Abstract
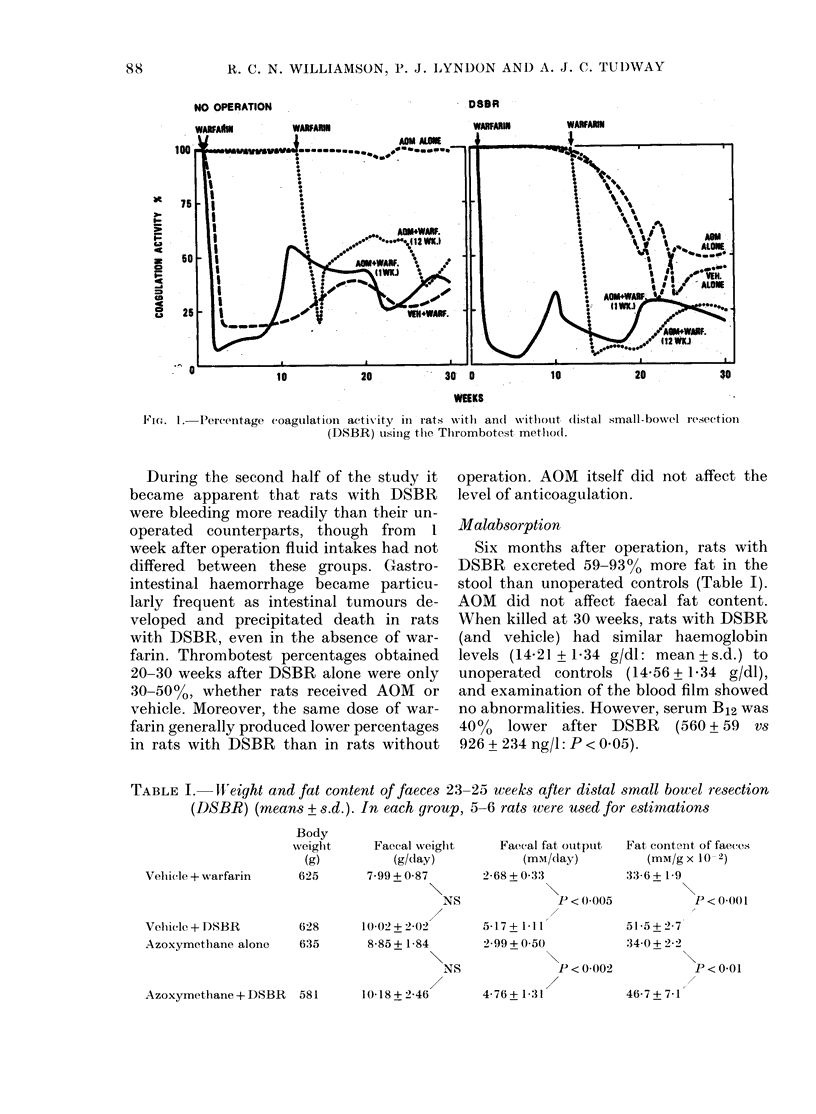
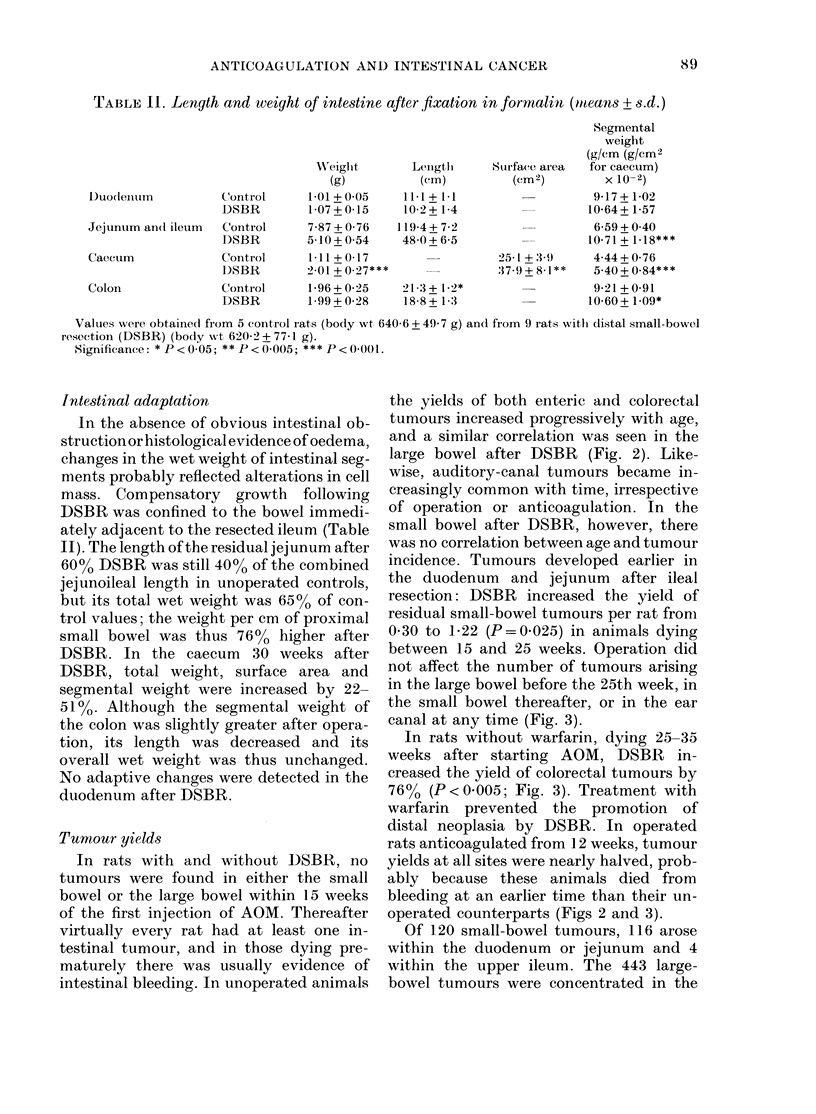
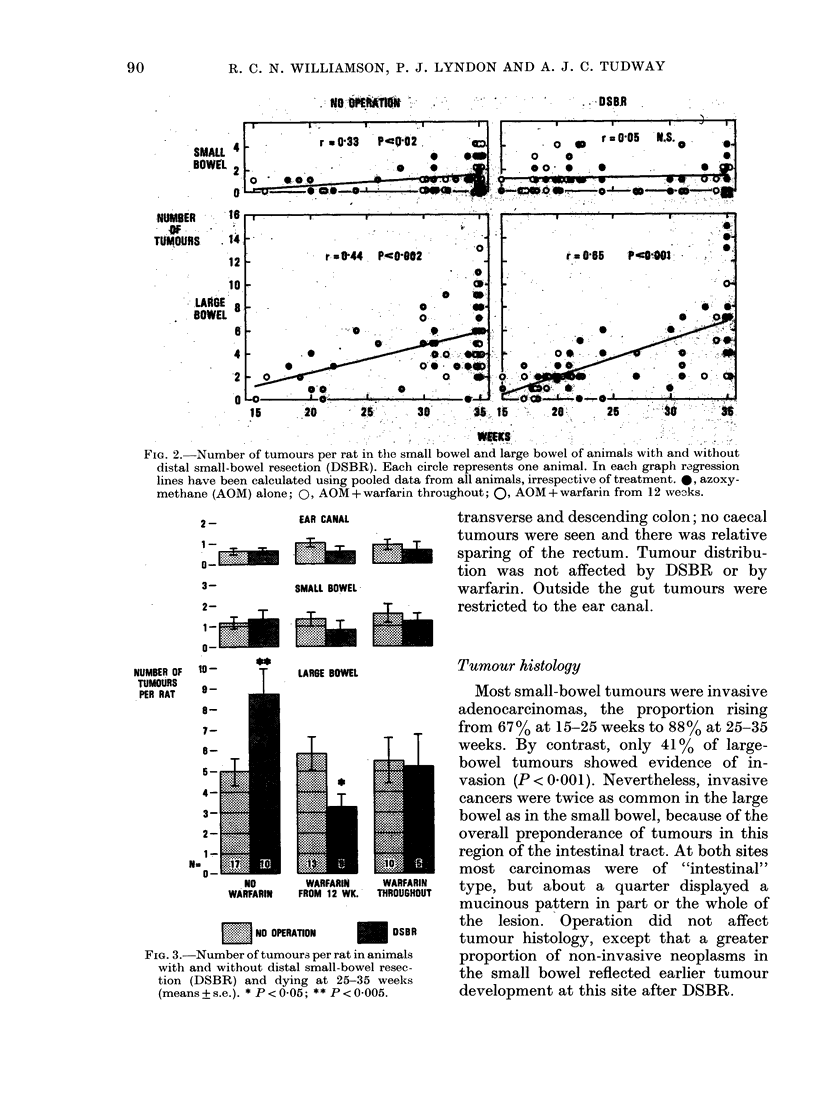
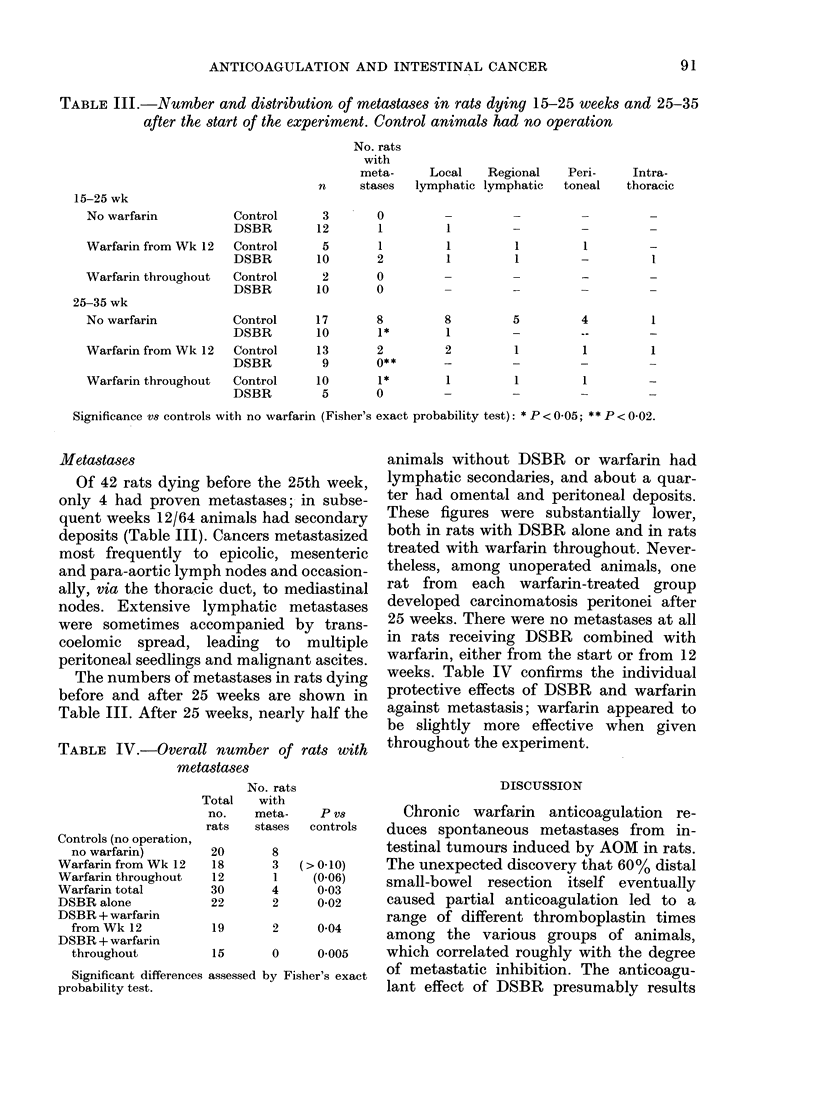
The possibility that anticoagulation with warfarin might inhibit the development of spontaneous metastases from intestinal carcinomas induced by azoxymethane (AOM) was tested in Sprague-Dawley rats with and without 60% distal small-bowel resection (DSBR). Warfarin (0.5 mg/l) was added to the drinking water from 1 week or 12 weeks postoperatively, and thromboplastin times were measured thereafter. AOM was given by 12 weekly s.c. injections (10 mg/kg/week), starting 1 week after DSBR. Besides increasing the sensitivity of rats to warfarin, DSBR itself caused partial anticoagulation, probably because of vitamin K malabsorption: at 30 weeks faecal fat was 59-93% higher, while serum B12 was 40% lower (> 0.05 P > 0.005). Adaptive growth of the jejunum and caecum after DSBR was manifested by 22-76% increases in segmental weight and surface area (P < 0.001). DSBR produced a 4-fold increase in duodenojejunal tumours at 15-25 weeks (P = 0.025) and a 76% increase in colorectal tumours at 25-35 weeks (P < 0.005). Eight of 20 control rats dying after 15 weeks had lymphatic metastases, compared with 0 of 15 rats with DSBR plus warfarin from week 1 (P = 0.005). The overall prevalence of metastases was reduced by both DSBR and warfarin, when assessed independently. Intestinal carcinogenesis induced by AOM is enhanced by the adaptive response to DSBR, but anticoagulation inhibits spontaneous metastases in this model.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AGOSTINO D., CLIFFTON E. E. Anticoagulants and the development of pulmonary metastases. Anticoagulant effect on the Walker 256 carcinosarcoma in rats. Arch Surg. 1962 Apr;84:449–453. doi: 10.1001/archsurg.1962.01300220073012. [DOI] [PubMed] [Google Scholar]
- AMUNDSEN M. A., SPITTELL J. A., Jr, THOMPSON J. H., Jr, OWEN C. A., Jr Hypercoagulability associated with malignant disease and with the postoperative state. Evidence for elevated levels of antihemophilic globulin. Ann Intern Med. 1963 Apr;58:608–616. doi: 10.7326/0003-4819-58-4-608. [DOI] [PubMed] [Google Scholar]
- Barthold S. W., Jonas A. M. Morphogenesis of early 1, 2-dimethylhydrazine-induced lesions and latent period reduction of colon carcinogenesis in mice by a variant of Citrobacter freundii. Cancer Res. 1977 Dec;37(12):4352–4360. [PubMed] [Google Scholar]
- Brown J. M. A study of the mechanism by which anticoagulation with warfarin inhibits blood-borne metastases. Cancer Res. 1973 Jun;33(6):1217–1224. [PubMed] [Google Scholar]
- Campbell R. L., Singh D. V., Nigro N. D. Importance of the fecal stream on the induction of colon tumors by azoxymethane in rats. Cancer Res. 1975 May;35(5):1369–1371. [PubMed] [Google Scholar]
- Compston J. E., Creamer B. The consequences of small intestinal resection. Q J Med. 1977 Oct;46(184):485–497. [PubMed] [Google Scholar]
- ENGELL H. C. Cancer cells in the blood; a five to nine year follow up study. Ann Surg. 1959 Apr;149(4):457–461. doi: 10.1097/00000658-195904000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FISHER B., FISHER E. R. Experimental studies of factors which influence hepatic metastases. VII. Efect of anticoagulants. Surgery. 1961 Jul;50:240–247. [PubMed] [Google Scholar]
- GROSSI C. E., AGOSTINO D., CLIFFTON E. E. The effect of human fibrinolysin on pulmonary metastases of Walker 256 carcinosarcoma. Cancer Res. 1960 Jun;20:605–608. [PubMed] [Google Scholar]
- Green R., Newmark P. A., Musso A. M., Mollin D. L. The use of chicken serum for measurement of serum vitamin B12 concentration by radioisotope dilution: discription of method and comparison with microbiological assay results. Br J Haematol. 1974 Jul;27(3):507–526. doi: 10.1111/j.1365-2141.1974.tb06816.x. [DOI] [PubMed] [Google Scholar]
- Hagmar B., Norrby K. Evidence for effects of heparin on cell surfaces influencing experimental metastases. Int J Cancer. 1970 Jan 15;5(1):72–84. doi: 10.1002/ijc.2910050110. [DOI] [PubMed] [Google Scholar]
- Hilgard P., Schulte H., Wetzig G., Schmitt G., Schmidt C. G. Oral anticoagulation in the treatment of a spontaneously metastasising murine tumour (3LL). Br J Cancer. 1977 Jan;35(1):78–86. doi: 10.1038/bjc.1977.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoover H. C., Jr, Jones D., Ketcham A. S. The optimal level of anticoagulation for decreasing experimental metastases. Surgery. 1976 Jun;79(6):625–630. [PubMed] [Google Scholar]
- Loeliger E. A., Meuwisse-Braun J. B., Muis H., Buitendijk F. J., Veltkamp J. J., Hemker H. C. Laboratory control of oral anticoagulants. Definition of therapeutic range in terms of different thromboplastin preparations. Thromb Diath Haemorrh. 1970 Jun 30;23(3):569–584. [PubMed] [Google Scholar]
- MICHAELS L. CANCER INCIDENCE AND MORTALITY IN PATIENTS HAVING ANTICOAGULANT THERAPY. Lancet. 1964 Oct 17;2(7364):832–835. doi: 10.1016/s0140-6736(64)90685-3. [DOI] [PubMed] [Google Scholar]
- Nundy S., Malamud D., Obertop H., Sczerban J., Malt R. A. Onset of cell proliferation in the shortened gut. Colonic hyperplasia after ileal resection. Gastroenterology. 1977 Feb;72(2):263–266. [PubMed] [Google Scholar]
- Nygaard K. Resection of the small intestine in rats. 3. Morphological changes in the intestinal tract. Acta Chir Scand. 1967;133(3):233–248. [PubMed] [Google Scholar]
- O'MEARA R. A. Coagulative properties of cancers. Ir J Med Sci. 1958 Oct;394:474–479. [PubMed] [Google Scholar]
- Oscarson J. E., Veen H. F., Ross J. S., Malt R. A. Ileal resection potentiates 1,2-dimethylhydrazine-induced colonic carcinogenesis. Ann Surg. 1979 Apr;189(4):503–508. [PMC free article] [PubMed] [Google Scholar]
- Poggi A., Mussoni L., Kornblihtt L., Ballabio E., de Gaetano G., Donati M. B. Warfarin enantiomers, anticoagulation, and experimental tumour metastasis. Lancet. 1978 Jan 21;1(8056):163–164. doi: 10.1016/s0140-6736(78)90472-5. [DOI] [PubMed] [Google Scholar]
- Pound A. W., McGuire L. J. Repeated partial hepatectomy as a promoting stimulus for carcinogenic response of liver to nitrosamines in rats. Br J Cancer. 1978 Apr;37(4):585–594. doi: 10.1038/bjc.1978.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan J. J., Ketcham A. S., Wexler H. Reduced incidence of spontaneous metastases with long-term Coumadin therapy. Ann Surg. 1968 Jul;168(1):163–168. doi: 10.1097/00000658-196807000-00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terpstra O. T., Peterson-Dahl E., Ross J. S., Williamson R. C., Malt R. A. Distal colonic hyperplasia after colostomy closure: a promoter of chemical carcinogenesis. Surg Forum. 1979;30:130–131. [PubMed] [Google Scholar]
- Thornes R. D., Edlow D. W., Wood S., Jr Inhibition of locomotion of cancer cells in vivo by anticoagulant therapy. I. Effects of sodium warfarin on V2 cancer cells, granulocytes, lymphocytes and macrophages in rabbits. Johns Hopkins Med J. 1968 Dec;123(6):305–316. [PubMed] [Google Scholar]
- WOOD S., Jr Pathogenesis of metastasis formation observed in vivo in the rabbit ear chamber. AMA Arch Pathol. 1958 Oct;66(4):550–568. [PubMed] [Google Scholar]
- Ward J. M. Morphogenesis of chemically induced neoplasms of the colon and small intestine in rats. Lab Invest. 1974 Apr;30(4):505–513. [PubMed] [Google Scholar]
- Williamson R. C., Bauer F. L., Oscarson J. E., Ross J. S., Malt R. A. Promotion of azoxymethane-induced colonic neoplasia by resection of the proximal small bowel. Cancer Res. 1978 Oct;38(10):3212–3217. [PubMed] [Google Scholar]
- Williamson R. C., Bauer F. L., Ross J. S., Malt R. A. Proximal enterectomy stimulates distal hyperplasia more than bypass or pancreaticobiliary diversion. Gastroenterology. 1978 Jan;74(1):16–23. [PubMed] [Google Scholar]
- Williamson R. C., Bauer F. L., Ross J. S., Watkins J. B., Malt R. A. Enhanced colonic carcinogenesis with azoxymethane in rats after pancreaticobiliary diversion to mid small bowel. Gastroenterology. 1979 Jun;76(6):1386–1392. [PubMed] [Google Scholar]
- Williamson R. C., Bauer F. L., Terpstra O. T., Ross J. S., Malt R. A. Contrasting effects of subtotal enteric bypass, enterectomy, and colectomy on azoxymethane-induced intestinal carcinogenesis. Cancer Res. 1980 Mar;40(3):538–543. [PubMed] [Google Scholar]
- Williamson R. C. Intestinal adaptation (first of two parts). Structural, functional and cytokinetic changes. N Engl J Med. 1978 Jun 22;298(25):1393–1402. doi: 10.1056/NEJM197806222982505. [DOI] [PubMed] [Google Scholar]



