Scholar]
- 23.Hicks, S., S. Carrington, R. Kaswan, S. Adam, J. Bara, and A. Corfield. 1997. Demonstration of discrete secreted and membrane-bound ocular mucins in the dog. Exp. Eye Res. 64:597-607. [DOI] [PubMed] [Google Scholar]
- 24.Holden, B. A., D. La Hood, T. Grant, J. Newton-Howes, C. Baleriola-Lucas, M. D. P. Willcox, and D. F. Sweeney. 1996. Gram-negative bacteria can induce contact lens related acute red eye (CLARE) responses. C.L.A.O. J. 22:47-52. [PubMed] [Google Scholar]
- 25.Homer, K. A., S. Kelley, J. Hawkes, D. Beighton, and M. C. Grootveld. 1996. Metabolism of glycoprotein-derived sialic acid and N-acetylglucosamine by Streptococcus oralis. Microbiology 142:1221-1230. [DOI] [PubMed] [Google Scholar]
- 26.Homer, K. A., R. A. Whiley, and D. Beighton. 1994. Production of specific glycosidase activities by Streptococcus intermedius strain UNS35 grown in the presence of mucin. J. Med. Microbiol. 41:184-190. [DOI] [PubMed] [Google Scholar]
- 27.Houdret, N., R. Ramphal, A. Scharfman, J.-M. Perini, M. Filliat, G. Lamblin, and P. Roussel. 1989. Evidence for the in vivo degradation of human respiratory mucins during Pseudomonas aeruginosa infection. Biochim. Biophys. Acta 992:96-105. [DOI] [PubMed] [Google Scholar]
- 28.Jumblatt, M. M., R. W. McKenzie, and J. E. Jumblatt. 1999. MUC5AC mucin is a component of the precorneal tear film. Investig. Ophthalmol. Visual Sci. 40:43-49. [PubMed] [Google Scholar]
- 29.Kernacki, K., J. Hobden, L. Hazlett, R. Fridman, and R. Berk. 1995. In vivo bacterial protease production during Pseudomonas aeruginosa corneal infection. Investig. Ophthalmol. Visual Sci. 36:1371-1378. [PubMed] [Google Scholar]
- 30.Lamont, I. L., P. A. Beare, U. Ochsner, A. I. Vasil, and M. L. Vasil. 2002. Siderophore-mediated signaling regulates virulence factor production in Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. USA 99:7072-7077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lyezak, J. B., C. L. Cannon, and G. B. Pier. 2000. Establishment of Pseudomonas aeruginosa infection: lessons from a versatile opportunist. Microb. Infect. 2:1051-1060. [DOI] [PubMed] [Google Scholar]
- 32.MacFarlane, S., M. J. Hopkins, and G. T. Macfarlane. 2001. Toxin synthesis and mucin breakdown are related to the swarming phenomenon in Clostridium septicum. Infect. Immun. 69:1120-1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Matsumoto, K., N. Shams, L. Hanninen, and K. Kenyon. 1992. Proteolytic activation of corneal matrix metalloproteinase by Pseudomonas aeruginosa elastase. Curr. Eye Res. 11:1105-1109. [DOI] [PubMed] [Google Scholar]
- 34.Milton, J. D., and J. M. Rhodes. 1998. Quantification of intestinal mucins. Methods Mol. Med. 9:255-261. [DOI] [PubMed] [Google Scholar]
- 35.Moncada, D., Y. Yu, K. Keller, and K. Chadee. 2000. Entamoeba histolytica cysteine proteinases degrade human colonic mucin and alter its function. Arch. Med. Res. 31:S224-S225. [DOI] [PubMed] [Google Scholar]
- 36.Morrissey, J. H. 1981. Silver stain for proteins in polyacrylamide gels: a modified procedure with enhanced uniform sensitivity. Anal. Biochem. 117:307-310. [DOI] [PubMed] [Google Scholar]
- 37.Nichols, B. A., M. L. Chiappino, and C. R. Dawson. 1985. Demonstration of the mucous layer of the tear film by electron microscopy. Investig. Ophthalmol. Visual Sci. 26:464-473. [PubMed] [Google Scholar]
- 38.O'Callaghan, R., L. Engel, J. Hobden, M. Callegan, L. Green, and J. Hill. 1996. Pseudomonas keratitis. The role of an uncharacterised exoprotein, protease IV, in corneal virulence. Investig. Ophthalmol. Visual Sci. 37:534-543. [PubMed] [Google Scholar]
- 39.Paugh, J., F. Stapleton, L. Keay, and A. Ho. 2001. Tear exchange under hydrogel contact lenses: methodological considerations. Investig. Ophthalmol. Visual Sci. 42:2813-2820. [PubMed] [Google Scholar]
- 40.Pearson, J., A. Allen, and S. Parry. 1981. A 70 000-molecular-weight protein isolated from purified pig gastric mucus glycoprotein by reduction of disulphide bridges and its implication in the polymeric structure. Biochem. J. 197:155-162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pfugfelder, S. C., Z. Liu, D. Monroy, D.-Q. Li, M. E. Carvajal, S. A. Price-Schiavi, N. Idris, A. Solomon, A. Perez, and K. L. Carraway. 2000. Detection of sialomucin complex (MUC4) in human ocular surface epithelium and tear fluid. Investig. Ophthalmol. Visual Sci. 41:1316-1326. [PubMed] [Google Scholar]
- 42.Ponez, L., N. Jentoft, M.-C. D. Ho, and D. G. Dearborn. 1988. Kinetics of proteolysis of hog gastric mucin by human neutrophil elastase and by Pseudomonas aeruginosa elastase. Infect. Immun. 56:703-704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Prydal, J. I., P. Artal, H. Woon, and F. W. Campbell. 1992. Study of human precorneal tear film thickness and structure using laser interferometry. Investig. Ophthalmol. Visual Sci. 33:2006-2011. [PubMed] [Google Scholar]
- 44.Schaefer, F., O. Bruttin, L. Zografos, and Y. Guex-Crosier. 2001. Bacterial keratitis: a prospective clinical and microbiological study. Br. J. Ophthalmol. 85:842-847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Scharfman, A., S. Degroote, J. Beau, G. Lamblin, P. Roussel, and J. Mazurier. 1999. Pseudomonas aeruginosa binds to neoglycoconjugates bearing mucin carbohydrate determinants and predominantly to sialyl-Lewis x conjugates. Glycobiology 9:757-764. [DOI] [PubMed] [Google Scholar]
- 46.Schroten, H., F. G. Hanisch, R. Plogmann, J. Hacker, G. Uhlenbruck, R. Nobis-Bosch, and V. Wahn. 1992. Inhibition of adhesion of S-fimbriated Escherichia coli to buccal epithelial cells by human milk fat globule membrane components: a novel aspect of the protective function of mucins in nonimmunoglobulin function. Infect. Immun. 60:2893-2899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schultz, C. L., D. W. Morck, S. G. McKay, M. E. Olson, and A. Buret. 1997. Lipopolysaccharide induced acute red eye and corneal ulcers. Exp. Eye Res. 64:3-9. [DOI] [PubMed] [Google Scholar]
- 48.Slomiany, A., K. Okazaki, S. Tamura, and B. Slomiany. 1991. Identity of mucin's “118-kDa link protein” with fibronectin fragment. Arch. Biochem. Biophys. 286:383-388. [DOI] [PubMed] [Google Scholar]
- 49.Thornton, D., D. Holmes, J. Sheehan, and I. Carlstedt. 1989. Quantitation of mucus glycoproteins blotted onto nitrocellulose membranes. Anal. Biochem. 182:160-164. [DOI] [PubMed] [Google Scholar]
- 50.Thornton, D., M. Howard, P. Devine, and J. Sheehan. 1995. Methods for separation and deglycosylation of mucin subunits. Anal. Biochem. 227:162-167. [DOI] [PubMed] [Google Scholar]
- 51.Thuruthyil, S. J., H. Zhu, and M. D. P. Willcox. 2001. Serotype and adhesion of Pseudomonas aeruginosa isolated from contact lens wearers. Clin. Exp. Ophthalmol. 29:147-149. [DOI] [PubMed] [Google Scholar]
- 52.Tsuboi, Y., Y. Kim, M. M. Paparella, N. Chen, P. A. Schachern, and J. Lin. 2001. Pattern changes of mucin gene expression with pneumococcal otitis media. Int. J. Pediatr. Otorhinolaryngol. 61:23-30. [DOI] [PubMed] [Google Scholar]
- 53.Twining, S., S. Kirschner, L. Mahnke, and D. Frank. 1993. Effect of Pseudomonas aeruginosa elastase, alkaline protease, and exotoxin A on corneal proteinases and proteins. Investig. Ophthalmol. Visual Sci. 34:2699-2712. [PubMed] [Google Scholar]
- 54.Van Klinken, B.-W., A. Einerhand, H. Büller, and J. Dekker. 1998. Strategic biochemical analysis of mucins. Anal. Biochem. 265:103-116. [DOI] [PubMed] [Google Scholar]
- 55.Windle, H. J., A. Fox, N. Eidhin, and D. Kelleher. 2000. The thioredoxin system of Helicobacter pylori. J. Biol. Chem. 275:5081-5089. [DOI] [PubMed] [Google Scholar]
- 56.Wong, T.-Y., T.-P. Ng, K.-S. Fong, and D. T. H. Tan. 1997. Risk factors and clinical outcomes between fungal and bacterial keratitis: a comparative study. C.L.A.O. J. 23:275-281. [PubMed] [Google Scholar]
- 57.Zhu, H., S. J. Thuruthyil, and M. D. P. Willcox. 2001. Determination of quorum-sensing signal molecules and virulence factors of Pseudomonas aeruginosa strains isolated from contact lens-induced microbial keratitis. J. Med. Microbiol. 51:1063-1070. [DOI] [PubMed] [Google Scholar]