Abstract
The immune response to pneumococcal surface structures during colonization was examined in a model of experimental human pneumococcal carriage. Healthy uncolonized adults were given a type 23F or 6B pneumococcus, and a portion of these subjects became colonized (6 of 14 with type 23F and 6 of 8 with type 6B). Sera from colonized and uncolonized subjects were used to determine the titer of antibody specific to pneumococcal surface components under consideration in development of noncapsular polysaccharide-based vaccines. These vaccine candidates included pneumococcal surface protein A (PspA), choline binding protein A (CbpA), lipoteichoic acid, immunoglobulin A1 (IgA1) protease, pneumolysin, proteinase maturation protein A, and pneumococcal surface adhesin A. Only the two related choline binding proteins, PspA and CbpA, were immunogenic in colonized subjects as determined by a statistically significant rise in the serum IgG titer. The serum IgG response to PspA was shown previously to correlate inversely with susceptibility to carriage and was localized to a region within the N-terminal portion of PspA. This region is highly variable in amino acid sequence between pneumococcal strains. Despite the sequence diversity in the immunodominant regions of both PspA and CbpA, a significant strain-to-strain cross-reactivity in the serum IgG response following experimental human carriage was observed. These findings support the need for further investigation of the human antibody response to PspA and CbpA and the potential use of one or both of these proteins as novel vaccine antigens for the prevention of pneumococcal colonization.
The only known reservoir for Streptococcus pneumoniae (the pneumococcus) is the mucosal surface of the human nasopharynx. Pneumococcal infection occurs when the organism spreads beyond this niche into normally sterile parts of the respiratory tract or, in the most serious cases, into the bloodstream. Colonization, therefore, is the crucial first step in the pathogenesis of all pneumococcal disease (1).
The rising problem of antimicrobial resistance has emphasized the need for preventive strategies against this common pathogen. The immunodominant antigen of the pneumococcus is the capsular polysaccharide (PnPS), of which there are 90 known types. Antibody to the PnPS of a given type provides protection that is generally limited to pneumococcal isolates of the homologous type (10). Immunization with a mixture of PnPS of the most prevalent types protects adults from infection, but because young children fail to generate a T-cell-dependent response to polysaccharide antigens, this prophylactic strategy is unsuccessful in the population at highest risk of disease. This finding has led to the recent introduction of a vaccine based on conjugate technology that couples PnPS to an immunogenic carrier protein, resulting in a shift to a T-cell-dependent immune response (3). To produce an epidemiologically effective PnPS-protein conjugate vaccine, however, multiple types of the PnPSs must be conjugated to a protein carrier. This requirement results in a complex and expensive multicomponent vaccine with restricted potential efficacy because of the limited number of PnPS types that can be included in any single formulation, the possibility of serotype replacement, and the high titer type-specific protective antibody response to some, but not all, types (3, 9, 17). A pneumococcal protein vaccine that would specifically interfere with carriage would avoid many of the problems associated with vaccines based on PnPS and could potentially have the greatest impact on the prevention of disease.
A number of pneumococcal cell-surface or secreted components that have been shown to induce opsonophagocytic antibodies or to offer some degree of protection in murine models are currently under investigation as novel vaccine candidates. These structures include the phosphorylcholine epitope found on lipoteichoic acid (LTA), choline binding protein A (CbpA) (also referred to as pneumococcal surface protein C, or PspC), pneumolysin (Ply), proteinase maturation protein A (PpmA), pneumococcal surface adhesin A (PsaA), pneumococcal surface protein A (PspA), and a group of surface proteins recently identified using a whole-genomic approach to vaccine candidate discovery (2, 4, 13, 19, 20, 21, 23, 24). These studies, however, have depended on the use of mice, which are not naturally colonized by S. pneumoniae and which, when subjected to infection via artificial routes, are variably susceptible to a limited number of pneumococcal types (15).
In order to avoid the limitations of animal models, an experimental model of human colonization in healthy adults was described and the antibody response during carriage was examined (11). In this previously reported study, asymptomatic colonization was detected in 6 of 14 subjects and continued for 27 to 122 days following intranasal inoculation of 103 to 104 CFU of a low-passage, broth subculture of a clinical isolate of type 23F. There was minimal serum antibody response to pneumococcal polysaccharides (PnPS) during experimental carriage and no correlation between the amount of PnPS-specific antibodies in serum collected prior to inoculation and the likelihood of an individual to become colonized. All of the colonized subjects, in contrast, developed a serum immunoglobulin G (IgG) and secretory IgA response to the PspA of the inoculum strain, whereas seven of eight subjects who did not become colonized had preexisting antibody to this protein. This observation raised the possibility that the immune response to PspA might be protective against colonization in humans. Analysis of the immune response to PspA, however, was limited, since the strain used as the inoculum contained a frameshift mutation leading to a truncation of PspA at amino acid 189. The presence of this mutation in PspA was not appreciated prior to the challenge study. In one of six colonized subjects, the truncated PspA reverted to a full-length protein due to a second frameshift mutation. This mutation, which occurred during carriage, as well as the truncation of PspA in the clinical isolate used as the inoculum provided evidence of selective pressure on this protein. In a separate challenge study using a low-passage, broth subculture of an unrelated type 6B isolate, six of eight volunteers became colonized (11).
The goal of this report was to assess the immunodominant region of PspA and to determine the antibody response of a select group of potential vaccine candidates in humans prior to and during experimental carriage. We show that there was an increase in serum IgG to CbpA during human carriage and that the immune response to both CbpA and the N-terminal portion of PspA induces measurable strain-to-strain cross-reactivity.
MATERIALS AND METHODS
Serum samples.
Serum samples were obtained from human subjects enrolled in an Institutional Review Board-approved study of experimental pneumococcal carriage at the Respiratory Pathogens Research Unit, Baylor College of Medicine, as described previously (11). Briefly, 14 healthy adults found to be free of pneumococci in three sets of preinoculation nasal wash and throat swab specimens (NW/TS) collected over a 2-week period were given a 0.2-ml intranasal inoculum (0.1 ml into each naris) containing 5,000 to 17,000 CFU of a low-passage, broth subculture of a type 23F clinical isolate, P833. In a separate study, eight healthy adults were given a 0.2-ml intranasal inoculum (0.1 ml into each naris) containing 44,000 to 78, 000 CFU of a low-passage, broth subculture of a type 6B clinical isolate, P826. A range of doses was used to establish an optimal colonizing dose. NW/TS were cultured for pneumococci twice weekly in the first 2 weeks after inoculation and then weekly up to a month following inoculation or continuing until three consecutive sets of specimens were negative for pneumococci. Pneumococcal isolates were serotyped to determine whether they matched the inoculum strain. Serum samples were obtained before and 1 month following inoculation and then every 2 weeks until pneumococcal carriage was no longer detected.
Recombinant protein expression.
For expression of recombinant proteins from P833, PCR products containing the nucleotide sequences of interest (PspA and CbpA) were amplified from genomic DNA. The primers used contained EcoRI and NdeI sites allowing for the addition of unique restriction enzyme sites for cloning (Table 1). After digestion with restriction enzymes, the PCR products were ligated into pET28b vectors and subsequently transformed into Escherichia coli strain BL21. His-tagged proteins were expressed in the presence of isopropyl-β-d-thiogalactopyranoside (IPTG) and purified on an activated His-Bind Resin column (Novagen) by standard procedures. Purity of the preparations was confirmed by Coomassie stain of sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and Western blot.
TABLE 1.
Primers used to generate recombinant His-tagged proteins for use in ELISAsa
| Construct | 5′ primer sequence | 3′ primer sequence |
|---|---|---|
| PspA31-87 | GGAATTCCATATGGAAGAAGCTCCC | CCGGAATTCTCTCAGCTTCTTCTG |
| PspA31-162 | GGAATTCCATATGGAAGAAGCTCCC | GGAATTCTAAGGAACTACTTTTGCTTGTTCC |
| PspA87-162 | GGGTTTCCATATGACTGAAGAGAAAGCAAAGCTT | GGAATTCTAAGGAACTACTTTTGCTTGTTCC |
| PspA202-274 | AAAGCTGTTGAAGCCAAACAA | ACTCAACTCTTCAAGTTTTGATAG |
| PspA183-627 | GGGTTTCCATATGGAACCAGAACTTGCTAAAAAGTAG | GGAATTCGCGCGTCGACGGCTTAAACCCATTCACCATTGG |
| PpmA22-313 | CCATGGCTAGCCACCATCACCATCACCATTCGAAAGGGTCAGAAGGTGC | TCATGGATCCGGACTATTCGTTTGATGTAC |
| IgA1 protease671-1987 | CAACATCACATATGGGACAAACAGAACCAGAG | ACTTAGATCTTAATGATGGTGGTGATGATGGGCTTTAAAGATTGCTCTC |
| CbpA (P833) | AGTGTAGTAGTTGCTAGTCTTGTT | GGAATTCCCACATACCGTTTCTTGTTTC |
Primers are listed 5′→3′.
The gene fragment encoding the C-terminal 292 residues of PpmA was amplified from S. pneumoniae D39 with primers which incorporate flanking NheI and BamHI restriction sites (Table 1). A 3,948-bp gene fragment which encodes amino acids 671 to 1987 of the IgA1 protease (Iga) was amplified from S. pneumoniae TIGR4 with primers which incorporate flanking NdeI and BglII restriction sites and with a C-terminal His6 tag (Table 1). The amplified DNA was cloned into a pET11a expression vector (Stratagene, LaJolla, Calif.) and electrotransformed into E. coli BL21(DE3). The recombinant protein was expressed and purified by Ni+ affinity chromatography with the HisTrap kit (Amersham Pharmacia, Uppsala, Sweden) according to the manufacturer's recommendations. Purity of the preparations was confirmed by Coomassie stain of SDS-PAGE and Western blot.
Other antigens.
Recombinant CbpA (amino acids 39 to 443) was expressed from The Institute for Genomic Research (TIGR) type 4 genome sequence. Ply was expressed and purified as described previously (14). Purified LTA was provided by Werner Fischer (Erlagen University, The Netherlands). rPsaA and rPspA (extending from the N terminus into the proline-rich region), which represent PspAs from clades 2, 3, and 4, were provided by Aventis-Pasteur.
ELISA.
Purified protein was used to coat 96-well Immulon 2 High binding plates (DYNEX Technologies) at 0.5 μg/ml in coating buffer (0.015 M Na2CO3, 0.035 M NaHCO3 [pH 9.6]). Purified LTA was used at a dilution of 0.3 nM/ml in coating buffer. After overnight incubation at 4°C, the plates were washed with phosphate-buffered saline-Tween 20, blocked with 1% bovine serum albumin (BSA) for 1 h at 37°C, and washed again. Threefold serial dilutions of human serum in 1% BSA were added and then incubated overnight at 4°C. Antigen-specific antibodies were detected by goat anti-human IgG-alkaline phosphatase (Sigma-Aldrich) and developed with pNPP, and the absorbance at 415 nm was recorded. Titers were determined by calculating the sample dilution at which the absorbance was equal to 0.3. Antibody to the type 23F and 6B capsular polysaccharides was detected by an ELISA protocol established by the Centers for Disease Control (6).
The standard protein ELISA, described above, was modified as follows to determine the percent inhibition of antibody binding. Dilutions of human sera were incubated for 1 h at room temperature with 5 μg of PspA31-189, PspA182-623, or BSA (no-inhibitor group)/ml prior to their addition to the ELISA plate. The amount of inhibitor added was calculated by determining the amount of inhibitor necessary to yield a 70% reduction in serum IgG binding to the same protein as solid phase on the plate. Titers were determined as described above. Percent inhibition for each subject was determined by dividing the titer in the presence of inhibitor by the titer in the absence of inhibitor (BSA) and multiplying by 100.
Western blot analysis.
Whole-cell bacterial sonicates made from strain P833 were used in Western blot analysis by separating 10 μg of total protein/lane on SDS-PAGE gels (12.5%). After electrophoretic transfer onto an Immobilon-P membrane (Millipore), transfer was visualized by staining membranes with Ponceau S (Sigma-Aldrich). Individual lanes were incubated 16 h at 25°C with pre- or postinoculum sera (diluted 1:10,000) from individual subjects. Where indicated, soluble inhibitor (CbpA [P833], PspA31-189, or BSA [negative control]) was added at a concentration of 1 to 10 μg/ml as specified. Protein-specific antibodies were detected with goat anti-human IgG antisera conjugated to alkaline phosphatase (Sigma-Aldrich).
Statistical analysis.
Antibody titers prior to and after colonization were compared using a two-tailed paired t test. Preinoculation antibody titers were compared between two different subject groups using a two-tailed t test. Statistical significance was defined as a P value of ≤ 0.05.
RESULTS
Serum IgG antibody response to pneumococcal surface antigens.
In order to study pneumococcal carriage in the human nasopharynx, healthy adult volunteers were given an intranasal inoculum containing either serotype 23F or 6B pneumococci. A portion of the subjects became colonized (6 of 14 for serotype 23F and 6 of 8 for serotype 6B), and the carrier state persisted as detected by culture of serial NW/TS specimens for as long as 122 days. Sera from the colonized and uncolonized groups were used to measure the titer of antibody specific to pneumococcal vaccine candidates, including CbpA, LTA, Ply, PpmA, PsaA, and Iga.
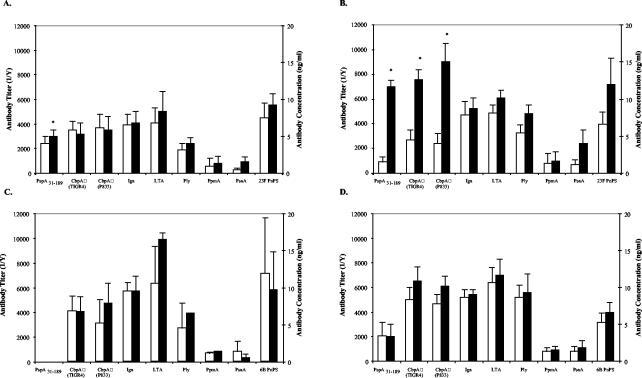
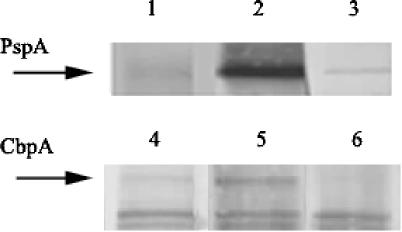
Among the subjects colonized with serotype 23F, significant increases in IgG titer to these surface antigens during carriage were observed for PspA, as previously reported (11), and for CbpA (P < 0.02) (Fig. 1B). However, in contrast to the previously reported higher PspA titers in subjects that resisted colonization with 23F than in those that became colonized (11), no correlation exists between preinoculation titer to CbpA and susceptibility to colonization with the serotype 23F strain (Fig. 1A and B, open bars). The fold increase in titer to CbpA during experimental carriage was similar to that previously described for PspA and contrasted with a lack of a significant increase in antibody against the homotypic capsular polysaccharide during carriage (11). Western analysis using whole-cell lysates of the inoculum strain confirmed the specificity of the human immune response to CbpA and PspA during experimental carriage (Fig. 2). Antibody reactivity to CbpA during carriage was inhibited by the addition of soluble recombinant CbpA expressed on the basis of the sequence spanning the variable alpha-helical domain and a portion of the conserved proline-rich domain of the inoculum strain (P833); likewise, the immune response to PspA was inhibited with soluble PspA31-189.
FIG. 1.
Serum IgG response to candidate pneumococcal vaccine antigens. Subjects either remained uncolonized (A and C) (eight and six subjects, respectively) or became colonized (B and D) (six subjects each) following intranasal challenge with a serotype 23F (A and B) or 6B (C and D) isolate. The antigens indicated on the x axis were used as the solid phase in ELISA to determine the specific IgG titers in the subjects prior to inoculation (open bars) and at 1 month or the first time point that colonization was no longer detected (solid bars). Values shown are the mean antibody titers (1/Y) ± standard errors of the mean for all subjects in a particular group for three independent experiments. Types 23F or 6B capsular polysaccharide (PnPS)-specific IgG concentrations are expressed in nanograms per milliliter ± standard errors of the mean. *, statistical significance exists (P ≤ 0.05) between pre- and postinoculation antibody levels.
FIG. 2.
Western blot analysis of whole-cell lysates of strain P833. Serum from individual subjects (FS1 and FS11) colonized with the serotype 23F isolate (P833) were used to visualize the IgG response during carriage to PspA or CbpA (as indicated by arrows). Lanes: 1, FS1 preinoculation serum; 2, FS1 serum from 2 weeks after the last detected colonization; 3, FS1 serum from 2 weeks after the last detected colonization preincubated with 1 μg of recombinant P833-derived PspA31-189/ml; 4, FS11 preinoculation serum; 5, FS11 serum from 2 weeks after the last detected colonization; 6, FS11 serum from 2 weeks after the last detected colonization preincubated with 10 μg of P833-derived recombinant CbpA/ml.
In order to determine if the observed serum IgG response to CbpA was strain specific, the corresponding recombinant CbpA fragment was generated based on the sequence of the TIGR4 genome sequence strain. CbpA, like PspA, has a substantial degree of strain-to-strain sequence variability in the N terminus (8). Among the subjects colonized, the magnitude of the immune response to the unrelated TIGR4 CbpA was similar to that observed to the CbpA from the inoculum strain, P833. This cross-reactivity was unexpected considering the degree of sequence variation between these two strains in the N terminus (Fig. 3B). Only a limited number of contiguous stretches of amino acid sequence with similarity or identity greater than eight residues in length exist in the immunogenic region examined (these included amino acids 210 to 219, 227 to 233, 426 to 446, 448 to 474, 476 to 488, 492 to 502, and 505 to 532). For subjects challenged with the serotype 6B isolate, no significant increase in IgG titer to any of the antigens tested, including CbpA, was observed during carriage (Fig. 1D). Neither of the recombinant CbpAs tested, however, were generated from the 6B challenge strain, suggesting that cross-reactivity in the antibody response observed between CbpAs of P833 and TIGR4 was limited. Preinoculation titers to CbpA from subjects challenged with the serotype 6B isolate did not correlate with susceptibility to carriage, although the uncolonized group was small (n = 2) (Fig. 1C and D, open bars).
FIG. 3.
Linear structure of mature PspA (A) and CbpA (B) showing conserved (unshaded) and variable (shaded) domains (7, 8). The region of PspA of highest strain-to-strain amino acid sequence heterogeneity (hatched) within the variable alpha-helical domain is indicated. Recombinant fragments used in ELISAs to determine region-specific IgG titers are shown below the linear structure and are labeled by amino acid position relative to the signal sequence. Amino acid sequences of P833 for regions relevant to this study are shown beneath each linear structure. Only sequence differences between P833 and other strains used in the study are indicated relative to the position in P833, with similar amino acids shown in outline. For PspA, this includes representative strains of clade family members from clades 1 to 4. The sequence used for clade 1 was derived from the 23F inoculum strain (P833, GenBank accession no. AY072940) and spans from the signal sequence through the site of truncation for this strain. The accession numbers for clades 2 to 4 PspA were M74122, AF01816, and U89711, respectively. For CbpA, this includes strains P833 (accession no. AF068647) and TIGR4 (accession no. AAK76241) and spans from the signal sequence to the end of the alpha-helical domain.
Analysis of the serum IgG antibody response to regions of PspA.
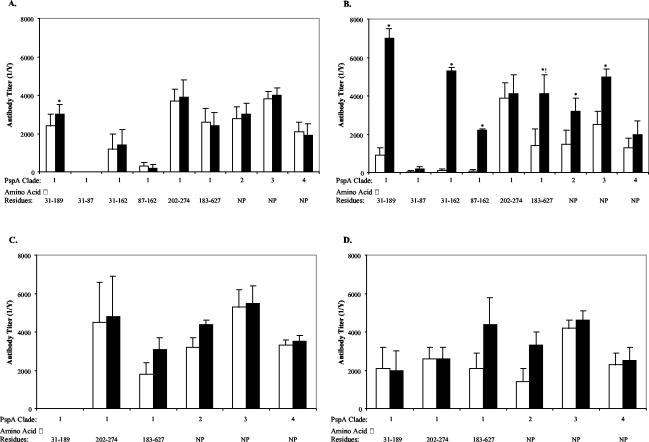
To build upon the previously observed relationship of higher PspA-specific antibody titers and resistance to colonization with 23F (11), antibody responses to smaller portions of PspA during experimental human carriage were further investigated. The N-terminal amino acid sequences of 20 PspA molecules from sequence databases, each from a different pneumococcal isolate, were compared with the prematurely truncated form of PspA expressed by the inoculum strain, P833 (data not shown). rPspA fragments were then generated based on regions of relative variability based on the sequence of P833 (Fig. 3A). As previously described, individuals colonized with the 23F pneumococcus (P833) showed significant increases in the serum IgG response to PspA31-189 as measured by ELISA (11). In addition, significant increases in the serum IgG response to a fragment spanning the first 131 amino acids of the mature protein and a fragment of 75 amino acids within this region (PspA31-162 [P ≤ 0.001] and PspA87-162 [P ≤ 0.05]), which include the most hypervariable portion, were also seen (Fig. 4B). In contrast, there was no increase in antibody titer to the PspA31-87, a region of lesser variability. Together, these results suggest that the fragment between amino acids 88 and 162 of the mature protein is the most immunogenic region. No increase in antibody titer was anticipated for PspA202-274 or PspA183-627, since the inoculated strain contained a truncation at amino acid 189. In fact, a significant increase in titer to PspA183-627 was observed, but this was attributable to antibody levels in the single individual whose colonizing strain reverted to a full-length form of PspA. No significant correlation between levels of preexisting antibody to any of the subfragments of PspA31-189 and susceptibility to colonization was observed (Fig. 4A and B, open bars). Unexpectedly, a significant increase in IgG titer to PspA31-189 in uncolonized subjects was observed and may be attributed to exposure to PspA31-189 during the inoculation procedure even though carriage was not detected in NW/TS collected beginning 3 days after inoculation. Subjects colonized with the type 6B strain demonstrated no increase in serum IgG to P833 PspA fragments over time, suggesting that PspA-specific antibody responses may be strain specific (Fig. 4C and D).
FIG. 4.
Serum IgG response to regions within PspA of the 23F inoculum strain and PspAs of different strains. Subjects either remained uncolonized (A and C) (eight and two subjects, respectively) or became colonized (B and D) (six subjects each) following intranasal challenge with a serotype 23F (A and B) or 6B (C and D) isolate. All recombinant fragments of PspA were derived from the 23F inoculum strain P833 (clade 1 family member). Whole PspA proteins representative of clades 2 to 4 family members or the PspA fragment indicated on the x axis were used as the solid phase in ELISA to determine the specific IgG titers in the subjects prior to inoculation (open bars) and at 1 month or within 2 weeks of the time that colonization was no longer detected (solid bars). Values shown are the mean antibody titers (1/Y) ± standard errors of the mean for all subjects in a particular group for three independent experiments. *, statistical significance exists (P ≤ 0.05) between pre- and postinoculation antibody levels; !, exclusion of the one individual colonized with P833 that reverted during carriage to full-length PspA would eliminate the statistically significant increase in antibody titer in this group; NP, the indicated PspAs extend from the N terminus into the proline-rich domain and do not contain the choline binding domain.
Cross-reactivity in the serum IgG antibody response to PspA.
The presence of antibodies recognizing the N terminus of PspA correlated with protection from colonization with pneumococci expressing the identical PspA (11). Since the N terminus of PspA is highly variable between strains, the ability of these antibodies to recognize heterotypic PspAs was assessed. PspA sequences from different strains may be grouped into several distinct families based upon similarity in a 72-amino acid fragment referred to as the clade-defining region (12). The antibody responses to PspA31-189 from P833, a clade 1 isolate, and three rPspAs (minus the complete proline-rich and choline binding domains) (Fig. 3A), each of which represented a different clade, were compared. For subjects challenged with 23F or 6B, titers of cross-reactive PspA-specific antibody were similar in the colonized and uncolonized groups prior to inoculation (Fig. 4). Type 23F-colonized subjects gained significant levels of serum IgG during the period of colonization that cross-reacted with a clade 2 (P ≤ 0.001) and a clade 3 (P ≤ 0.02) PspA but did not recognize a clade 4 PspA. Therefore, a significant level of cross-reactivity of antibody recognizing epitopes within the fragment N-terminal to amino acid 190 for these clade 1, 2, and 3 strains must exist. Because the inoculum strain was truncated at amino acid 189, it was not possible to determine the levels of cross-reactivity to the clade-defining or other more conserved regions. Type 6B-colonized subjects, in contrast, did not demonstrate a significant increase in antibody titers specific to any of the PspAs of other clades.
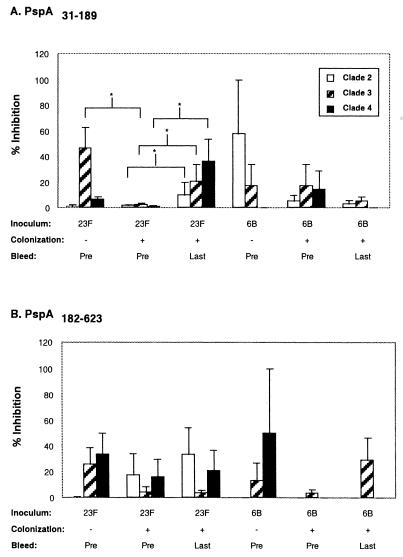
PspA-specific antibody cross-reactivity was also assessed using an inhibition ELISA. rPspA31-189 and rPspA183-627 derived from P833 were used in solution to inhibit antibody binding to solid-phase clade 2, 3, or 4 PspAs (Fig. 5). Type 23F-colonized subjects showed a trend towards an increase in PspA31-189-inhibitable antibody against PspA from each of the other clades during carriage (Fig. 5A). In addition, binding of clade 3 cross-reactive antibodies was significantly inhibited by PspA31-189 in the subjects that did not become colonized with the type 23F strain (P < 0.04 when compared to other clades). Levels of cross-reactive antibody were greater than predicted considering the degree of sequence variation among these PspAs (Fig. 3A). Among the clades of PspA considered in this study, PspA from P833 and clade 3 pneumococci were the most similar in amino acid sequence as revealed by dendrogram analysis performed by ClustalW alignment (MacVector, Accelrys, Burlington, Mass.). The only identical regions to the PspA of P833 of sufficient length to form a potential B-cell epitope were with the clade 3 PspA (amino acids 74 to 83 and 171 to 179) and clade 4 PspA (amino acids 171 to 179). Limited stretches of similar sequences between PspA from P833 and that of the other PspAs included amino acids 43 to 50 and 68 to 89 for the clade 2 strain; amino acids 57 to 62 and 74 to 90 for the clade 3 strain; and amino acids 31 to 37, 43 to 50, 52 to 62, and 74 to 84 for the clade 4 strain. However, both the sequence analysis and inhibition ELISAs indicated that there was unlikely to be a single, broadly cross-protective epitope within the N terminus of PspA. In addition, none of these short stretches of identity or similarity were within the most immunogenic portions of PspA31-189 or PspA87-162. All subject groups, regardless of inoculated strain or colonization status, contained some degree of cross-reactive antibody inhibitable by the more conserved portions of PspA (amino acids 182 to 623) (Fig. 5B). However, a correlation between preexisting antibody to these regions and susceptibility to carriage has not yet been established in humans.
FIG. 5.
Inhibition ELISA showing IgG binding to PspAs representative family members of clades 2 to 4 in the presence of recombinant PspA31-189 (A) and PspA182-627 (B) derived from the clade 1 family member (serotype 23F, inoculum strain P833). Sera tested were obtained before inoculation (Pre) from subjects that either did not (−) or did (+) subsequently become colonized and from previously colonized subjects within 2 weeks after colonization ceased (Last). Serum was incubated with the selected inhibitor (5 μg/ml) prior to addition to the ELISA plates. The percentage drop in antibody titer was determined by comparison with corresponding sera incubated with no inhibitor. Values shown are the mean percent inhibition of binding to the solid phase ± standard errors of the mean for all subjects in a particular group. *, statistical significance exists (P ≤ 0.05) between indicated groups.
DISCUSSION
At least two surface components of the pneumococcus, the choline-anchored proteins PspA and CbpA, were shown to be immunogenic during experimental human colonization. This model of pneumococcal carriage allowed for direct comparison of preinoculation and postcolonization antibody specificities and titers in the natural host. Our study focused on the serum IgG response rather than mucosal antibody. The possibility remains, therefore, that a significant mucosal antibody response to the antigens tested may not correlate with the serum IgG response examined in this study. Despite the relatively small number of colonized individuals in the experiment using a type 23F clinical isolate (n = 6), a significant rise in serum IgG during carriage to these two proteins was observed. The presence of an immune response during carriage confirmed the expression of PspA and CbpA by the pneumococcus in the commensal state in humans. In addition, our results support prior work demonstrating that PspA and CbpA elicit an IgG response during murine colonization and in vitro infection of human adenoidal B cells (5, 25). The rise in serum antibody to PspA and CbpA during experimental human colonization contrasted with the lack of a significant increase in serum IgG to the homotypic PnPS (for both the type 23F and 6B inocula), thought to be the immunodominant antigen of the pneumococcus. However, only adults were challenged in our study, and they may have been colonized with types 6B and 23F pneumococci previously. Thus, these results may not reflect those found in uncolonized children.
Other surface components that have been proposed as vaccine candidates did not appear to be immunogenic during experimental human carriage. A positive association during childhood between the exposure to S. pneumoniae that occurs with increasing age and serum IgG titers to PsaA and Ply has been described (18, 22). No rise in serum IgG titer to either PsaA or Ply was observed in our study using experimental adult carriage. The lack of significant immunogenicity of PsaA in our carriage study also contrasts with previous reports showing an increase in antibody when acute and convalescent titers during acute otitis media were compared (22). Although only PspA and CbpA were immunogenic during carriage in our study, there was detectable preinoculation antibody to all the other surface components tested. Since colonization does not appear to elicit an IgG response, the presence of antibody to LTA, PLY, PsaA, Iga, and PpmA could be from past infection or prior exposure to cross-reactive antigens.
One advantage in the use of experimental human carriage is having a uniform and characterized inoculum strain. This enabled the analysis of strain-specific immune responses in a population. For instance, there is considerable strain-to-strain variability in the sequences of PspA and CbpA in the N-terminal regions shown to be immunogenic in this study. The antigenic region of the N-terminal portion of PspA was determined to be the most highly variable portion of the protein between amino acids 88 and 162. In the six individuals colonized with the type 6B strain, there was no significant rise in IgG titer to either PspA or CbpA. However, the recombinant PspA and CbpA used to assess this response were based on proteins expressed from a heterologous isolate, suggesting that strain-to-strain diversity may account for the absence of rise in IgG titer. The immune response to highly conserved regions was not analyzed for either protein. The conserved choline-binding domain of CbpA was excluded because of the difficulty in expression of recombinant, full-length protein. The immunogenicity of regions C-terminal to amino acid 189 of PspA, which encompass the clade-defining, proline-rich, and conserved choline-binding regions, was not analyzed because the strain used for inoculation (P833) was truncated at this site. Strain-to-strain diversity is less likely to explain the lack of immunogenicity of the other vaccine candidates tested, including LTA, Ply, PsaA, and PpmA, which are highly conserved in amino acid sequence. Iga, however, has a region of significant diversity which, if immunodominant, could account for our inability to detect a rise in antibody titers during carriage, since the recombinant Iga used was derived from another strain. Also, difficulties in the expression of the full-length (>2,000 amino acid) Iga resulted in our use of a product that lacked a large portion of the N-terminal region. Hence, antibody that might be specific for that region was not detected in our analysis.
Another aspect of this study was to assess the cross-reactivity of the immune response of immunogenic surface components during carriage. For both PspA and CbpA, there was a serum IgG response during colonization with 23F that was cross-reactive with the corresponding protein expressed from another strain(s). This cross-reactivity antibody was generated during colonization with a single strain and is unlikely to represent undetected colonization by other by other serotype(s) in this closely monitored group. The amount of cross-reactivity was unexpected considering the degree of sequence diversity for the regions tested. This observation, therefore, could be due to the presence of common short linear peptides that act as B-cell epitopes or conformational epitopes of different primary sequence. For the group challenged with the type 6B inoculum, there was no detectable increase in IgG response to either PspA or CbpA expressed from other strains. This suggests that the cross-reactivity in the IgG response to these variable regions, although detectable with some PspA and CbpA proteins, is limited. In this regard, we demonstrated in the group colonized with the 23F inoculum that the amount of cross-reactivity to N-terminal regions of PspA from four unrelated strains was proportional to the similarity in their amino acid sequence.
We also assessed whether there was any positive correlation between preinoculation IgG titers to any surface component and susceptibility to becoming colonized. Among the surface molecules tested, a correlation was noted only with IgG titers to PspA, as previously described (11). Although CbpA was found to be immunogenic, there was no similar correlation between IgG titers and susceptibility to becoming colonized in the relatively small cohort examined. The lack of this correlation for PsaA in our study differs from the findings of Obaro et al., who showed lower titers to PsaA in colonized compared to uncolonized infants (18).
Our study was not designed to determine whether the presence of IgG to any of the components examined is protective from pneumococcal colonization. Our finding that PspA is immunogenic during human colonization and that there is some degree of cross-reactivity in this immune response is supportive of the approach to immunization based on the use of combinations of multiple PspAs proposed by McDaniel et al. and Nabors et al. (12, 16). Current information about the use of multiple PspAs in protection has been generated in animal studies and is based on the immune response to the clade-defining region (12). Immune responses to the clade-defining region during carriage could not be compared in our study because of the strain used. There were, however, significant amounts of preexisting antibody to more conserved regions of PspA, including the clade-defining region in many of the subjects participating in this study. For human immunization, it remains unclear how many unique PspA molecules would be needed to elicit a broadly protective immune response. In addition, a preventive strategy relying on PspA should consider the stability of its expression in the natural host, which recent evidence suggests might be an issue (11). A protein-based vaccine, therefore, may need to include additional protein components. In this regard, our results suggest that the protective potential of CbpA also merits further evaluation. Finally, our study indicates that experimental human pneumococcal carriage, which allowed us to assess immunogenicity of vaccine candidates in the natural host for this pathogen, could be useful in facilitating human protection studies for developing improved pneumococcal vaccines.
Acknowledgments
We thank Aventis-Pasteur, W. Fischer, and P. Hermans for providing antigens. We also thank Yolanda Rayford for technical support in performance of the volunteer studies.
This work was supported by grants from the US Public Health Service to J.N.W. (AI44231 and AI38446) and from the Respiratory Pathogens Research Unit, Baylor College of Medicine (NO1-AI-65298).
Editor: V. J. DiRita
REFERENCES
- 1.Austrian, R. 1986. Some aspects of the pneumococcal carrier state. J. Antimicrob. Chemother. 18:35-45. [DOI] [PubMed] [Google Scholar]
- 2.Balachandran, P., A. Brooks-Walter, A. Virolainen-Julkunen, S. Hollingshead, and D. Briles. 2002. Role of pneumococcal surface protein C in nasopharyngeal carriage and pneumonia and its ability to elicit protection against carriage of Streptococcus pneumoniae. Infect. Immun. 70:2526-2534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Black, S., H. Shinefield, B. Fireman, E. Lewis, P. Ray, J. Hansen, L. Elvin, K. Ensor, J. Hackell, G. Siber, F. Malinoski, D. Madore, I. Chang, R. Kohberger, W. Watson, R. Austrian, K. Edwards, et al. 2000. Efficacy, safety and immunogenicity of heptavalent pneumococcal conjugate vaccine in children. Pediatr. Infect. Dis. J. 19:187-195. [DOI] [PubMed] [Google Scholar]
- 4.Briles, D., E. Ades, J. Paton, J. Sampson, G. Carlone, R. Huebner, E. Virolainen, E. Swiatlo, and S. Hollingshead. 2000. Intranasal immunization of mice with a mixture of the pneumococcal proteins PsaA and PspA is highly protective against nasopharyngeal carriage of Streptococcus pneumoniae. Infect. Immun. 68:796-800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Briles, D. E., J. Yother, and L. S. McDaniel. 1988. Role of pneumococcal surface protein A in the virulence of Streptococcus pneumoniae. Rev. Infect. Dis. 10:S372-S374. [DOI] [PubMed] [Google Scholar]
- 6.Concepcion, N., and C. Frasch. 2001. Pneumococcal type 22F polysaccharide absorption improves the specificity of a pneumococcal-polysaccharide enzyme-linked immunosorbent assay. Clin. Diagn. Lab. Immunol. 8:266-272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hollingshead, S., R. Becker, and D. Briles. 2000. Diversity of PspA: mosaic genes and evidence for past recombination in Streptococcus pneumoniae. Infect. Immun. 68:5889-5900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Iannelli, F., M. R. Oggioni, and G. Pozzi. 2002. Allelic variation in the highly polymorphic locus pspC of Streptococcus pneumoniae. Gene. 284:63-71. [DOI] [PubMed] [Google Scholar]
- 9.Lipsitch, M., J. Dykes, S. Johnson, E. Ades, J. King, D. Briles, and G. Carlone. 2000. Competition among Streptococcus pneumoniae for intranasal colonization in a mouse model. Vaccine 18:2895-2901. [DOI] [PubMed] [Google Scholar]
- 10.MacLeod, C. M., R. G. Hodges, M. Heidelberger, and W. G. Bernhard. 1945. Prevention of pneumococcal pneumonia by immunization with specific capsular polysaccharides. J. Exp. Med. 82:445-465. [PMC free article] [PubMed] [Google Scholar]
- 11.McCool, T. L., T. Cate, G. Moy, and J. N. Weiser. 2002. The immune response to pneumococcal proteins during experimental human carriage. J. Exp. Med. 195:359-365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McDaniel, L. S., B. A. Ralph, D. O. McDaniel, and D. Briles. 1994. Localization of protection-eliciting epitopes on PspA of Streptococcus pneumoniae between amino acid residues 192 and 260. Microb. Pathog. 17:323-337. [DOI] [PubMed] [Google Scholar]
- 13.McDaniel, L. S., W. D. Waltman, B. Gray, and D. E. Briles. 1987. A protective monoclonal antibody that reacts with a novel antigen of pneumococcal teichoic acid. Microb. Pathog. 3:249-260. [DOI] [PubMed] [Google Scholar]
- 14.Mitchell, T. J., J. A. Walker, F. K. Saunders, P. W. Andrew, and G. J. Boulnois. 1989. Expression of the pneumolysin gene in Escherichia coli: rapid purification and biological properties. Biochim. Biophys. Acta 1007:67-72. [DOI] [PubMed] [Google Scholar]
- 15.Morch, E. 1943. Serological studies on the pneumococci. Oxford University Press, London, United Kingdom.
- 16.Nabors, G. S., P. A. Braun, D. J. Herrmann, M. L. Heise, D. J. Pyle, S. Gravenstein, M. Schilling, L. M. Ferguson, S. K. Hollingshead, D. E. Briles, and R. S. Becker. 2000. Immunization of healthy adults with a single recombinant pneumococcal surface protein A (PspA) variant stimulates broadly cross-reactive antibodies to heterologous PspA molecules. Vaccine 18:1743-1754. [DOI] [PubMed] [Google Scholar]
- 17.Obaro, S. 2000. Confronting the pneumococcus: a target shift or bullet change? Vaccine 19:1211-1217. [DOI] [PubMed] [Google Scholar]
- 18.Obaro, S. K., R. A. Adegbola, J. A. Tharpe, E. W. Ades, K. P. McAdam, G. Carlone, and J. S. Sampson. 2000. Pneumococcal surface adhesin A antibody concentration in serum and nasopharyngeal carriage of Streptococcus pneumoniae in young African infants. Vaccine 19:411-412. [DOI] [PubMed] [Google Scholar]
- 19.Ogunniyi, A., R. Folland, D. Briles, S. Hollingshead, and J. Paton. 2000. Immunization of mice with combinations of pneumococcal virulence proteins elicits enhanced protection against challenge with Streptococcus pneumoniae. Infect. Immun. 68:3028-3033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Overweg, K., A. Kerr, M. Sluijter, M. Jackson, T. Mitchell, A. De Jong, R. De Groot, and P. Hermans. 2000. The putative proteinase maturation protein A of Streptococcus pneumoniae is a conserved surface protein with potential to elicit protective immune responses. Infect. Immun. 68:4180-4188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Paton, J. C., R. A. Lock, C-J. Lee, J. P. Li, A. M. Berry, T. J. Mitchell, P. W. Andrew, D. Hansman, and G. J. Boulnois. 1991. Purification and immunogenicity of genetically obtained pneumolysin toxoids and their conjugation to Streptococcus pneumoniae type 19F polysaccharide. Infect. Immun. 59:2297-2304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rapola, S., V. Jantti, R. Haikala, R. Syrjanen, G. M. Carlone, J. S. Sampso, D. E. Briles, J. C. Paton, A. K. Takala, T. M. Kilpi, and H. Kayhty. 2000. Natural development of antibodies to pneumococcal surface protein A, pneumococcal surface adhesin A, and pneumolysin in relation to pneumococcal carriage and acute otitis media. J. Infect. Dis. 182:1146-1152. [DOI] [PubMed] [Google Scholar]
- 23.Rosenow, C., P. Ryan, J. N. Weiser, S. Johnson, P. Fontan, A. Ortqvist, and H. R. Masure. 1997. Contribution of novel choline-binding proteins to adherence, colonization and immunogenicity of Streptococcus pneumoniae. Mol. Microbiol. 25:819-829. [DOI] [PubMed] [Google Scholar]
- 24.Wizemann, T., J. Heinrichs, J. Adamou, A. Erwin, C. Kunsch, G. Choi, S. Barash, C. Rosen, H. Masure, E. Tuomanen, A. Gayle, Y. Brewah, W. Walsh, P. Barren, R. Lathigra, M. Hanson, S. Langermann, S. Johnson, and S. Koenig. 2001. Use of a whole genome approach to identify vaccine molecules affording protection against Streptococcus pneumoniae infection. Infect. Immun. 69:1593-1598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang, Q., S. Choo, and A. Finn. 2002. Immune responses to novel pneumococcal proteins pneumolysin, PspA, PsaA, and CbpA in adenoidal B cells from children. Infect. Immun. 70:5363-5369. [DOI] [PMC free article] [PubMed] [Google Scholar]