Abstract
Phage type 99 of Salmonella enterica subsp. enterica serovar Typhimurium variant Copenhagen strains isolated from pigeons were examined for the presence of genotypic and phenotypic characteristics. The pulsed-field gel electrophoresis patterns obtained with XbaI and BlnI from 38 pigeon strains were compared with those obtained from 89 porcine, poultry, and human strains of variant Copenhagen. Identical patterns with XbaI and four closely related patterns with BlnI were obtained with the pigeon strains, whereas 16 XbaI patterns were found with the other strains. The XbaI patterns of the pigeon strains showed a low genetic similarity to the patterns of the porcine, poultry, and human strains and invariably showed a low-molecular-weight band that was absent in the majority of the other strains. The virulence genes shdA, spvR, pefA, sopE, and spvB were uniformly present in six pigeon isolates representing the genetic diversity found with BlnI. These six pigeon-derived strains were highly cytotoxic for pigeon macrophages compared to three porcine strains. After experimental infection of pigeons with a pigeon strain, clinical symptoms, fecal shedding, and colonization of internal organs were more pronounced than those after infection with a porcine strain. These data suggest that the phage type 99 strains used in this study are highly adapted to pigeons and should be classified as a host-restricted lineage of the serovar Typhimurium.
Salmonella enterica subsp. enterica serovar Typhimurium is able to colonize and cause disease in a very broad range of animals. However, the phage types 2 and 99 of the O5-negative variant of serovar Typhimurium are almost exclusively isolated from pigeons (for a review, see reference 17). A certain degree of host adaptation of pigeon strains of serovar Typhimurium variant Copenhagen has thus been postulated on numerous occasions (e.g., references 24 and 15). Detailed data on the underlying mechanisms, however, are lacking. The purpose of the present study was to document host specificity of pigeon-derived serovar Typhimurium variant Copenhagen strains and to determine the bacterial characteristics which are associated with the host adaptation of these strains to pigeons.
The pulsed-field gel electrophoresis (PFGE) patterns of 38 Salmonella serovar Typhimurium variant Copenhagen strains, isolated either from pigeon fecal samples or from pigeons at necropsy, were determined. The isolates derived from different flocks. Both the serotype and the phage type of these strains were determined (21). For comparison of PFGE patterns, 89 strains from poultry (10 strains), pigs (73 strains), and humans (6 strains) were used. The bacteria were grown while being shaken overnight at 37°C in Luria-Bertani broth (LB). The XbaI and BlnI PFGE patterns were determined for all 145 Salmonella serovar Typhimurium variant Copenhagen strains using previously described PFGE methods with some slight modifications (11, 16). The patterns were grouped in a dendrogram with GelCompar II (Applied Maths, St.-Martens-Latem, Belgium) by using the Dice coefficient and the unweighted pair group method using arithmetic averages clustering algorithm.
The presence of the virulence genes shdA, spvR, spvB, pefA, and sopE in six Salmonella serovar Typhimurium variant Copenhagen strains isolated from pigeons was determined by separate PCRs (Table 1). The following specific primers were designed for the virulence genes investigated on the basis of the sequences deposited in the EMBL database: shdAf (5′-CTG ACG TTA AGC GGC GAT AA-3′) and shdAr (5′-CGT CAA CGT CTG TCA GTG TA-3′), pefAf (5′-ACA CGC TGC CAA TGA AGT GA-3′) and pefAr (5′-ACT GCG AAA GAT GCC ACA GA-3′), spvRf (5′-CAG GTT CCT TCA GTA TCG CA-3′) and spvRr (5′-TTT GGC CGG AAA TGG TCA GT-3′), spvBf (5′-CGC AGT ATA ACG ACA GCG AT-3′) and spvBr (5′-GCT AGT CCA GAG GTA CAG AT-3′), and sopEf (5′-CAG ACC CGT GAA GCT ATA CT-3′) and sopEr (5′-AAT TGC TGT GGA GTC GGC AT-3′). The specificity of each primer pair for its respective virulence gene was assayed by sequencing the amplicon of at least one strain, purified with a High Pure PCR product purification kit (Roche Diagnostics, Mannheim, Germany) on an Applied Biosystems ABI 310 sequencer with an ABI Prism Dye Terminator cycle sequencing ready reaction kit (Perkin Elmer Applied Biosystems, Foster City, Calif.). The specific amplicons were separated by agarose gel electrophoresis in 1.5% Seakem ME agarose (FMC Bioproducts, Rockland, Maine) and visualized by staining with ethidium bromide.
TABLE 1.
Origins, phage types, and PFGE-BlnI patterns of serovar Typhimurium variant Copenhagen strains used in the in vitro and in vivo studiesa
| Strain | Origin | Phage type | PFGE-BlnI type |
|---|---|---|---|
| DAB1 | Pigeon | 99 | II |
| DAB29 | Pigeon | 99 | IV |
| DAB48 | Pigeon | 99 | I |
| DAB69 | Pigeon | 99 | III |
| DAB121 | Pigeon | 99 | II |
| DAB153 | Pigeon | 99 | I |
| MB2113 | Pig | 120 | ND |
| MB2150 | Pig | 208 | ND |
| MB2157 | Pig | NT | ND |
ND, not determined; NT, nontypeable.
The in vitro behavior of Salmonella serovar Typhimurium variant Copenhagen strains in peritoneal macrophages from pigeons was examined by using pigeon peritoneal macrophages collected by a previously described method (5). The cells were resuspended in a solution containing Dulbecco modified Eagle medium (Gibco) supplemented with 10% heat-inactivated fetal bovine serum (Gibco), 100 mg of streptomycin/ml (Gibco), 100 IU of penicillin/ml (Gibco), and 1% nonessential amino acids (Gibco). Cell purity was determined using nonspecific esterase (Sigma Diagnostics, St. Louis, Mo.) and Haemacolor (Merck, Darmstadt, Germany) staining, and 105 nonspecific-esterase-positive cells were seeded in the wells of a 96-well plate, incubated overnight (41°C, 5% CO2), and then rinsed three times to remove antibiotics and nonadherent cells. Six isolates of serovar Typhimurium variant Copenhagen from pigeons were used. The strains belonged to phage type 99 and to four different PFGE-BlnI types (Table 1). For comparison, three porcine isolates of three different phage types (120, 208, and nontypeable) were included. For use in the in vitro assays, the bacteria were grown overnight in LB at 37°C. The pigeon macrophages were exposed, at a multiplicity of infection of 10, to bacteria from the six pigeon and the three porcine isolates. The plate was centrifuged (350 × g, 10 min, 41°C) and incubated for 1 h at 41°C in 5% CO2. Then, gentamicin (Gibco) was added to the wells at a final concentration of 50 μg/ml and the wells were incubated for another hour. At this time point (0 h postinoculation) and at 4 and 24 h postinoculation, the wells were rinsed to remove the gentamicin, the macrophages were lysed by adding 1% Triton X-100 (Acros, Geel, Belgium), and the number of CFU was counted.
In order to assess the cytotoxic effects of Salmonella, the macrophages were exposed to the pigeon and the porcine strains as described above. At 24 h postinoculation, 20 μl of the cell proliferation agent WST-1 (Roche) was added to each well and absorbencies were measured at 450 nm (Titertek, Helsinki, Finland). The number of cells per well was determined using a standard curve, which was prepared with a dilution series of the cell suspension.
For the study of the in vivo behavior of Salmonella serovar Typhimurium variant Copenhagen in pigeons, a porcine and a pigeon isolate exhibiting low (phage type 208) and high (phage type 99) macrophage cytotoxicities, respectively, were selected. The bacteria were grown in LB overnight at 37°C. Twelve clinically healthy adult pigeons were inoculated. The animals derived from a breeding colony free of Salmonella and were negative for the presence of Salmonella bacteria in their feces and for the presence of agglutinating antibodies to Salmonella. Each animal was housed individually. The experiment was carried out with the approval of the ethical committee of the Faculty of Veterinary Medicine, Ghent University. Each pigeon was inoculated in the crop with 5 × 108 CFU of the bacterial suspension of either the pigeon or the porcine strain in 1 ml of inoculum. The numbers of Salmonella CFU per gram of feces were determined at days 1, 2, 3, 4, 6, 8, and 10 postinoculation. If negative after direct plating, the samples were preenriched in buffered peptone water (Oxoid) and then enriched in tetrathionate brilliant green broth (Oxoid). The animals were examined daily for the presence of clinical symptoms such as apathy, diarrhea, and polyuria. At day 10 postinoculation, blood was collected from each pigeon and the titer of agglutinating antibodies in the serum was determined using a twofold dilution series of serum and glass slide agglutination with inactivated bacteria. From each animal, the liver, spleen, lung, heart, kidney, ovary (if present), and small and large intestines were collected, homogenized, and examined for the number of CFU of serovar Typhimurium variant Copenhagen per gram of tissue. The bacterial counts per organ were compared using the Mann-Whitney U test (SPSS, Chicago, Ill.). Levels of fecal shedding between pigeons inoculated with the pigeon strain and pigeons inoculated with the porcine strain were compared using a linear mixed-effect model, with the pigeon as a random factor and an autoregressive correlation structure of the first order (S-plus, Amsterdam, The Netherlands). The probability level for significance was a P of <0.05.
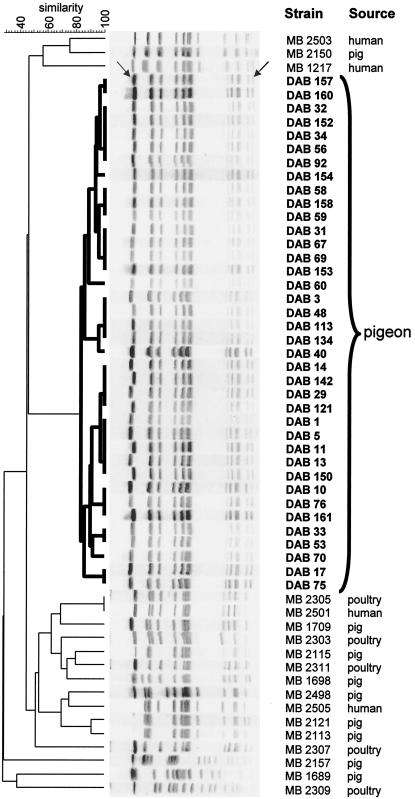
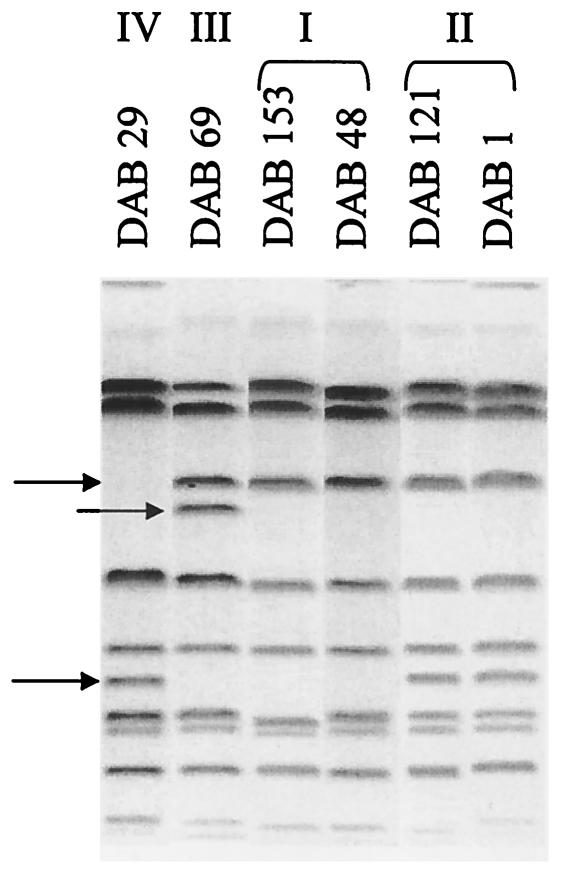
All the Salmonella strains collected from pigeons belonged to serovar Typhimurium variant Copenhagen phage type 99. The PFGE analysis of these pigeon strains with XbaI showed a uniform pattern (at least 85% similarity), which was, however, distinct from the XbaI patterns observed with serovar Typhimurium variant Copenhagen isolates from other animal sources (poultry and pigs) and from human infections (similarity of 45% or lower) (Fig. 1). The 89 isolates from poultry, pigs, and humans belonged to 16 different XbaI patterns. The pigeon isolates invariably showed the presence of a low-molecular-weight band (less than 50 kb) in the XbaI pattern, which was absent in the majority of the other isolates shown in Fig. 1. Contrary to the results of the PFGE analysis with XbaI, the PFGE analysis with BlnI showed four different patterns within the collection of pigeon isolates (Fig. 2): 33 strains belonged to BlnI type I, while BlnI types II, III, and IV included 4 strains, 3 strains, and 1 strain, respectively. BlnI types II, III, and IV could be attributed to only a one- or two-band difference from the bands of type I observed in the majority of the pigeon isolates. The virulence typing of six of these pigeon isolates (Table 1) belonging to these four BlnI types showed that the virulence genes shdA, spvR, spvB, pefA, and sopE were uniformly present in these strains.
FIG. 1.
Dendrogram obtained by unweighted pair group method using arithmetic averages clustering of the PFGE-XbaI patterns of serovar Typhimurium variant Copenhagen strains from pigeons and other sources on the basis of the Dice coefficient. The highest-molecular-weight band (indicated by the left arrow) was omitted from the numerical analysis. A low-molecular-weight band (indicated by the right arrow) was invariably present in all pigeon isolates. The dendrogram shows the genetic relationships between the various isolates and the clonal nature of the pigeon isolates (indicated in bold on the dendrogram) in particular.
FIG. 2.
PFGE-BlnI patterns of pigeon isolates of serovar Typhimurium variant Copenhagen used in in vivo and in vitro studies. The strains represent the closely related BlnI types I to IV, which were designated on the basis of the polymorphic bands indicated by arrows.
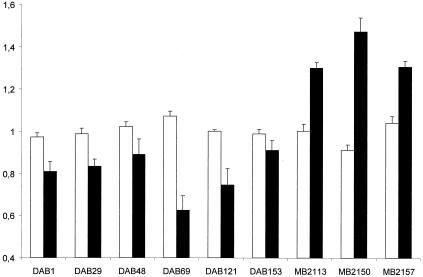
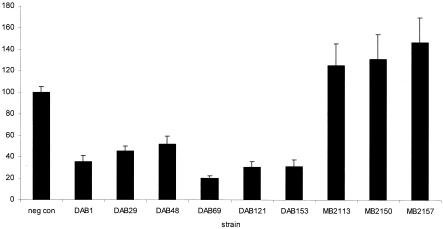
During the first 4 h postinoculation, the numbers of Salmonella bacteria in the macrophages remained stable. At 24 h postinoculation, the numbers of Salmonella bacteria from the three porcine strains were increased whereas the bacterial numbers of the six pigeon isolates were decreased (Fig. 3). In contrast to the porcine strains, the pigeon strains were cytotoxic for the macrophages at 24 h postinoculation (Fig. 4).
FIG. 3.
Intracellular numbers of either pigeon isolates (DAB series) or porcine isolates (MB series) of serovar Typhimurium variant Copenhagen in pigeon peritoneal macrophages at 0, 4, and 24 h postinoculation. The ratio of the log10 transformation of bacterial numbers recovered was determined for the time points 4 h versus 0 h (white bars) and 24 h versus 4 h postinoculation (black bars). In order to reduce interexperiment variation, the ratios obtained in one experiment were divided by the overall mean ratio of this experiment. Each bar represents the mean result of four independent experiments ± the standard error of the mean. Each experiment was conducted in triplicate.
FIG. 4.
Cytotoxic effects of either pigeon isolates (DAB series) or porcine isolates (MB series) of serovar Typhimurium variant Copenhagen on pigeon peritoneal macrophages. The bars represent the average percentages of viable cells at 24 h postinoculation ± the standard errors of the means compared to those of the uninfected control (negative control). Each bar represents the average result of three independent experiments conducted in triplicate.
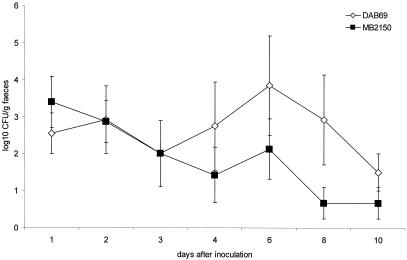
Whereas clinical symptoms were not noticed in any of the pigeons inoculated with the porcine strain, four of six pigeons inoculated with the pigeon strain suffered from severe diarrhea from day 2 postinoculation. In one of these animals, hemorrhagic diarrhea was observed. Two of the pigeons inoculated with the pigeon strain showed symptoms of general illness and died at 9 days postinoculation. Upon necropsy, none of the pigeons exposed to the porcine strain showed gross pathological lesions. In the pigeons inoculated with the pigeon strain, splenomegaly and/or hepatomegaly were noticed. In one animal, granulomas were observed on the kidneys. Agglutinating antibodies were present in all of the pigeons inoculated with the pigeon strain (mean log2 titer, 4) and in three of the six pigeons inoculated with the porcine strain (mean log2 titer for the antibody-positive animals, 5). Results of the bacterial determination of the numbers of CFU per gram of feces and the numbers of CFU per gram of tissue are summarized in Fig. 5 and Table 2. During the first 3 days postinoculation, the excretion levels were comparable for the two strains. From 4 to 10 days postinoculation, the pigeon strain was shed at a higher level than the porcine strain (P < 0.05). At 10 days postinoculation, three of the four remaining pigeons inoculated with the pigeon strain shed Salmonella with the feces, compared to one of the six pigeons inoculated with the porcine strain. Very high numbers of Salmonella bacteria (up to 6 × 107 CFU/g) were found in the internal organs of the two pigeons that died after inoculation with the pigeon strain.
FIG. 5.
The mean number of CFU per gram of feces ± the standard error of the mean after inoculation of 12 pigeons with either the serovar Typhimurium variant Copenhagen strain from pigeons (DAB69) or that from pigs (MB2150). At day 9 postinfection, two pigeons inoculated with DAB69 died.
TABLE 2.
Percentages of Salmonella-positive samples of organs and the mean numbers of CFU per gram of tissuea
| Organ | DAB69
|
MB2150
|
||
|---|---|---|---|---|
| % Positive | Mean log10 CFU/g ± SEM | % Positive | Mean log10 CFU/g ± SEM | |
| Liver | 100 | 3.15 ± 1.23b | 33 | 0.17 ± 0.17 |
| Spleen | 83 | 2.88 ± 1.36b | 0 | 0 |
| Kidney | 100 | 3.49 ± 1.20b | 0 | 0 |
| Lung | 83 | 2.87 ± 1.35b | 0 | 0 |
| Ovary | 100 | 1.76 ± 0.54b | 0 | 0 |
| Intestine | 83 | 3.01 ± 1.44 | 83 | 0.58 ± 0.15 |
Measurements taken after inoculation of 12 pigeons either with the serovar Typhimurium variant Copenhagen strain from pigeons (DAB69; no. of pigeons, 6) or with that from pigs (MB2150; no. of pigeons, 6). Two pigeons inoculated with DAB69 died at 9 days postinoculation. The remaining 10 pigeons were necropsied at day 10 postinoculation.
This value is significantly different from that found for the same organ in pigeons inoculated with MB2150 (P < 0.05).
Phenotypic and molecular characterization of the pigeon isolates of Salmonella showed a high degree of clonality. All the isolates belonged to serovar Typhimurium variant Copenhagen phage type 99. This finding is in agreement with previous reports (17). The 38 phage type 99 strains showed PFGE patterns obtained with XbaI that were identical or almost identical and could be divided into four very closely related groups (one- to two-band difference only) based on their PFGE patterns with BlnI. Moreover, virulence typing revealed a very homogenous presence of the virulence genes examined. Antibiotic resistance patterns confirm the homogeneity of Salmonella isolates from pigeons (8). The clonal nature of the pigeon isolates contrasts with the 16 different PFGE types with XbaI that were distinguished in the poultry, porcine, and human isolates, showing the genetic diversity found with XbaI for serovar Typhimurium variant Copenhagen in these species. A numerical analysis of the XbaI patterns showed only a low level of genetic relatedness between the pigeon isolates and the isolates from other sources. The clonal nature of the pigeon strains resembles the relatively low level of genetic diversity of known host-restricted serovars (22).
The ability to survive inside macrophages is associated with host adaptation in mammals and birds (1, 7, 20, 25). In mammalian species, the intracellular survival of Salmonella in macrophages eventually leads to killing of the host macrophage in an apoptosis-like way (3, 4, 10, 12, 14, 18, 23, 27). The pigeon strains were found in smaller numbers in the pigeon peritoneal macrophages after 24 h than the porcine strains. However, these lower bacterial numbers coincided with a higher cytotoxicity of the pigeon strains for the macrophages. Hence, the lower number of bacteria recovered after 24 h is instead due to an enhanced killing of macrophages, which in turn release the intracellular bacteria in the gentamicin-containing environment. Therefore, adaptation of the pigeon isolates of serovar Typhimurium to pigeons appears to be associated with enhanced macrophage cytotoxicity. Possibly, enhanced cytotoxicity allows the pigeon-adapted strains of serovar Typhimurium to spread more quickly once the internal organs, such as liver and spleen, are colonized. The importance of host macrophage cell death during a systemic infection in mice was proposed earlier (13).
The pigeon isolate was more virulent for pigeons than the porcine strain of serovar Typhimurium variant Copenhagen, resulting in a typhoid fever-like syndrome with a higher mortality rate, severe clinical symptoms, and higher bacterial counts in the internal organs. This finding is in agreement with the hypothesis that increased adaptation of a Salmonella serovar to a certain host is associated with increased virulence in that particular host (2). Prolonged and enhanced fecal shedding of Salmonella bacteria was noticed in the pigeons inoculated with the pigeon isolate. This epidemiologically important feature enables this particular strain to maintain Salmonella infections more efficiently in a population of pigeons than the porcine strain (9).
Interestingly, the pigeon strain was found in the ovaries of infected pigeons, unlike the porcine strain. Association of Salmonella with the genital tracts of pigeons has been mentioned before (6) and is a characteristic of other known host-adapted and host-restricted serovars, such as serovars Abortusovis in sheep, Pullorum in poultry, and Dublin in cattle (22, 26). The possibility of vertical transmission promotes the maintenance of this pigeon-adapted strain in the pigeon population.
Both the porcine and the pigeon strains induced seroconversion in at least some of the inoculated pigeons. Hence, care should be taken in interpreting the results of serologic examination of pigeons in the diagnosis of salmonellosis as proposed earlier (19), since transient infections with avirulent strains may also produce positive results.
In conclusion, the clonal nature of the pigeon isolates of serovar Typhimurium demonstrated using PFGE, phage typing, and virulence typing, as well as the enhanced virulence of these strains for pigeon macrophages and for pigeons, and their association with the pigeon genital tract suggest that these isolates of serovar Typhimurium variant Copenhagen phage type 99 represent a separate lineage within the serovar Typhimurium. Opposed to other lineages of serovar Typhimurium, which are generally accepted to be unrestricted, this lineage could be classified as host restricted (according to the classification of Uzzau et al. [22]).
Acknowledgments
The skillful technical assistance of Elly Engels, Venessa Eeckhaut, and Gunter Massaer is greatly appreciated. We thank Nadine Botteldoorn (Center for Agricultural Research, Melle, Belgium), Lieven De Zutter (Ghent University, Faculty of Veterinary Medicine), and J.-M. Collard (Scientific Institute for Public Health, Brussels, Belgium) for the porcine, poultry, and human strains of serovar Typhimurium variant Copenhagen, respectively.
This study was funded by research grants from Ghent University, from BOF, and from FWO-Flanders to F. Pasmans.
Editor: B. B. Finlay
REFERENCES
- 1.Barrow, P. A., M. B. Huggins, and M. A. Lovell. 1994. Host specificity of Salmonella infection in chickens and mice is expressed in vivo primarily at the level of the reticuloendothelial system. Infect. Immun. 62:4602-4610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bäumler, A. J., R. M. Tsolis, T. A. Ficht, and L. G. Adams. 1998. Evolution of host adaptation in Salmonella enterica. Infect. Immun. 66:4579-4587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boise, L. H., and C. M. Collins. 2001. Salmonella-induced cell death: apoptosis, necrosis or programmed cell death? Trends Microbiol. 9:64-67. [DOI] [PubMed] [Google Scholar]
- 4.Chen, L. M., K. Kaniga, and J. E. Galan. 1996. Salmonella spp. are cytotoxic for cultured macrophages. Mol. Microbiol. 21:1101-1115. [DOI] [PubMed] [Google Scholar]
- 5.Dom, P., P. De Herdt, F. Haesebrouck, and R. Ducatelle. 1992. Chemiluminescence properties of porcine and pigeon phagocytes. Comm. Fac. Agric. Sci. Univ. Ghent 57:1843-1850.
- 6.Duchatel, J. P., J. M. De Ree, and H. Vindevogel. 1996. Premiers essais de vaccination de pigeons contre la paratyphose au moyen de vaccins inactivés adjuvés aqueux. Ann. Med. Vet. 140:161-163. [Google Scholar]
- 7.Fields, P. I., R. V. Swanson, C. G. Haidaris, and F. Heffron. 1986. Mutants of Salmonella typhimurium that cannot survive within the macrophage are avirulent. Proc. Natl. Acad. Sci. USA 83:5189-5193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kimpe, A., A. Decostere, A. Martel, F. Haesebrouck, and L. A. Devriese. 2002. Prevalence of antimicrobial resistance among pigeon isolates of Streptococcus gallolyticus, Escherichia coli and Salmonella enterica serotype Typhimurium. Avian Pathol. 31:393-397. [DOI] [PubMed] [Google Scholar]
- 9.Kingsley, R. A., K. van Amsterdam, N. Kramer, and A. J. Bäumler. 2000. The shdA gene is restricted to serotypes of Salmonella enterica subspecies I and contributes to efficient and prolonged fecal shedding. Infect. Immun. 68:2720-2727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Knodler, L. A., and B. B. Finlay. 2001. Salmonella and apoptosis: to live or let die? Microbes Infect. 3:1321-1326. [DOI] [PubMed] [Google Scholar]
- 11.Liebisch, B., and S. Schwarz. 1996. Molecular typing of Salmonella enterica subsp. enterica serovar Enteritidis isolates. J. Med. Microbiol. 44:52-59. [DOI] [PubMed] [Google Scholar]
- 12.Lindgren, S. W., I. Stojiljkovic, and F. Heffron. 1996. Macrophage killing is an essential virulence mechanism of Salmonella typhimurium. Proc. Natl. Acad. Sci. USA 93:4197-4201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Monack, D. M., D. Hersh, N. Ghori, D. Bouley, A. Zychlinsky, and S. Falkow. 2000. Salmonella exploits caspase-1 to colonize Peyer's patches in a murine typhoid model. J. Exp. Med. 192:249-258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Monack, D. M., B. Raupach, A. E. Hromockyj, and S. Falkow. 1996. Salmonella typhimurium invasion induces apoptosis in infected macrophages. Proc. Natl. Acad. Sci. USA 93:9833-9838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nastasi, A., C. Mammina, and M. R. Villafrate. 1993. Epidemiology of Salmonella typhimurium: ribosomal DNA analysis of strains from human and animal sources. Epidemiol. Infect. 110:553-565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Olsen, J. E., M. N. Skov, E. J. Threlfall, and D. J. Brown. 1994. Clonal lines of Samonella enterica serotype Enteritidis documented by IS200-, ribo-, pulsed-field gel electrophoresis and RFLP typing. J. Med. Microbiol. 40:15-22. [DOI] [PubMed] [Google Scholar]
- 17.Rabsch, W., H. L. Andrews, R. A. Kingsley, R. Prager, H. Tschäpe, L. G. Adams, and A. J. Bäumler. 2002. Salmonella enterica serotype Typhimurium and its host-adapted variants. Infect. Immun. 70:2249-2255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Santos, R. L., R. M. Tsolis, A. J. Bäumler, R. Smith III, and L. G. Adams. 2001. Salmonella enterica serovar Typhimurium induces cell death in bovine monocyte-derived macrophages by early sipB-dependent and delayed sipB-independent mechanisms. Infect. Immun. 69:2293-2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schmeer, N., R. Busche, H. Krauss, and R. Weiss. 1983. Untersuchungen über den Zusammenhang zwischen Antikörperstatus und Infektionsgeschehen bei der Ornithose und Salmonellose von Reisetauben. Berl. Muench. Tieraerztl. Wochenschr. 96:234-238. [PubMed] [Google Scholar]
- 20.Schwan, W. R., X.-Z. Huang, L. Hu, and D. J. Kopecko. 2000. Differential bacterial survival, replication, and apoptosis-inducing ability of Salmonella serovars within human and murine macrophages. Infect. Immun. 68:1005-1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Threlfall, E. J., and A. J. Frost. 1990. The identification, typing and fingerprinting of Salmonella: laboratory aspects and epidemiological applications. J. Appl. Bacteriol. 68:5-16. [DOI] [PubMed] [Google Scholar]
- 22.Uzzau, S., D. J. Brown, T. Wallis, S. Rubino, G. Leori, S. Bernard, J. Casadesús, D. J. Platt, and J. E. Olsen. 2000. Host adapted serotypes of Salmonella enterica. Epidemiol. Infect. 125:229-255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van der Velden, A. W. M., S. W. Lindgren, M. J. Worley, and F. Heffron. 2000. Salmonella pathogenicity island 1-independent induction of apoptosis in infected macrophages by Salmonella enterica serotype Typhimurium. Infect. Immun. 68:5702-5709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Van Leeuwen, W., and P. A. M. Guinée. 1975. Frequency distribution of S. typhimurium phage types in various countries. Zentbl. Bakteriol. Hyg. Abt. 1 Orig. A 230:320-335. [PubMed] [Google Scholar]
- 25.Vladoianu, I. R., H. R. Chang, and J. C. Pechere. 1990. Expression of host resistance to Salmonella typhi and Salmonella typhimurium: bacterial survival within macrophages of murine and human origin. Microb. Pathog. 8:83-90. [DOI] [PubMed] [Google Scholar]
- 26.Wigley, P., A. Berchieri, Jr., K. L. Page, A. L. Smith, and P. A. Barrow. 2001. Salmonella enterica serovar Pullorum persists in splenic macrophages and in the reproductive tract during persistent, disease-free carriage in chickens. Infect. Immun. 69:7873-7879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhou, X., N. Mantis, X. R. Zhang, D. A. Potoka, S. C. Watkins, and H. R. Ford. 2000. Salmonella typhimurium induces apoptosis in human monocyte-derived macrophages. Microbiol. Immunol. 44:987-998. [DOI] [PubMed] [Google Scholar]