Abstract
Although Mycobacterium marinum is closely related to Mycobacterium tuberculosis H37Rv genomically, the clinical outcome in humans is quite different for M. marinum and M. tuberculosis infections. We investigated possible factors in the host macrophages for determining differential pathological responses to M. tuberculosis and M. marinum using an in vitro model of mycobacterial infection. Using suppression-subtractive hybridization, we identified 12 differentially expressed genes in the human monocytic cell line U937 infected with M. tuberculosis and M. marinum. Of those genes, the most frequently recovered transcript encoded interleukin-8 (IL-8). Northern hybridization revealed that IL-8 mRNA was highly upregulated in M. tuberculosis-infected U937 cells compared with M. marinum-infected cells. In addition, enzyme-linked immunosorbent assay showed that IL-8 protein secretion was significantly elevated in M. tuberculosis-infected U937 cells, human primary monocytes, and monocyte-derived macrophages compared with that in M. marinum-infected cells. The depressed IL-8 expression was unique in M. marinum-infected cells compared with cells infected with other strains of mycobacteria, including M. tuberculosis H37Ra, Mycobacterium bovis BCG, or Mycobacterium smegmatis. When the expression of NF-κB was assessed in mycobacterium-infected U937 cells, IκBα proteins were significantly degraded in M. tuberculosis-infected cells compared with M. marinum-infected cells. Collectively, these results suggest that differential IL-8 expression in human macrophages infected with M. tuberculosis and M. marinum may be critically associated with distinct host responses in tuberculosis. Additionally, our data indicate that differential signal transduction pathways may underlie the distinct patterns of IL-8 secretion in cells infected by the two mycobacteria.
Mycobacterium marinum is the mycobacterial species that is most closely related to the Mycobacterium tuberculosis complex, with a sequence homology of 99.4% (34), and it is the cause of tuberculosis (TB) in ectothermic hosts, such as fish and frogs (7). It was first isolated from saltwater fish with a TB-like systemic disease (3). The typical M. marinum lesion seen on histopathological examination is the granuloma (37), which is similar to the histopathology seen in human TB (25). M. marinum, a relatively rapidly growing pathogen in humans and animals, is used to study the molecular pathogenesis of chronic mycobacterioses, including TB (32).
In humans, M. marinum causes only localized nodular and ulcerated lesions on the surfaces of the extremities (19). The mycobacteria were reported to localize on these relatively cool surfaces because the optimal growth temperature for M. marinum was thought to be between 25 and 35°C (7). However, M. marinum can persist (4) and replicate (22, 27) in murine macrophages, as well as in a number of epithelial cell lines (30, 31). In addition, it adapted to optimal growth at 37°C and caused disseminated systemic disease when injected into mouse footpads or tail veins, with a disease pattern similar to that of TB (9). In warm-blooded animals such as humans, dissemination of M. marinum disease to systemic organs occurs extremely rarely, even in immunocompromised populations (28). Although previous studies have suggested that the species spectrum of M. marinum pathogenicity is related to its low optimum growth temperature (27), factors for differential host responses in human monocytes infected with M. marinum and M. tuberculosis have not been well established.
Since the complete genome sequence of M. tuberculosis was well characterized in 1998 (8), several comparative studies of differentially expressed genes have been reported (6). Using DNA microarray analysis, 14 regions that were absent from Mycobacterium bovis BCG Pasteur relative to M. tuberculosis and two deletions specific for particular BCG substrains were identified (5). Using an mRNA differential-display assay, six cDNAs were found that were highly homologous to M. tuberculosis genes but were absent from its avirulent mutant, H37Ra (28). Genomic subtractive hybridization was performed between M. bovis and M. bovis BCG, and it identified three regions (RD1 to -3) that were deleted during the attenuation of M. bovis BCG (20). Although a recent study with DNA microarrays exploring the responses of human macrophages suggested that interleukin-12 (IL-12) production was inhibited during the M. tuberculosis-specific response (27), little is known about the differential expression of host genes that are induced in response to different mycobacteria.
In the present study, we used suppression-subtractive hybridization (SSH) to investigate the ability of M. marinum to enter human macrophages and to characterize differentially expressed genes in the human monocytic cell line U937 infected with either M. tuberculosis or M. marinum. Of the 12 differentially expressed genes found, we focused on IL-8 expression in U937 cells and human primary monocytes. IL-8 mRNA expression and protein secretion were more significantly elevated in M. tuberculosis-infected U937 cells than in M. marinum-infected cells. IL-8 secretion was further compared with that in cells infected with other mycobacterial strains, including M. tuberculosis H37Ra, M. bovis BCG, and Mycobacterium smegmatis. In addition, the levels of expression of nuclear factor κB (NF-κB) in U937 cells infected with the mycobacteria were compared. The data suggest that IL-8 and its underlying mechanisms are differentially regulated in human macrophages infected with different mycobacteria, suggesting distinct roles for IL-8 in the host-parasite relationship in TB.
MATERIALS AND METHODS
Bacteria.
Cultures of M. tuberculosis (kindly provided by R. L. Friedmann, University of Arizona, Tucson), M. bovis BCG (ATCC 35734), M. tuberculosis H37Ra (ATCC 25177), M. smegmatis, M. marinum (ATCC 927), and two clinical strains of M. marinum (KIT 21103 and KIT 21104) isolated from biopsy specimens from typical lesions on the hand or forearm at the Korean Institute of Tuberculosis were grown to late log phase in Middlebrook 7H10 agar (Difco, Detroit, Mich.) medium supplemented with 10% OADC (oleic acid, albumin, dextrose, catalase; Becton Dickinson Immunocytometry, San Jose, Calif.) and 0.05% Tween 80 (Sigma, St. Louis, Mo.). Batch cultures were aliquoted and stored at −70°C. Representative vials were thawed, and viable CFU were enumerated on Middlebrook 7H10 agar. Heat-killed mycobacteria were prepared by incubating M. tuberculosis and M. marinum suspensions at 80°C for 20 min and then storing them at −70°C.
For infection, bacteria were resuspended in phosphate-buffered saline (PBS) and sonicated (Bandelin, Berlin, Germany) for 3 to 5 min at 35 kHz to disrupt aggregates. Prior to infection, the bacteria were opsonized as follows: 109 viable organisms were suspended in 1 ml of RPMI 1640 (Gibco-BRL, Gaithersburg, Md.) containing 50% AB+ serum and rocked for 30 min at 37°C. The bacteria were then resuspended in 1 ml of RPMI 1640, and clumps were disrupted by multiple passages through a 25-gauge needle. To control for potential nonspecific phagocytosis effects, the cells were incubated with 0.005% latex beads (uptake, 10 to 15 particles/cell). Infection with Salmonella enterica serovar Typhimurium was used as a positive control for phagocytosis.
Differentiation of U937 cells and preparation of human monocytes and monocyte-derived macrophages (MDM).
The human monocytic cell line U937 (ATCC CRL 1593; American Type Culture Collection, Rockville, Md.) was maintained in complete medium (RPMI 1640 [Gibco-BRL] with 10% fetal bovine serum [Gibco-BRL], sodium pyruvate, nonessential amino acids, penicillin G [100 IU/ml], and streptomycin [100 μg/ml]). The U937 cells were treated with 4 nM phorbol myristate acetate (Sigma) for 72 h to induce differentiation into macrophage-like cells and washed with phosphate-buffered saline (PBS) three times.
Human peripheral blood mononuclear cells were isolated from healthy human volunteer donors using Histopaque-1077 (Sigma) gradient centrifugation. The mononuclear cells were incubated for 1 h in polystyrene tissue culture dishes at 37°C in 5% CO2. Nonadherent cells were removed by washing the dishes three times with PBS at 37°C, and adherent cells, typically >90% CD14+ by fluorescence-activated cell sorter analysis (Becton Dickinson, San Jose, Calif.), were cultivated in complete medium at 37°C in 5% CO2. Human MDM were obtained by culturing adherent monocytes in 96-well tissue cultures plates with 0.1 ng of granulocyte-macrophage colony-stimulating factor (Sigma)/ml for 5 days in complete medium.
In vitro infection of U937 cells, human monocytes, and MDM.
Differentiated U937 cells, adherent human monocytes, and MDM were washed three times with Ca2+- and Mg2+-free PBS, and adherent monolayers were replenished with complete medium without antibiotics. The cells were incubated overnight without stimulation at 37°C in a 5% CO2 atmosphere. After the overnight incubation, U937 cells (105/ml), monocytes, and MDM (2 × 105/ml) were infected with mycobacteria using opsonized bacteria-to-cell ratios of 1:1, 10:1, and 20:1, respectively.
Assessment of phagocytosis.
Phagocytosis of mycobacteria was determined by a modification of a fluorescence-quenching technique described previously (12). Briefly, the opsonized bacteria (109/ml) were labeled by incubation with 0.5 mg of fluorescein isothiocyanate (FITC; Sigma) per ml in 0.1 M carbonate buffer (pH 9.0) at 37°C for 2 h. Thereafter, the FITC-labeled mycobacteria were washed twice with PBS to remove unbound FITC and suspended in fresh 7H9 broth supplemented with 0.05% Tween 80 and 10% OADC. The mycobacterium-infected cells were washed extensively and resuspended in sodium acetate buffer (0.05 M; pH 4.5) containing 0.06% trypan blue for 5 min at 4°C. Analysis of phagocytosis was performed with a FACSCalibur flow cytometer (Becton Dickinson).
SSH and slot blot hybridization.
SSH was performed between cDNAs from U937 cells infected with M. marinum and M. tuberculosis after 24 h using a PCR-Select cDNA Subtraction kit (Clontech, Palo Alto, Calif.) according to the manufacturer's recommendations. Briefly, tester DNA was digested with the restriction endonuclease RsaI, and the fragments were marked by ligation to specialized oligonucleotide adapters. When the marked DNA was denatured and hybridized to excess unmarked driver DNA that had been digested with the same enzyme, most tester sequences formed heterohybrids with the driver. In the forward subtraction, cDNA from M. marinum-infected U937 cells was used as a driver and cDNA from M. tuberculosis-infected U937 cells was used as a tester. The subtracted fragments were then inserted into the pGEM-T East plasmid vector (Promega, Madison, Wis.). Individual transformants carrying subtracted cDNA fragments were isolated from white colonies on 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside-isopropyl-1-thio-β-d-galactopyranoside agar plates.
The slot blot hybridization (PCR-Select Differential Screening kit; Clontech) was carried out according to the manufacturer's protocol. Briefly, after PCR amplification of the Luria-Bertani culture, PCR products were slot blotted onto two sets of membranes (one set for hybridization with the radiolabeled M. tuberculosis-infected U937 cell cDNA probe and the other with the radiolabeled M. marinum-infected U937 cell cDNA probe). The radiolabeled probe was added to the hybridization buffer (ExpressHyb; Clontech), and the membranes were hybridized overnight at 72°C, followed by low- and high-stringency washes at 68°C. The membranes were exposed to films for up to 1 day, and the signals of identical clones were compared.
Northern blot analysis of subtracted cDNA and sequencing.
RNA isolation and Northern blot analysis were performed as previously described (33). The probes were generated by PCR using secondary primers from the colonies corresponding to the dots differentially expressed by dot blot differential screening. Candidate clones were sequenced on an automated PRISM 310 genetic analyzer (ABI-Perkin-Elmer), and a homology search of the blast database (http://www.ncbi.nlm.nih.gov/BLAST) was done.
Enzyme-linked immunosorbent assay (ELISA).
Supernatants were collected from cultures of U937 cells and human monocytes infected with mycobacteria for 18, 48, or 96 h and frozen at −70°C. The frozen supernatants were thawed at room temperature, and cytokine levels were measured with commercial IL-8 assay kits (PharMingen, San Diego, Calif.) according to the manufacturer's instructions. The differences between duplicate wells were consistently <10% of the mean.
Western blot analysis.
Western blot analysis was performed as previously described (35). To detect phosphorylated IκBα, the nitrocellulose membranes were incubated with phospho-IκBα-specific rabbit polyclonal antibody (Santa Cruz Biotechnology, Santa Cruz, Calif.), followed by incubation with the appropriate horseradish peroxidase-conjugated secondary antibody. Bands were visualized by enhanced-chemiluminescence analysis (Amersham, Arlington Heights, Ill.).
Statistical methods.
The results are presented as the mean ± standard deviation (SD). Statistical significance was calculated using analysis of variance or Student's t test.
RESULTS
Phagocytosis of mycobacteria by U937 cells.
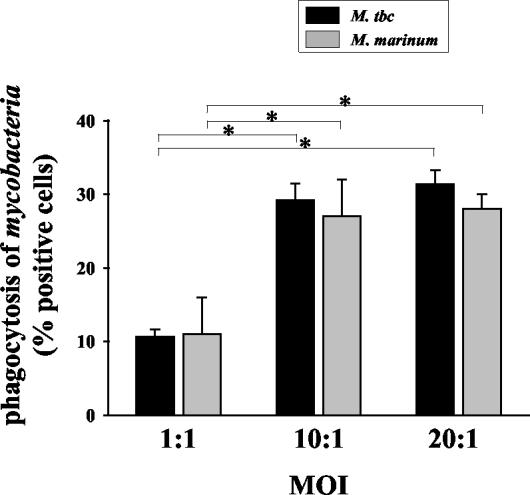
Flow cytometry and FITC-labeled bacteria were used to assess the phagocytosis of M. tuberculosis and M. marinum by U937 cells that were induced to differentiate by phorbol myristate acetate. As shown in Fig. 1, when the multiplicity of infection (MOI) was 1, M. tuberculosis and M. marinum infected 10.6% ± 1.0% and 11.0% ± 5.0% of cells, respectively. When the MOI was 10, phagocytosis rates in U937 cells increased dramatically (29.2% ± 2.2% for M. tuberculosis and 27.0% ± 5.0% for M. marinum; P < 0.05 for both mycobacteria) compared with an MOI of 1. Because the percentage of phagocytic cells infected with an MOI of 20 was not different from cells infected with an MOI of 10 (P > 0.05), we used an MOI of 10 for SSH in this study. The phagocytosis rates were not significantly different in cells infected with M. tuberculosis and M. marinum, regardless of the MOI.
FIG. 1.
Phagocytosis of mycobacteria by U937 cells. Differentiated U937 cells were incubated with opsonized mycobacteria (bacteria/U937 cell ratios, 1:1, 10:1, or 20:1) for 2 h. Then, the cells were washed free of bacteria and added to U937 cultures for 24 h. The cells were fixed with 2% paraformaldehyde for 24 h before fluorescence-activated cell sorter analysis. The values are the means + SD of three independent experiments. *, P < 0.05. M. tbc, M. tuberculosis.
Identification of differentially expressed genes in M. tuberculosis-infected U937 cells and M. marinum-infected U937 cells.
We used the SSH technique to investigate differentially expressed genes in human U937 cells infected with M. tuberculosis and M. marinum for 24 h. Using M. tuberculosis-infected U937 cells as a tester, 32 clones with cDNA inserts were selected by SSH. To verify differential expression in these clones, an initial screening was performed by slot blot hybridization. A clone was considered a candidate for M. tuberculosis-infected U937 cell-specific cDNA if it was induced at least fivefold in the membrane hybridized with M. tuberculosis-infected U937 cell cDNA compared to the membrane hybridized with M. marinum-infected U937 cell-specific cDNA. Of the 32 clones that were analyzed, 12 (37.5%) showed a stronger hybridization signal with radiolabeled cDNA derived from M. tuberculosis-infected U937 cell cDNA (data not shown).
To characterize the 12 differentially expressed clones, the inserts were automatically sequenced, and the output sequences were compared to BLAST databases in order to find homologies with known genes. As shown in Table 1, most were identified as genes that showed high homology to known sequences in GenBank. Two cDNAs, clones A1 and A6, were identified as the isoleucine-tRNA synthetase gene. Clone A2 was identified as the adenylate kinase 2 gene, and clone A3 was the ribosomal protein L19 gene. Among the known genes, thioredoxin reductase 1 and IL-10 receptor beta genes were also identified. Two of the clones were not sequenced in this study. Our failure to identify the DNA sequences of these clones could be a result of sequencing method limitations. Interestingly, we discovered that IL-8 genes were repeatedly found in M. tuberculosis-infected U937 cells (three clones indicated IL-8 genes). Because IL-8 is a potent proinflammatory chemotactic factor that induces important immune responses for antimycobacterial defenses (2), we focused on the IL-8 that is induced early at infection sites.
TABLE 1.
Description of subtracted clones representing characterized genes
| Clone no. | Length (bp) | Genea | E valueb |
|---|---|---|---|
| A1 | 1,910 | Human isoleucine-tRNA synthetase; mRNA | 7.00E-67 |
| A2 | 2,135 | Human adenylate kinase 2 (AK2); mRNA | 0 |
| A3 | 597 | Human ribosomal protein L19 (RPL19); mRNA | E-122 |
| A5 | 1,605 | Human IL-8; clone MGC:9211 | 0 |
| A6 | 1,910 | Human isoleucine-tRNA synthetase; mRNA | 2.00E-64 |
| A8 | 1,560 | Human IL-8; mRNA and human mRNA for monocyte-derived neutrophil chemotactic factor | 0 |
| A9 | NAc | ||
| A11 | 1,605 | Human IL-8; clone MGC:9211 | 0 |
| B1 | NA | ||
| B3 | 1,659 | Human IL-10 receptor beta (IL10RB); mRNA | E-130 |
| C2 | 3,794 | Human thioredoxin reductase 1 (TXNRD1); mRNA | E-115 |
| C3 | 2,848 | Human poly(A)-binding protein cytoplasmic 1 (PABPC1) | 5.00E-56 |
A common name of the gene.
Number of sequence alignments with an equivalent or superior score expected to have been found purely by chance.
NA, not analyzed.
To establish whether IL-8 expression was indeed increased due to gene transcription in M. tuberculosis-infected U937 cells, we analyzed expression by Northern blot hybridization of total RNA. Northern blot analysis revealed that the IL-8 mRNA levels were much higher in M. tuberculosis-infected U937 cells than in cells infected with M. marinum (Fig. 2). None of the differences in mRNA induction were due to variations in the RNA isolation procedure, since equivalent amounts of β-actin mRNA (a “housekeeping gene”) were expressed by monocytes infected with either of the two mycobacteria.
FIG. 2.
Northern blot analysis of differential signals in the screening. Each lane contains 20 μg of total RNA from M. tuberculosis-infected U937 cells and M. marinum-infected U937 cells/ml. The blot was hybridized with the IL-8 probe used for Northern analysis. A higher IL-8 expression level was observed in M. tuberculosis-infected cells. Loading equality was demonstrated using a β-actin probe (bottom).
M. marinum induces significantly less IL-8 protein than M. tuberculosis.
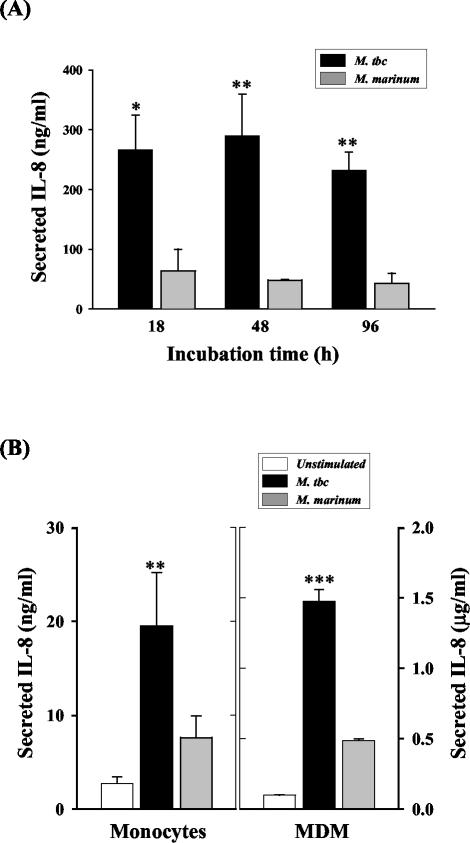
To confirm the SSH results, we used ELISA to measure the IL-8 concentrations in culture supernatants from U937 cells 18, 48, and 96 h after equivalent levels of infection with M. tuberculosis and M. marinum. As shown in Fig. 3A, IL-8 secretion in M. tuberculosis-infected cells was markedly increased after 18 h of culture. The IL-8 levels remained elevated for 18 to 48 h and were slightly decreased after 96 h of culture compared with 18 h. In contrast, IL-8 induction was significantly lower in M. marinum-infected cells than in M. tuberculosis-infected cells at 18 (P < 0.05), 48 (P < 0.01), and 96 (P < 0.01) h.
FIG. 3.
Kinetics of mycobacterium-induced IL-8 secretion from U937 cells. (A) U937 cells were infected with M. tuberculosis (M. tbc) or M. marinum (MOI = 10) for 2 h. The cell culture supernatants were harvested 18, 48, and 96 h following infection, and IL-8 protein was measured by ELISA. Statistical differences were measured for cells infected with M. tuberculosis compared to cells infected with M. marinum. *, P < 0.05; **, P < 0.01. (B) Human monocytes and MDM were infected with M. tuberculosis or M. marinum (MOI = 10) for 2 h. IL-8 was measured by ELISA in the culture supernatants 48 (for MDM) or 96 (for monocytes) h following infection. The cells infected with M. tuberculosis and M. marinum were compared statistically. *, P < 0.05; **, P < 0.01. The error bars indicate SD.
Since IL-8 production decreased in U937 cells after infection with M. marinum, parallel experiments were performed to measure IL-8 production by human monocytes and MDM infected with the two mycobacteria after 96 h of culture (Fig. 3B). Significant differences were observed between IL-8 secreted by human monocytes (P < 0.01) and MDM (P < 0.001) infected with M. tuberculosis and M. marinum, as seen in U937 cells.
Neither viability nor bacterial load influences IL-8 production by M. marinum-infected cells.
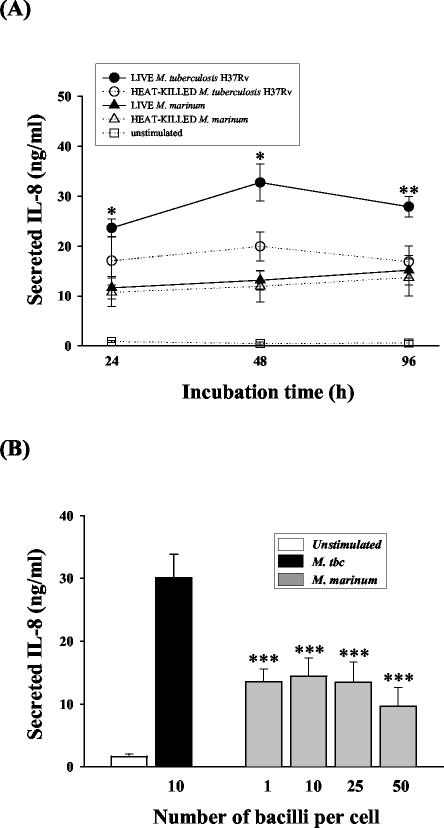
To investigate whether mycobacterial viability influences the induction of IL-8 during infection, we evaluated IL-8 production in human monocytes infected with live and dead M. tuberculosis and M. marinum. IL-8 production in monocytes infected with live M. tuberculosis was significantly higher after 24-, 48-, and 96-h incubations than in those infected with dead M. tuberculosis (P < 0.05 for both 24 and 48 h; P < 0.01 for 96 h). By contrast, no significant differences were observed between the IL-8 concentrations in monocytes infected with live and dead M. marinum (Fig. 4A). Importantly, IL-8 production by cells infected with live and dead M. marinum was significantly depressed on days 1 through 4 compared with that in cells infected with M. tuberculosis.
FIG. 4.
Effects of viability or bacterial load on M. marinum-induced IL-8 secretion by human monocytes. (A) Secretion of IL-8 by human monocytes infected with either live or killed mycobacteria. Cell supernatants were obtained 24, 48, or 96 h after infection with each mycobacterium, and IL-8 protein production was measured by ELISA. (B) IL-8 production by M. marinum at progressively higher MOIs (1, 10, 25, and 50). The IL-8 production data were obtained 48 h after infection with each mycobacterium. The data are expressed as the mean ± SD of triplicate supernatant samples. The cells infected with M. tuberculosis (M. tbc) and M. marinum were compared statistically. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Next, we evaluated IL-8 secretion by human monocytes infected with mycobacteria at various MOIs (1, 10, 25, and 50). Although the mean IL-8 production by M. marinum-infected monocytes increased slightly with bacterial load at MOIs of 1 and 10, the M. marinum-infected monocytes still secreted significantly less IL-8 than those infected with M. tuberculosis at an MOI of 10 (Fig. 4B). Therefore, we confirmed that neither the viability nor the MOI of M. marinum affects the ability to induce IL-8 production in human monocytes.
M. marinum induces significantly less IL-8 protein than other mycobacterial strains.
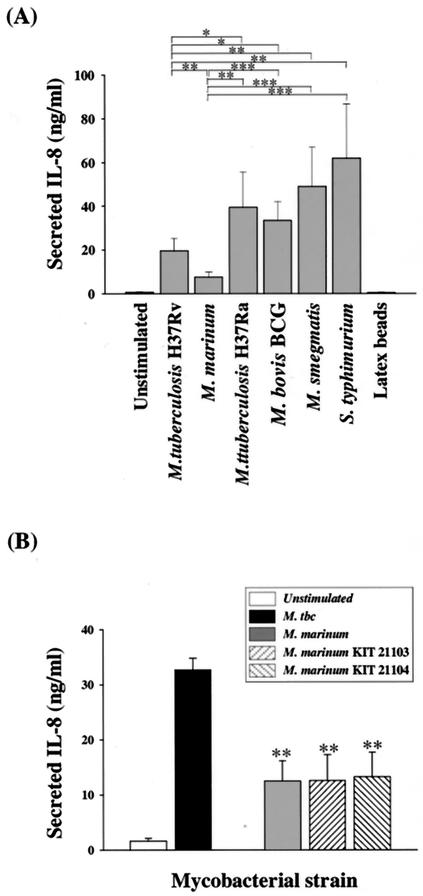
To determine whether IL-8 production varied in response to different mycobacterial strains, IL-8 levels were compared in human monocytes infected with three other mycobacterial strains (M. tuberculosis H37Ra, M. bovis BCG, and M. smegmatis) or S. enterica serovar Typhimurium or incubated with 0.005% latex beads. IL-8 induction by the three mycobacteria was significantly greater than induction by M. marinum at 96 h. M. marinum induced significantly lower IL-8 concentrations than M. tuberculosis H37Ra (P < 0.01), M. bovis BCG (P < 0.001), and M. smegmatis (P < 0.01) (Fig. 5A). S. enterica serovar Typhimurium-infected cells also had significantly increased IL-8 production compared with M. marinum (P < 0.01), whereas latex particles had no significant effect on IL-8 induction in human monocytes. In addition, IL-8 induction by M. tuberculosis was significantly lower than induction by M. tuberculosis H37Ra (P < 0.05), M. bovis BCG (P < 0.01), or M. smegmatis (P < 0.01).
FIG. 5.
Mycobacterium-induced IL-8 secretion from human monocytes. (A) Human monocytes were infected with M. tuberculosis, M. tuberculosis H37Ra, M. bovis BCG, M. smegmatis, M. marinum, or S. enterica serovar Typhimurium (MOI = 10) for 2 h. Cell culture supernatants were harvested 96 h following infection, and IL-8 protein was measured by ELISA. (B) IL-8 concentrations in the supernatants of human monocyte cultures collected 96 h following infection with M. tuberculosis (M. tbc), M. marinum ATCC 927, and two clinical isolates. Data are expressed as the mean + SD of triplicate supernatant samples. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
To investigate whether IL-8 suppression is a general feature of M. marinum, IL-8 levels in human monocytes infected with two clinical strains of M. marinum were measured and compared with those of cells infected with the strain in this study. As shown in Fig. 5B, the IL-8 levels in human monocytes infected with each strain of M. marinum were significantly lower than those of cells infected with M. tuberculosis H37Rv. In addition, there was no significant difference in IL-8 secretion among cells infected with different strains of M. marinum.
Activation of IκBα by mycobacterial strains.
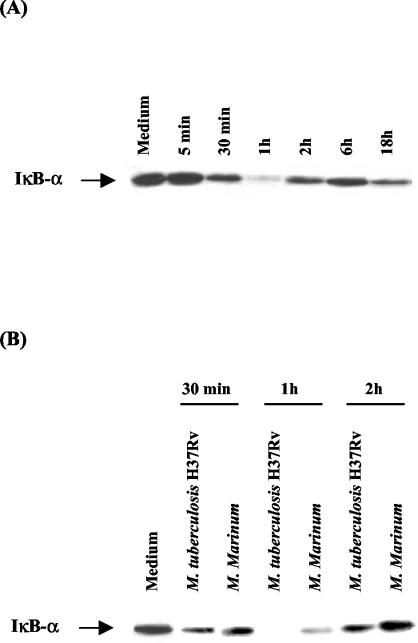
To identify possible mechanisms for the differential IL-8 production, we compared IκBα degradation in U937 cells infected with M. tuberculosis and M. marinum. Kinetic analysis of M. tuberculosis-induced IκBα degradation in U937 cells revealed gradual replacement of IκBα levels (Fig. 6A) up to 1 h. Partial recovery of IκBα levels was seen after the initial 1 h of infection.
FIG. 6.
Induction of IκBα in mycobacterium-infected U937 cells. Cytoplasmic extracts were assessed for IkBα activity by Western blot analysis. Protein from equal numbers of cells was loaded in each lane. (A) Kinetic analysis of IkBα activity in U937 cells infected with M. tuberculosis H37Rv. (B) Activation of IkBα by U937 cells infected with M. tuberculosis H37Rv and M. marinum. The MOI was 10.
Because M. tuberculosis-induced U937 cells showed decreased IκBα levels, indicating degradation, we next compared IκBα degradation in U937 cells infected with M. tuberculosis and M. marinum at a 10:1 bacteria-to-cell ratio. As shown in Fig. 6B, loss of IκBα reactivity was first observed at 30 min in all infected U937 cells, but more IκBα degradation was seen in cells infected with M. tuberculosis than in cells infected with M. marinum after 1 h of infection. Restoration of IκBα levels in all mycobacterium-infected U937 cells was seen after 2 h of culture. More IκBα degradation was seen in M. tuberculosis-infected cells than in M. marinum-infected cells at all time points. In contrast, stable levels of IκBα protein were observed at all time points in unstimulated U937 cells (data not shown), suggesting that IκBα degradation in U937 cells after infection depends on phagocytosis of mycobacteria.
DISCUSSION
Although M. marinum is very closely related to the M. tuberculosis complex according to DNA-DNA homology, little is known about M. marinum infection in human macrophages. We studied differential gene expression profiles in M. tuberculosis- and M. marinum-infected human monocytic cells using SSH. The expression of IL-8 mRNA and protein was greater in M. tuberculosis-infected cells than in cells infected with M. marinum. Like other mycobacteria, M. marinum can enter both macrophages and epithelial cells, and the levels of entry after infection are similar at 33 and 37°C (24). We used the human monocytic cell line U937 as an in vitro host model and found that M. marinum entered U937 cells efficiently. Our results demonstrate that the percentage of cells infected with M. marinum did not significantly differ from the percentage infected with M. tuberculosis at 37°C. Therefore, differences in IL-8 secretion between M. marinum and other mycobacterial strains are unlikely to have resulted from the level of entry; instead, they may represent differential host responses to the mycobacteria.
Using SSH, we obtained genes involved in the stress response (thioredoxin reductase, which is involved with the alteration of the redox status) and apoptosis (adenylate kinase 2), thioredoxin reductase genes, and the IL-8 gene in M. tuberculosis-infected U937 cell-specific cDNA. A previous study using SSH identified differentially expressed genes for Ym1 (a macrophage protein) and FIZZ1 (a novel cysteine-rich secreted protein) in activated macrophages infected with a Trypanosoma brucei brucei variant (26). Another study suggested that the regions deleted from M. bovis BCG show that genes classified as transcriptional regulators are lost disproportionately and that they may control the expression of genes required for virulence in M. tuberculosis and M. bovis (5). Our studies confirmed that SSH is useful for identifying genes that are differentially expressed in host cells infected with comparable mycobacteria. Further studies will characterize the functions of other differentially expressed genes observed in our study.
We focused on IL-8 because it has a central role in leukocyte recruitment to areas of granuloma formation in TB. IL-8 is a CXC chemokine that is also chemotactic for T lymphocytes, and it has a pivotal role in controlling cellular influx into sites of infection (2). At the cellular level, phagocytosis of M. tuberculosis by monocytic cells is an important stimulus for IL-8 secretion (2, 18, 37). Previous studies emphasized the roles of IL-8 in early host responses during M. tuberculosis infection in humans (14) and animals (17). Our present experiments showed that M. tuberculosis infection led to greater IL-8 production in U937 cells than M. marinum infection. Additionally, the differential expression of IL-8 secretion was repeatedly observed in human primary monocytes and MDM and persisted for 96 h. These findings strongly support the important role of IL-8 in the human immune response to M. tuberculosis infection and its possible role in host macrophages for determining the different pathological responses to M. tuberculosis and M. marinum.
We demonstrated that the depressed expression of IL-8 seen in M. marinum-infected cells was unaffected by the viability or MOI of the mycobacteria. A previous study indicated that the survival of M. smegmatis was significantly reduced 4 to 72 h postinfection in macrophages (13), whereas in the present study its IL-8 producing activity was significantly greater than that of M. tuberculosis or M. marinum. Our data are partially consistent with previous findings in that the levels of IL-8 produced from neutrophils incubated with M. tuberculosis were markedly lower than those produced following incubation with fast-growing M. smegmatis (11). However, the depressed IL-8 secretion by M. marinum-infected cells was unique, compared with that by cells infected with various strains of mycobacteria with high or low growth rates. Furthermore, depressed IL-8 production was clearly observed with two other clinical isolates of M. marinum, suggesting that decreased IL-8 secretion in M. marinum-infected cells is a general phenomenon with the species. Combined, these results suggest that the ability of mycobacteria to induce IL-8 production in monocytes does not reflect bacterial viability or growth rate but is a property of the mycobacterial strain.
Several studies have demonstrated that cytokine secretion is correlated with the virulence of mycobacteria. Our data show that M. tuberculosis-induced IL-8 levels are significantly lower than those induced by M. tuberculosis H37Ra, M. bovis BCG, and M. smegmatis. The relative virulences of different isolates of Mycobacterium avium have been linked to their capacities to induce proinflammatory cytokines from macrophages (36). In addition, virulent strains of mycobacteria were found to activate more tumor necrosis factor (TNF) production in alveolar macrophages than did attenuated strains (10). However, there are contrary findings. The responses of TNF-α and IL-10 in primary human alveolar macrophages to M. tuberculosis infection were found not to be correlated with microbial virulence (16). In addition, M. tuberculosis strains H37Rv and H37Ra induced comparable amounts of TNF-α in human alveolar macrophages (15). Therefore, consistent conclusions concerning the induction of IL-8 protein require the analysis of more strains.
Although little is known about the mechanisms controlling IL-8 secretion by mycobacteria in TB, a recent study indicated that protein tyrosine kinases regulate IL-8 secretion from M. tuberculosis-infected monocytes (1). Additionally, the ERK1/2 and p38 cascades play key roles in IL-8 production by monocytes stimulated with bacterial fractions (21). Since the IL-8 gene promoter contains a number of binding sites, including that of NF-κB (23), we compared IκBα degradation in cells infected with M. tuberculosis and M. marinum. Our data showed that the degradation of IκBα, the major cytoplasmic inhibitor of NF-κB, was more pronounced in M. tuberculosis-infected U937 cells than in M. marinum-infected cells. Previous studies demonstrated that virulent M. tuberculosis and avirulent M. tuberculosis H37Ra caused comparable degradation of IκBα in human monocytes (35). Another study, using different strains of Helicobacter pylori, showed that the abilities of bacteria to activate NF-κB activity are correlated with the levels of IL-8 that they induce (29). The results of previous studies and ours led us to hypothesize that the difference in IL-8 secretion between human monocytes infected with M. tuberculosis and M. marinum might be modulated by differential IκBα degradation.
In summary, this study is the first to demonstrate differential expression of IL-8 in human monocytes infected with M. tuberculosis and M. marinum. Further studies are needed to clarify the underlying mechanisms involved in depressed IL-8 expression by M. marinum-infected human macrophages.
Acknowledgments
This study was supported by a grant from the Korea Health 21 RandD Project, Ministry of Health and Welfare, Republic of Korea (01-PJ10-PG6-01GM03-002).
We thank R. L. Friedmann, University of Arizona, Tucson, for providing M. tuberculosis.
Editor: S. H. E. Kaufmann
REFERENCES
- 1.Ameixa, C., and J. S. Friedland. 2002. Interleukin-8 secretion from Mycobacterium tuberculosis-infected monocytes is regulated by protein tyrosine kinases but not by ERK 1/2 or p38 mitogen-activated protein kinases. Infect. Immun. 70:4743-4746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ameixa, C., and J. S. Friedland. 2001. Down-regulation of interleukin-8 secretion from Mycobacterium tuberculosis-infected monocytes by interleukin-4 and -10 but not by interleukin-13. Infect. Immun. 69:2470-2476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aronson, J. D. 1926. Spontaneous tuberculosis in salt water fish. J. Infect. Dis. 39:315-320. [Google Scholar]
- 4.Barker, L. P., K. M. George, S. Falkow, and P. L. C. Small. 1997. Differential trafficking of live and dead Mycobacterium marinum organisms in macrophages. Infect. Immun. 65:1497-1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Behr, M. A., M. A. Wilson, W. P. Gill, H. Salamon, G. K. Schoolnik, S. Rane, and P. M. Small. 1999. Comparative genomics of BCG vaccines by whole-genome DNA microarray. Science 284:1520-1523. [DOI] [PubMed] [Google Scholar]
- 6.Brosch, R., A. S. Pym, S. V. Gordon, and S. T. Cole. 2001. The evolution of mycobacterial pathogenicity: clues from comparative genomics. Trends Microbiol. 9:452-458. [DOI] [PubMed] [Google Scholar]
- 7.Clark, H. F., and C. C. Shepard. 1963. Effect of environmental temperatures on infection with Mycobacterium marinum (balnei) of mice and a number of poikilothermic species. J. Bacteriol. 86:1057-1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cole, S. T., R. Brosch, J. Parkhill, T. Garnier, C. Churcher, D. Harris, S. V. Gordon, K. Eiglmeier, S. Gas, C. E. Barry III, F. Tekaia, K. Badcock, D. Basham, D. Brown, T. Chillingworth, R. Connor, R. Davies, K. Devlin, T. Feltwell, S. Gentles, N. Hamlin, S. Holroyd, T. Hornsby, K. Jagels, and B. G. Barrell. 1998. Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence. Nature 393:537-544. [DOI] [PubMed] [Google Scholar]
- 9.Collins, F. M., V. Montalbine, and N. E. Morrison. 1975. Growth and immunogenicity of photochromogenic strains of mycobacteria in the footpads of normal mice. Infect. Immun. 11:1079-1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Engele, M., E. Stossel, K. Castiglione, N. Schwerdtner, M. Wagner, P. Bolcskei, M. Rollinghoff, and S. Stenger. 2002. Induction of TNF in human alveolar macrophages as a potential evasion mechanism of virulent Mycobacterium tuberculosis. J. Immunol. 168:1328-1337. [DOI] [PubMed] [Google Scholar]
- 11.Faldt, J., C. Dahlgren, and M. Ridell. 2002. Difference in neutrophil cytokine production induced by pathogenic and non-pathogenic mycobacteria. APMIS 110:593-600. [DOI] [PubMed] [Google Scholar]
- 12.Hmama, Z., R. Gabathuler, W. A. Jefferies, G. de Jong, and N. E. Reiner. 1998. Attenuation of HLA-DR expression is related to intracellular sequestration of immature class II heterodimers. J. Immunol. 161:4882-4893. [PubMed] [Google Scholar]
- 13.Hostetter, J. M., E. M. Steadham, J. S. Haynes, T. B. Bailey, and N. F. Cheville. 2002. Cytokine effects on maturation of the phagosomes containing Mycobacteriumavium subspecies paratuberculosis in J774 cells. FEMS Immunol. Med. Microbiol. 34:127-134. [DOI] [PubMed] [Google Scholar]
- 14.Juffermans, N. P., A. Verbon, S. J. van Deventer, H. van Deutekom, J. T. Belisle, M. E. Ellis, P. Speelman, and T. van der Poll. 1999. Elevated chemokine concentrations in sera of human immunodeficiency virus (HIV)-seropositive and HIV-seronegative patients with tuberculosis: a possible role for mycobacterial lipoarabinomannan. Infect. Immun. 67:4295-4297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Keane, J., M. K. Balcewicz-Sablinska, H. G. Remold, G. L. Chupp, B. B. Meek, M. J. Fenton, and H. Kornfeld. 1997. Infection by Mycobacterium tuberculosis promotes human alveolar macrophage apoptosis. Infect. Immun. 65:298-304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Keane, J., H. G. Remold, and H. Kornfeld. 2000. Virulent Mycobacterium tuberculosis strains evade apoptosis of infected alveolar macrophages. J. Immunol. 164:2016-2020. [DOI] [PubMed] [Google Scholar]
- 17.Larsen, C. G., M. K. Thomsen, B. Gesser, P. D. Thomsen, B. W. Deleuran, J. Nowak, V. Skodt, H. K. Thomsen, M. Deleuran, K. Thestrup-Pedersen, A. Harada, K. Matsushima, and T. Menne. 1995. The delayed-type hypersensitivity reaction is dependent on IL-8. Inhibition of a tuberculin skin reaction by an anti-IL-8 monoclonal antibody. J. Immunol. 155:2151-2157. [PubMed] [Google Scholar]
- 18.Lin, Y., M. Zhang, and P. F. Barnes. 1998. Chemokine production by a human alveolar epithelial cell line in response to Mycobacterium tuberculosis. Infect. Immun. 66:1121-1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Linell, F., and A. Norden. 1954. Mycobacterium balnei, a new acid fast bacillus occurring in swimming pools and capable of producing skin lesions in humans. Acta Tuberc. Scand. Suppl. 33:1-84. [PubMed] [Google Scholar]
- 20.Mahairas, G. G., P. J. Sabo, M. J. Hickey, D. C. Singh, and C. K. Stover. 1996. Molecular analysis of genetic differences between Mycobacterium bovis BCG and virulent M. bovis. J. Bacteriol. 178:1274-1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marie, C., S. Roman-Roman, and G. Rawadi. 1999. Involvement of mitogen-activated protein kinase pathways in interleukin-8 production by human monocytes and polymorphonuclear cells stimulated with lipopolysaccharide or Mycoplasma fermentans membrane lipoproteins. Infect. Immun. 67:688-693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mor, N. 1985. Multiplication of Mycobacterium marinum within phagolysosomes of murine macrophages. Infect. Immun. 48:850-852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mukaida, N., M. Shiroo, and K. Matsushima. 1989. Genomic structure of the human monocyte-derived neutrophil chemotactic factor IL-8. J. Immunol. 143:1366-1377. [PubMed] [Google Scholar]
- 24.Nau, G. J., J. F. Richmond, A. Schlesinger, E. G. Jennings, E. S. Lander, and R. A. Young. 2002. Human macrophage activation programs induced by bacterial pathogens. Proc. Natl. Acad. Sci. USA 99:1503-1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nau, G. J., P. Guilfoile, G. L. Chupp, J. S. Berman, S. J. Kim, H. Kornfeld, and R. A. Young. 1997. A chemoattractant cytokine associated with granulomas in tuberculosis and silicosis. Proc. Natl. Acad. Sci. USA 94:6414-6419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Raes, G., P. De Baetselier, W. Noel, A. Beschin, F. Brombacher, and G. G. Hassanzadeh. 2002. Differential expression of FIZZ1 and Ym1 in alternatively versus classically activated macrophages. J. Leukoc. Biol. 71:597-602. [PubMed] [Google Scholar]
- 27.Ramakrishnan, L., and S. Falkow. 1994. Mycobacterium marinum persists in cultured mammalian cells in a temperature-restricted fashion. Infect. Immun. 62:3222-3229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rindi, L., N. Lari, and C. Garzelli. 1999. Search for genes potentially involved in Mycobacterium tuberculosis virulence by mRNA differential display. Biochem. Biophys. Res. Commun. 258:94-101. [DOI] [PubMed] [Google Scholar]
- 29.Sharma, S. A., M. K. Tummuru, M. J. Blaser, and L. D. Kerr. 1998. Activation of IL-8 gene expression by Helicobacter pylori is regulated by transcription factor nuclear factor-kappa B in gastric epithelial cells. J. Immunol. 160:2401-2407. [PubMed] [Google Scholar]
- 30.Shepard, C. C. 1957. Growth characteristics of tubercle bacilli and certain other mycobacteria in HeLa cells. J. Exp. Med. 105:39-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shepard, C. C. 1958. A comparison of the growth of selected mycobacteria in HeLa, monkey kidney and human amnion cells in tissue culture. J. Exp. Med. 107:237-250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Talaat, A. M., R. Reimschuessel, S. S. Wasserman, and M. Trucksis. 1998. Goldfish, Carassius auratus, a novel animal model for the study of Mycobacterium marinum pathogenesis. Infect. Immun. 66:2938-2942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tchou-Wong, K. M., O. Tanabe, C. Chi, T. A. Yie, and W. N. Rom. 1999. Activation of NF-κB in Mycobacterium tuberculosis-induced interleukin-2 receptor expression in mononuclear phagocytes. Am. J. Respir. Crit. Care Med. 159:1323-1329. [DOI] [PubMed] [Google Scholar]
- 34.Tonjum, T., D. B. Welty, E. Jantzen, and P. L. Small. 1998. Differentiation of Mycobacterium ulcerans, M. marinum, and M. haemophilum: mapping of their relationships toM. tuberculosis by fatty acid profile analysis, DNA-DNA hybridization, and 16S rRNA gene sequence analysis. J. Clin. Microbiol. 36:918-925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Toossi, Z., B. D. Hamilton, M. H. Phillips, L. E. Averill, J. J. Ellner, and A. Salvekar. 1997. Regulation of nuclear factor-kappa B and its inhibitor I kappa B-alpha/MAD-3 in monocytes by Mycobacterium tuberculosis and during human tuberculosis. J. Immunol. 159:4109-4116. [PubMed] [Google Scholar]
- 36.Tse, H. M., S. I. Josephy, E. D. Chan, D. Fouts, and A. M. Cooper. 2002. Activation of the mitogen-activated protein kinase signaling pathway is instrumental in determining the ability of Mycobacterium avium to grow in murine macrophages. J. Immunol. 168:825-833. [DOI] [PubMed] [Google Scholar]
- 37.van Diujn, C., Jr. 1981. Tuberculosis in fish. J. Small Anim. Pract. 22:391-411. [DOI] [PubMed] [Google Scholar]