Abstract
The Plasmodium merozoite surface protein 1 (MSP1) is a leading vaccine candidate for protecting against the blood stage of malaria. Previous studies have shown that the 19-kDa carboxyl terminus of this protein is able to induce protective immunity in some monkey and mouse strains. We show that immunization with the recombinant Plasmodium yoelii 19-kDa fragment of MSP1 (MSP119) expressed in Saccharomyces cerevisiae (yMSP119) can induce protective antibodies in several inbred mouse strains and one outbred mouse strain. However, mice expressing the H-2s major histocompatibility complex haplotype are unable to generate yMSP119-specific antibodies. While synthetic peptides derived from MSP119 are immunogenic in B10.S mice, they cannot function as helper epitopes, and immunization with yMSP119 does not induce T cells that recognize the recombinant protein or synthetic peptides corresponding to its sequence. Nonresponsiveness could be overcome by using chemical linkers to conjugate yMSP119 to diphtheria toxoid (DT), resulting in immunogens capable of inducing protective yMSP119-specific antibodies in both MSP119-responsive and otherwise nonresponsive mouse strains. The ability of sera from mice immunized with the conjugate to inhibit binding of a protective monoclonal antibody (MAb 302) to yMSP119 correlated strongly with a delay in the prepatent period. Chemical conjugation of yMSP119 to DT may be a preferred method to enhance immunogenicity, as carrier priming experiments demonstrated that an existing immune response to DT enhanced a subsequent antibody response to yMSP119 after vaccination with yMSP119-DT. These results have important implications for the development of a malaria vaccine to protect a population with diverse HLAs.
Malaria remains one of the leading causes of morbidity and mortality in the tropics. Each year, 300 to 500 million cases of malaria occur, and 1 to 2 million of these cases result in death (90% of these deaths occur in Africa) (33). Existing control measures, such as chemoprophylaxis, are increasingly less efficacious, emphasizing the need to develop a successful vaccine against the disease.
Merozoite surface protein 1 (MSP1) is a leading vaccine candidate against the blood stage of malaria and has been evaluated extensively in rodent and primate models (7, 12, 13, 18, 19). It is expressed on the surface of the developing merozoite where it undergoes two proteolytic cleavages, the second of which generates a 19-kDa fragment (MSP119) that remains membrane bound and is carried on the surface of the merozoite into the newly invaded erythrocyte (2, 3). MSP119 is cysteine rich and highly conserved and contains two epidermal growth factor (EGF)-like domains (4). It is the first EGF-like domain in the 19-kDa fragment in Plasmodium yoelii that is the target of an immunoglobulin G3 (IgG3) protective monoclonal antibody (MAb 302) (5). MSP119 has been produced using a number of recombinant protein expression systems, including bacterial (7), mammalian (22), baculovirus (6), and yeast (15) models, which have all demonstrated some degree of success at producing antigens that are both immunogenic and protective against challenge with the malaria parasite. Immunity induced by MSP119 is thought to be dependent on a high antibody titer at the time of challenge (12, 13) and on an ongoing immune response induced by the malaria parasite following challenge (14), the specificity of which need not be directed at MSP119 (32).
Despite extensive investigation, a significant obstacle to the ultimate success of MSP119 as a vaccine is its small size, which may make it nonimmunogenic in a significant percentage of the population. Immunization with glutathione S-transferase (GST)-MSP119 expressed in Escherichia coli can protect some but not all H-2 congenic strains of mice following challenge with P. yoelii (31). Protection correlated with the genes present in the H-2 loci. Further studies found that immunization with MSP119 expressed in Saccharomyces cerevisiae (yMSP119), which lacked the GST molecule but contained six additional histidine residues, resulted in sterile and complete protection from challenge in two H-2 congenic mouse strains following either parenteral or intranasal immunization (12-14).
The problem of designing a vaccine that is universally recognized by a population with diverse HLAs is a challenge for malaria vaccine development. A number of studies have focused on the use of universal helper T-cell epitopes to provide help for B cells, thereby enhancing the immunogenicity of small-subunit-based vaccines (1, 17, 24). While conjugating B-cell epitopes (haptens) to proteins is a more classical approach to providing T-cell help (21) and one that would result in immunological responsiveness among a greater proportion of the population, some studies have suggested that prior exposure to the protein can result in a diminished response to the hapten following protein-hapten immunization (8, 11, 23, 26-28). However, this is not necessarily observed (8, 23, 29), and furthermore, protein-protein conjugates have not been studied extensively. If prior exposure to a protein vaccine (such as diphtheria toxoid [DT]) resulted in enhanced immunogenicity following subsequent immunization with a DT-protein vaccine, then this would be an additional strategy to develop a vaccine that is highly immunogenic in a large proportion of the population.
We defined genetic restriction of the immune response to yMSP119 in a number of inbred strains and one outbred strain of mice immunized with yMSP119. An inbred strain of mice that failed to generate any antibodies to yMSP119 was found to be responsive to two different DT conjugates and was protected from challenge. Furthermore, prior exposure to DT enhanced the subsequent immune response to yMSP119, when it was administered as a DT conjugate.
MATERIALS AND METHODS
Mice and parasite.
Female BALB/c (H-2d), C57BL/10 (H-2b), B10.BR (H-2k), B10.D2 (H-2d), and Quackenbush mice were 6 to 10 weeks old at the start of the experiments. Mice were obtained from the Animal Resources Centre, Willeton, Australia. Female B10.S (H-2s) mice were 10 to 15 weeks old at the start of experiments and were obtained from the Walter and Eliza Hall Institute, Melbourne, Australia. All mice were housed under specific-pathogen-free conditions. The experimental procedures described in this paper were approved by The Bancroft Centre Animal Ethics Committee.
The malaria parasite used was P. yoelii YM (lethal). The parasites were maintained by intraperitoneal passaging of 106 parasitized erythrocytes into recipient mice. The parasitized red blood cells were cryopreserved in liquid nitrogen.
yMSP119 protein and peptides.
Recombinant MSP119 corresponding to P. yoelii YM protein was produced with a C-terminal His6 tag in S. cerevisiae (31). It was provided as a generous gift by Anthony Stowers (National Institute of Allergy and Infectious Diseases, National Institutes of Health).
Ten peptides, 20 amino acids in length spanning the length of MSP119 and overlapping each other by 10 amino acids, were made by the Peptide Unit, Queensland Institute of Medical Research and Mimotopes Pty., Ltd., Victoria, Australia (30). The peptide sequences are listed in Table 1. The purity of the peptides was >85%.
TABLE 1.
Sequences of the 10 overlapping linear peptides derived from yMSP119
| Peptide | Sequence |
|---|---|
| 15 | NMDGMDLLGVDPKHVCVDTR |
| 16 | DPKHVCVDTRDIPKNAGCFR |
| 17 | DIPKNAGCFRDDNGTEEWRC |
| 18 | DDNGTEEWRCLLGYKKGEGN |
| 19 | LLGYKKGEGNTCVENNNPTC |
| 20 | TCVENNNPTCDINNGGCDPT |
| 21 | DINNGGCDPTASCQNAESTE |
| 22 | ASCQNAESTENSKKIICTCK |
| 23 | NSKKIICTCKEPTPNAYYEG |
| 24 | EPTPNAYYEGVFCSSSS |
Preparation of yMSP119-DT conjugate using SPDP.
First, yMSP119 and DT were mixed individually with N-succinimidyl 3-(2-pyridyldithio) propionate (SPDP). The N-succinimidyl moiety of SPDP coupled with the primary amine of yMSP119 and DT to form a stable amide bond. The resulting DT 2-pyridyl disulfide derivative was subsequently reduced to give DT molecules with free thiol groups. Finally, the 2-pyridyl disulfide-containing yMSP119 was mixed with the thiol-containing DT, yielding a yMSP119-DT conjugate linked via a disulfide bond. The conjugate was dialyzed against phosphate-buffered saline (PBS) overnight at 4°C to eliminate free yMSP119. This conjugate was named yMSP119-DT (SPDP). Determination of conjugate concentration was determined with a Bradford protein assay kit (Bio-Rad).
Preparation of yMSP119-DT conjugate using 6-maleimidocaproic acyl N-hydroxysuccinimide ester (MCS).
DT was dialyzed against a solution consisting of 50 mM triethanolamine, 0.15 M NaCl, and 1 mM EDTA (pH 8.0). A 10× molar excess of 2-iminothiolamine-HCl (Traut's reagent; Pierce) was added to DT prior to a 45-min incubation under N2 at room temperature (RT). The Traut's reagent reacted with the primary amines on DT, introducing sulfhydryl functional groups. Spare Traut's reagent was separated from the iminothiolated protein by dialysis against PBS.
Initially, the conjugation process was optimized to determine the amount of chemical linker to add to the reaction mixture. Consequently, yMSP119 suspended in phosphate buffer was added to a 2× molar excess of MCS (Sigma) dissolved in dimethyl formamide and allowed to react for 1 h at RT. The N-hydroxysuccinimide moieties of MCS coupled with the primary amines of yMSP119 to form a stable amide bond. The MCS-yMSP119 solution was dialyzed overnight at 4°C against a solution containing 0.1 M phosphate and EDTA (pH 7.0).
The iminothiolated DT was added to MCS-yMSP119 in a 2× molar excess and was allowed to react for 1 h at RT. The free maleimide group of MCS reacted with the sulfhydryl group of the iminothiolated DT, forming a stable thioether linkage. This yMSP119-DT conjugate was then dialyzed against PBS overnight at 4°C to eliminate free yMSP119. The conjugate was named yMSP119-DT (MCS). The conjugate concentration was determined with a Bradford protein assay kit (Bio-Rad).
Analysis of yMSP119-DT conjugate.
To ensure yMSP119 had conjugated to DT, the products were run on a sodium dodecyl sulfate-polyacrylamide gel (4 to 20% polyacrylamide) (Gradipore). The protein bands were visualized by staining with Coomassie blue and by Western blotting. The yMSP119-DT conjugate on the Western blot was detected by either MAb 302 (20), polyclonal yMSP119 hyperimmune serum (HIS) or DT HIS raised in mice. A horseradish peroxidase (HRP)-conjugated goat anti-mouse IgG was used as the secondary antibody.
yMSP119 and yMSP119 conjugate protection studies.
For the yMSP119 protection studies, groups of five mice were immunized subcutaneously (s.c.) with 20 μg of yMSP119 in complete Freund’s adjuvant (CFA). At day 0, equal amounts of emulsion (10 μg at each site) were injected into the base of the tail and abdomen. The mice were given booster doses. On day 21, a booster dose of 20 μg of yMSP119 in incomplete Freund’s adjuvant (IFA) was injected s.c. at the base of the neck. On days 42 and 56, a booster dose of 20 μg of yMSP119 in IFA was injected intraperitoneally (i.p.). The mice were given a final booster dose i.p. on day 63 with the same amount of yMSP119 in PBS.
Mice were challenged intravenously (i.v.) with 104 P. yoelii YM parasitized erythrocytes on day 70 of the immunization protocol. Parasitemia was monitored by microscopic examination of stained blood films every second day following challenge infection. Blood was collected by snipping the tail prior to each booster dose and every second day following challenge infection for the duration of the experiment and frozen until it was assayed for antibody levels.
Immunization studies with the yMSP119-DT conjugates [named yMSP119-DT (MCS) and yMSP119-DT (SPDP)] followed the same schedule and dosage as described above for yMSP119, except that the mice received two additional booster doses in PBS administered i.p. on days 114 and 120. (The two extra immunizations helped to increase the yMSP119-specific antibody levels). In the yMSP119-DT conjugate experiment, the mice that were immunized with yMSP119 alone also received the two additional booster doses. The mice received 20 μg of protein in each immunization and were challenged with 104 P. yoelii YM parasitized erythrocytes 7 days (day 127) after the final booster dose.
Determination of the effect of prior exposure to DT.
Groups of mice (C57BL/10 and B10.S mice; five mice per group) were immunized s.c. at the base of the tail and abdomen with 20 μg of yMSP119, yMSP119-DT (SPDP), DT, or PBS in CFA (10 μg at each site). Twenty-one days later, the mice were given s.c. booster doses of 20 μg of yMSP119-DT (SPDP) in IFA at the base of the neck. Blood samples were collected for the duration of the experiment, and serum samples were obtained for determination of DT- and yMSP119-specific antibody responses.
Priming mice with MSP119 or its peptides.
The mice were injected s.c. at two sites with 20 μg of MSP119, 20 μg of peptide, or PBS emulsified in CFA. Three weeks later, the mice were given s.c. booster doses of 20 μg of yMSP119 emulsified in IFA. Blood was collected from the tail tip immediately prior to the booster dose and every 2 days for the length of the experiments. Serum was frozen until assayed for antibody levels.
Lymphocyte proliferation assays.
Groups of 10 mice were immunized in the hind footpads with pools of five sequential overlapping peptides emulsified in CFA. They received 20 μg of each peptide in the immunization, with a cumulative total of 100 μg of peptide in each immunization. Seven to nine days later, the draining popliteal and inguinal lymph nodes were removed. Cells were suspended at 2.5 × 106 cells/ml in Eagle minimum essential medium containing 2-mercaptoethanol (diluted 1:1,000) and 2% normal mouse serum, and 200 μl of this suspension was added to 96-well plates. Cells were cultured in triplicate with various concentrations of peptides, antigens, or mitogens for 72 h at 37°C in 5% CO2. The plates were pulsed with [3H]thymidine (Amersham Pharmacia), and incorporation of radiolabel was measured 18 to 24 h later by β-emission spectroscopy.
Antibody assay.
Serum antibody levels were assayed by enzyme-linked immunosorbent assays (ELISAs). The wells in 96-well plates were coated with 0.5 μg of yMSP119 per ml in coating buffer and incubated overnight at 4°C. The plates were then blocked with PBS containing 1% bovine serum albumin for 1 h at 37°C. Serum previously diluted 1:10 in PBS was added to the wells and serially diluted, and the plates were incubated for 1 h at 37°C. After the wells were washed with PBS containing 0.05% Tween 20, a 1:3,000 dilution of HRP-conjugated goat anti-mouse IgG (The Binding Site) was added and incubated for 1 h. Following further washing, substrate solution [2,2′-azinobis(3-ethylbenthiazolinesulfonic acid) (ABTS); Sigma] was added. After 30 min, the optical density was determined at 405 nm. For antibody isotyping ELISAs, HRP-conjugated goat anti-mouse IgG1, IgG2a, IgG2b, and IgG3 were used (The Binding Site).
For the inhibition ELISAs, coating, blocking, and addition of diluted sera were done as described above. However, an extra step involving the incubation of MAb 302 (1 μg/ml) for 1 h was performed after the serum had been washed from the plate. The MAb 302 was then washed from the plates prior to the addition of a 1:3,000 dilution of HRP-conjugated goat anti-mouse IgG3 (The Binding Site) to detect the level of MAb 302 binding to yMSP119.
Statistical analysis.
Data from the yMSP119-DT experiments were analyzed using Spearman rank correlation, Kruskal-Wallis test, or Mann-Whitney test. Values for P of <0.05 in the Kruskal-Wallis or Mann-Whitney test were considered statistically significant. The total area under the curve was used as an indicator of total antibody produced.
RESULTS
Determination of antibody response and protection in different strains of mice following vaccination with yMSP119.
To assess the roles of H-2 genes on the immunogenicity of yMSP119, five inbred mouse strains, C57BL/10 (H-2b), BALB/c (H-2d), B10.D2 (H-2d), B10.BR (H-2k), and B10.S (H-2s), and one outbred strain (Quackenbush) were immunized with yMSP119. Following completion of the immunization schedule, mice were challenged with P. yoelii YM.
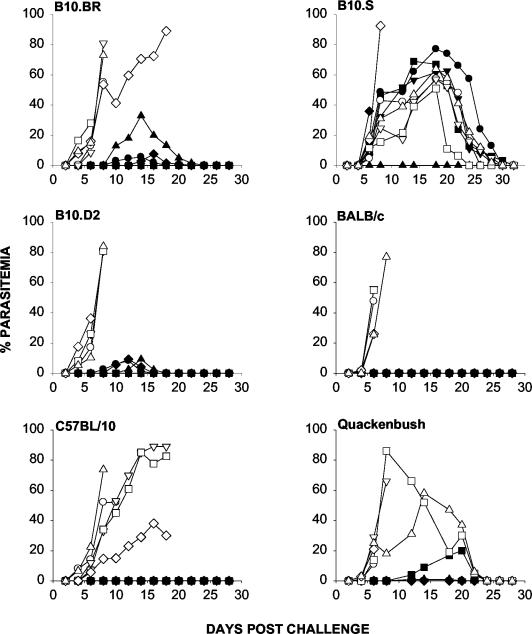
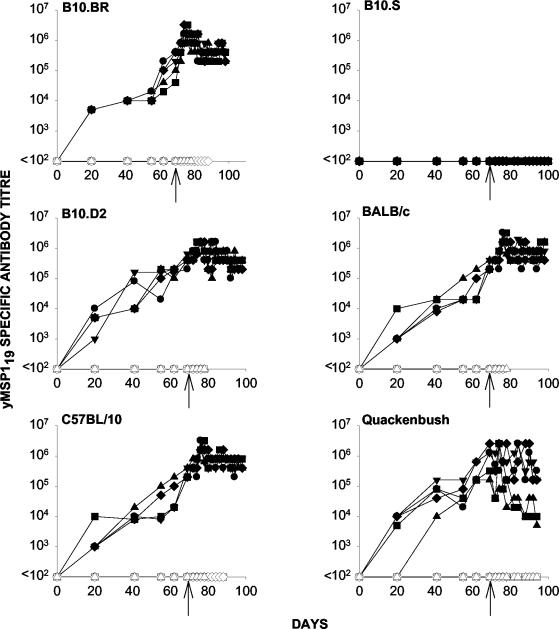
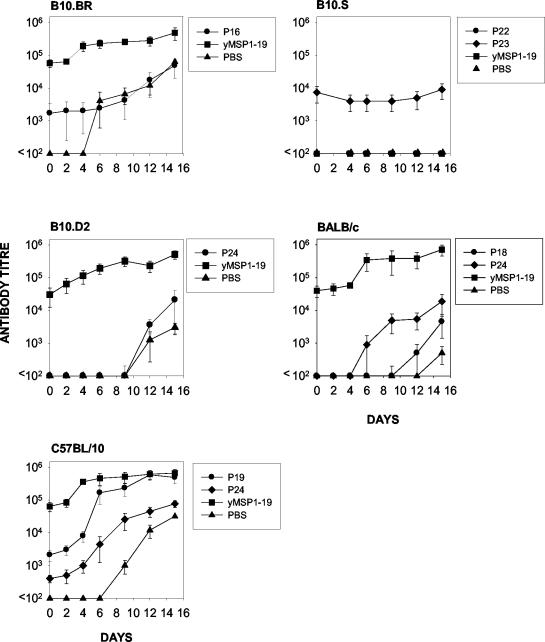
Survival was observed in all yMSP119-immunized groups tested, although the levels of protection (as determined by peak parasitemia) varied in the different mouse strains (Fig. 1). Control mice (immunized with PBS) and B10.S mice immunized with yMSP119 developed a high parasitemia. Parasites were not detected in BALB/c and C57BL/10 mice immunized with yMSP119 (Fig. 1). With the exception of B10.S mice, all strains generated yMSP119-specific antibodies following immunization (Fig. 2).
FIG. 1.
The course of parasitemia in different strains of mice immunized with yMSP119 (closed symbols) and PBS (open symbols) in FA and subsequently challenged with P. yoelii YM. Mice were immunized on day 0 with 20 μg of yMSP119 in CFA and then given booster doses of 20 μg of yMSP119 in IFA on days 21, 42, and 56. A final booster dose of 20 μg of yMSP119 in PBS was given on day 63. Mice were challenged i.v. on day 70 with 104 P. yoelii YM parasitized parasitized red blood cells. Symbols depict parasitemia in individual mice.
FIG. 2.
yMSP119-specific antibody titers as measured by ELISA for mice immunized with yMSP119 (closed symbols) and PBS (open symbols) in FA and subsequently challenged with P. yoelii YM. Mice were immunized on day 0 with 20 μg of yMSP119 in CFA and then given booster doses of 20 μg of yMSP119 in IFA on days 21, 42, and 56. A final booster dose of 20 μg of yMSP119 in PBS was given on day 63. Mice were challenged i.v. on day 70 with 104 P. yoelii parasitized red blood cells (indicated by arrows). Day 70 on these graphs is equivalent to day 0 in Fig. 1. Symbols depict antibody titer in individual mice.
Determination of T-cell proliferative responses for peptides derived from yMSP119.
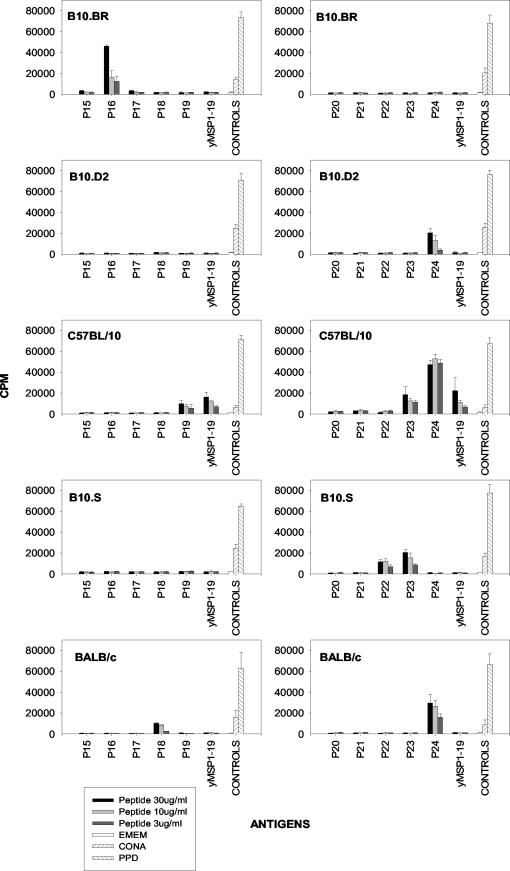
To determine the T-cell determinants on yMSP119, mice were immunized with pools of synthetic overlapping peptides spanning yMSP119 (Table 1). Peptide pool 1 contained peptides 15, 16, 17, 18, and 19, and peptide pool 2 contained peptides 20, 21, 22, 23, and 24. The draining lymph nodes were taken 7 to 9 days later to assess responsiveness.
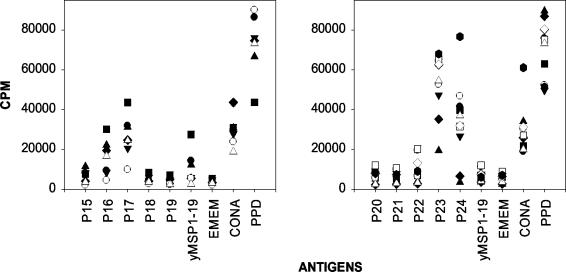
As had been previously shown (30), C57BL/10 mice responded to peptides 19, 23, 24, and yMSP119, whereas BALB/c mice responded to peptides 18 and 24 (Fig. 3). We now show that B10.D2 mice, with the same H-2 haplotype as BALB/c mice, responded to peptide 24, but not peptide 18. We observed that B10.BR (H-2k) mice responded to peptide 16, while B10.S (H-2s) mice responded to peptides 22 and 23 (Fig. 3). C57BL/10 mice were the only inbred mouse strain able to respond to yMSP119 following peptide immunization. Quackenbush (outbred) mice had different responses, but each mouse recognized multiple peptides. The most frequent responses were to peptides 16, 17, 23, and 24 (Fig. 4). They were also able to respond to yMSP119.
FIG.3.
Antigens recognized by T cells from the draining lymph nodes of different strains of mice immunized with pools of overlapping peptides derived from yMSP119. T-cell proliferative responses of mice immunized with peptide pool 1 (peptides 15 to 19 [P15 to P19]) (left graphs) or with peptide pool 2 (peptides 20 to 24 [P20 to P24]) (right graphs) are shown. Cells were incubated with various concentrations (30, 10, or 3 μg/ml) of peptide or with purified protein derivative (PPD), concanavalin A (CONA), and Eagle minimum essential medium as controls. Bars are the means plus 95% confidence intervals (error bars) for the 10 mice in each group.
FIG. 4.
Antigens recognized by T cells from draining lymph nodes of Quackenbush mice immunized with pools of overlapping peptides derived from yMSP119. T-cell proliferative responses of mice immunized with peptide pool 1 (peptides 15 to 19 [P15 to P19]) (left) or with peptide pool 2 (peptides 20 to 24 [P20 to P24]) (right) are shown. The cells were plated in triplicate, and each symbol is the mean of an individual mouse's response to each peptide. The peptide and protein concentration used to stimulate the T cells was 30 μg/ml. Cells were incubated with purified protein derivative (PPD), concanavalin A (CONA), and Eagle minimum essential medium as controls.
As B10.S mice did not produce antibodies following yMSP119 immunization yet responded to peptides following immunization with peptide pool 2, they were also immunized with the recombinant protein to determine peptide and protein responses. Following protein immunization, T cells from B10.S mice did not respond to any of the peptides or yMSP119 (data not shown).
Induction of T-cell help by MSP119 epitopes.
We then asked whether proliferative T-cell epitopes could activate helper T cells. Mice were primed with putative peptide epitopes, and 3 weeks later, the mice were given booster doses of yMSP119 using an established assay (10, 30). yMSP119-specific antibody responses were then measured to determine whether priming with the peptide resulted in a more rapid antibody response after the yMSP119 booster dose.
At the time of the yMSP119 booster dose, mice that had previously been immunized with yMSP119 or with one of several peptides had yMSP119-specific antibody that was detectable by ELISA (Fig. 5). BALB/c mice primed with p18 or p24 and C57BL/10 mice primed with p19 or p24 had an earlier and more rapid yMSP119 antibody response than those primed with PBS, in agreement with previous studies (30). However, in B10.S, B10.BR, and B10.D2 mice primed with proliferative peptide epitopes, the rate of development of the yMSP119 antibody response was similar to that in mice primed with PBS, indicating that the peptides did not contain T-helper epitopes.
FIG. 5.
yMSP119-specific antibody responses in different strains of mice primed with the immunodominant peptide(s) specific for that mouse strain, yMSP119, or PBS and then given a booster dose of yMSP119 on day 0. Bars are the means ± 95% confidence intervals (error bars) for the five mice in each group. Peptides 16 (P16), 18 (P18), 19 (P19), 22 (P22), 23 (P23), and 24 (P24) were used to prime mice.
Preparation and immunogenicity of recombinant protein-DT conjugate.
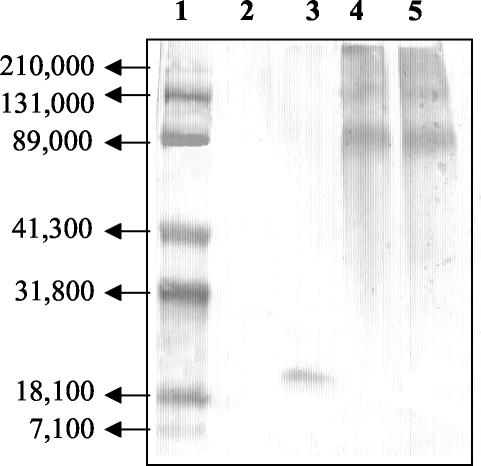
The above data indicated that B10.S mice were absolute nonresponders, and there was no evidence that they recognized any helper T-cell epitopes on yMSP119. To attempt to induce a yMSP119-specific antibody response in these mice, yMSP119 was chemically conjugated to DT. Two different conjugation procedures utilizing two different chemical linkers were used, SPDP and MCS. (Their chemistry is outlined in Materials and Methods.) The results of Western blot analysis showed that both conjugates reacted with yMSP119 HIS (Fig. 6). These bands were also recognized by MAb 302 (a protective monoclonal antibody that recognizes a conformational epitope in the first EGF-like domain of MSP119 [20]) and DT HIS [data not shown]. The absence of a band at a molecular weight of 19,000 indicated that the mixture contained only conjugated yMSP119 (Fig. 6). Responder C57BL/10 mice and nonresponder B10.S mice were immunized with PBS, DT, yMSP119, yMSP119-DT (MCS), or yMSP119-DT (SPDP) and challenged with malaria parasites to assess the ability of the conjugates to elicit a protective antibody response.
FIG. 6.
Western blot of yMSP119-DT conjugates probed with yMSP119-specific HIS. Lane 1, molecular weight markers; lane 2, DT; lane 3, yMSP119; lane 4, yMSP119-DT (MCS); lane 5, yMSP119-DT (SPDP).
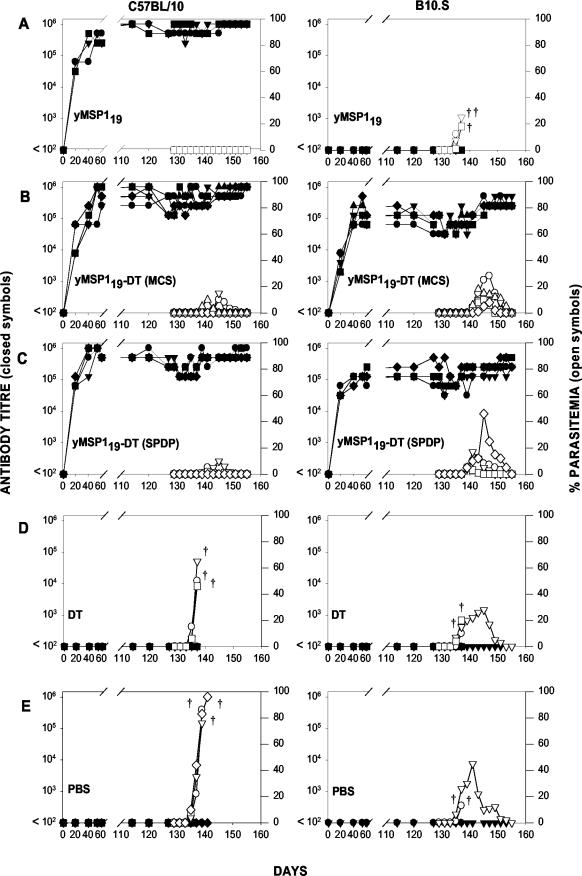
C57BL/10 mice immunized with yMSP119 produced specific antibodies and were protected from lethal challenge, whereas the B10.S mice immunized with yMSP119 did not produce yMSP119-specific antibodies and all these mice succumbed to challenge infection (Fig. 7A). However, both strains of mice immunized with either yMSP119-DT (MCS) or yMSP119-DT (SPDP) produced yMSP119-specific antibodies and were protected (Fig. 7B and C). C57BL/10 mice immunized with yMSP119-DT (MCS) and yMSP119-DT (SPDP) developed higher yMSP119-specific antibody titers (mean maximum antibody titers of 1,024,000 and 1,024,000, respectively) than B10.S mice (mean maximum antibody titers of 358,400 and 384,000, respectively) and exhibited a higher degree of protection (mean peak parasitemias of 7.2 and 4%, respectively) than B10.S mice (mean peak parasitemias of 14.8 and 20%, respectively) (Fig. 7B and C). Mice immunized with DT or PBS and the B10.S mice immunized with yMSP119 were not protected (Fig. 7D and E). Sera from mice immunized with yMSP119-DT (MCS) or yMSP119-DT (SPDP) recognized conjugated yMSP119 and DT (data not shown).
FIG.7.
Immunogenicity of yMSP119-DT conjugates. yMSP119-specific antibody titers (left axis, closed symbols) and the course of parasitemia (right axis, open symbols) in C57BL/10 (left) and B10.S (right) mice immunized with yMSP119, yMSP119-DT (MCS), yMSP119-DT (SPDP), DT, or PBS. Mice were challenged i.v. with 104 P. yoelii YM parasitized red blood cell on day 127. Antibody titers and parasitemias were determined for the duration of the experiment, which concluded on day 155. Termination of the parasitemia and antibody titer curves on the graphs before day 155 indicates that the mouse succumbed to malaria infection and is represented by daggers.
The yMSP119-specific antibody response in both strains of conjugate-immunized mice was predominantly IgG1 and IgG2b, irrespective of which conjugate was used for immunization (data not shown).
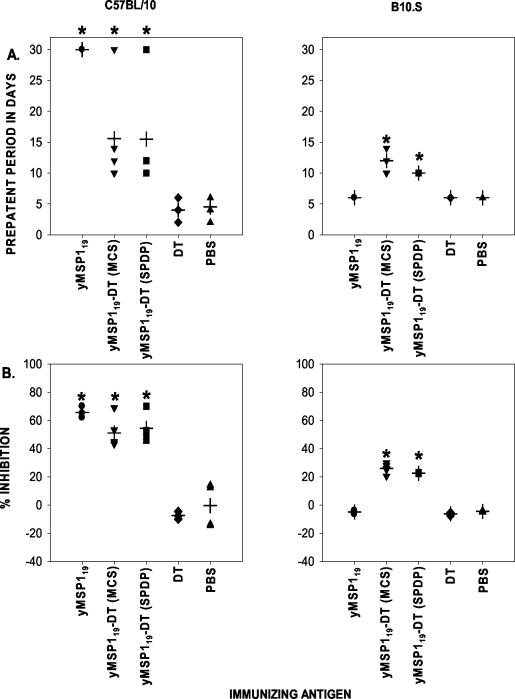
After the immunized mice were challenged, there was a significant delay (P < 0.05) in the prepatent period (Fig. 8A) —the best indicator of effective MSP119-specific antibody function (14). The mean prepatent period was longest in the C57BL/10 mice immunized with yMSP119 (Fig. 8A). C57BL/10 and B10.S mice immunized with yMSP119-DT (MCS) or yMSP119-DT (SPDP) had extended prepatent periods that were significantly different (P < 0.05) from the prepatent periods seen for the groups of mice immunized with PBS or DT and for B10.S mice immunized with yMSP119.
FIG. 8.
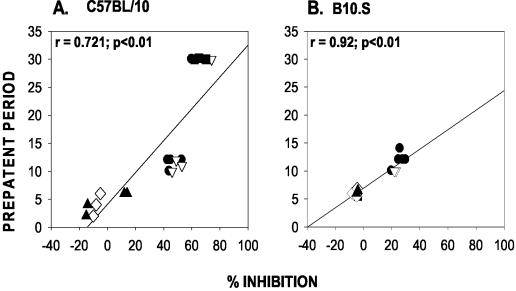
Ability of the immunizing antigens in the conjugation experiments to generate antibodies capable of affecting parasite growth and to bind to known protective epitopes. (A) Prepatent periods for the experimental groups. The median prepatent period of each group is indicated by a cross. Blood smears were examined until 30 days after parasite challenge, and mice that had not developed parasitemia by this time were allocated a prepatent period of 30 days. (B) Ability of sera from the experimental groups to inhibit the binding of MAb 302 to yMSP119. The median percent inhibition of each group is indicated by a cross. Values for experimental groups that were significantly different (P < 0.05) from the values for both control groups (mice immunized with DT or PBS and B10.S mice immunized with yMSP119) are indicated by asterisks.
Fine specificity of the antibody response.
MAb 302 binds to an epitope in the first EGF-like domain of MSP119 and confers protection when it is passively transferred to mice (20). Recognition of this epitope could be used as a predictive indicator of protection after challenge. Competition ELISAs were performed to determine whether serum samples from immunized mice recognized this epitope.
Sera from C57BL/10 mice immunized with yMSP119, yMSP119-DT (MCS), or yMSP119-DT (SPDP) were more effective at inhibiting the binding of MAb 302 to yMSP119 than the sera from yMSP119 conjugate-immunized B10.S mice (Fig. 8B). However, sera from B10.S mice immunized with yMSP119-DT (MCS) or yMSP119-DT (SPDP) could significantly inhibit the binding of MAb 302 (P < 0.05) compared with sera from C57BL/10 and B10.S mice immunized with DT or PBS and B10.S mice immunized with yMSP119 (Fig. 8B). Overall, the percent inhibition values for both mouse strains immunized with the conjugates and for C57BL/10 mice immunized with yMSP119 were significantly different (P < 0.05) from the values for the control groups immunized with DT or PBS and for B10.S mice immunized with yMSP119. In both C57BL/10 and B10.S mice, there was a significant positive correlation between the level of inhibition exhibited by the antibodies in the serum and the length of the prepatent period (r = 0.721 and 0.92, respectively; P < 0.01 for both groups) (Fig. 9).
FIG. 9.
Relationship between the ability of the antibodies in the sera from the different experimental groups to inhibit the binding of MAb 302 to yMSP119 and affect the length of the prepatent period in C57BL/10 and B10.S mice. Mice were immunized with yMSP119-DT (MCS) (•), yMSP119-DT (SPDP) (▿), and yMSP119 (▪) and with DT (⋄) and PBS (▴) as controls.
Determining the effect of an existing immune response to the carrier protein (DT) on a subsequent immune response to yMSP119.
While DT conjugation can overcome immunological nonresponsiveness, we were concerned that epitopic suppression as a result of prior exposure to DT may compromise a potential DT-based conjugate (11, 27). We thus asked whether an existing immune response to DT could interfere with the development of an immune response to yMSP119 after immunization with a yMSP119-DT conjugate. C57BL/10 and B10.S mice were immunized s.c. with 20 μg of yMSP119, yMSP119-DT (SPDP), DT, or PBS in CFA. Twenty-one days later, they were immunized s.c. with 20 μg of yMSP119-DT (SPDP) in IFA. Sera were tested for the production of yMSP119-specific antibodies on day 21 after immunization.
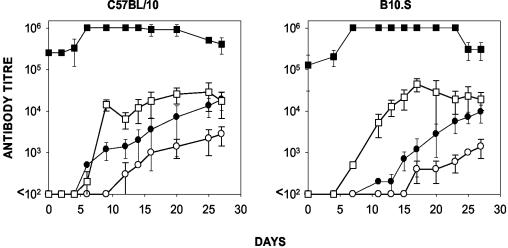
C57BL/10 and B10.S mice preimmunized with DT were able to produce yMSP119-specific antibodies after they were given booster doses of the conjugate (Fig. 10). Indeed, yMSP119-specific antibodies were detected earlier (P < 0.01 for both mouse strains) and more antibody was produced (P < 0.01 for both mouse strains) (Materials and Methods) in mice preimmunized with DT than in mice preimmunized with PBS, indicating that the presence of DT antibodies at the time of yMSP119-DT immunization did not interfere with the production of yMSP119-specific antibodies (Fig. 10) but instead enhanced it.
FIG. 10.
Effects of preexisting DT-specific antibodies on a subsequent immune response to yMSP119 in C57BL/10 and B10.S mice. The yMSP119-specific antibody titers (circles) and DT-specific antibody titers (squares) in mice preimmunized with DT (closed symbols) or PBS (open symbols) in CFA. The mice were given booster doses of yMSP119 in IFA 21 days later. Day 21 of the immunization schedule is shown as day 0 on these graphs. Each point represents the mean ± 95% confidence intervals (error bars) for five mice.
DISCUSSION
This study has demonstrated that it is possible to generate protective antibodies after immunization with yMSP119 in a number of inbred and outbred mouse strains with different H-2 genes. The inability to produce antibodies specific for yMSP119 after immunization would appear to be controlled by I-As, given that H-2s mice do not express an I-E molecule (Fig. 2). This immunological nonresponsiveness was related to an absence of T helper epitopes on yMSP119 recognized by B10.S mice and could be overcome by chemical modification of yMSP119 with a known source of T helper epitopes, DT. Furthermore, DT preimmunization markedly enhanced the response to the conjugate.
In a previous study using GST-MSP119, H-2k mice were not protected after immunization (31). They did not examine the ability of H-2s mice to respond to immunization with GST-MSP119. Interestingly, a different pattern of nonresponsiveness was observed in the experiment presented here, with H-2k mice exhibiting protection and H-2s mice failing to generate antibodies when immunized with yMSP119 alone. There are a number of possible explanations for the differences observed for recombinant proteins from the different expression systems. The yMSP119 was expressed in S. cerevisiae, as opposed to GST-MSP119, which is produced in E. coli. It is likely that the presence of a hexahistidine tail at the C-terminal end of the yMSP119 molecule created an epitope not present in the GST-MSP119 molecule that is recognized in the context of H-2k. These results highlight the significance of choosing the appropriate expression system and fusion partners for vaccine molecules.
As MSP119-mediated protection is reliant on high-titer antibodies present at the time of challenge (12, 13), T-cell studies were conducted to ascertain T helper responses and to determine whether there was a correlation between the T-cell determinants recognized and the subsequent antibody-mediated protection. The proliferative T-cell responses differed in the strains of mice, with T cells from each strain exhibiting a different pattern of response to yMSP119 peptides (Fig. 3). B10.D2 and BALB/c mice express the same alleles at the H-2 loci, yet it is interesting that T cells from B10.D2 mice respond only to peptide 24, while T cells from BALB/c mice respond to peptides 18 and 24. It is possible that regions outside the major histocompatibility complex (MHC) are also influencing the immune response to the peptides. The role of MHC- and non-MHC-associated genes in determining the immune response to malaria antigens was the subject of a recent review (25). The peptides recognized by B10.S mice after protein immunization are cryptic epitopes within yMSP119, as immunization with the recombinant protein did not produce a response to peptide 22 or 23. When the T-cell responses were examined in parallel with protection studies, there appeared to be no association between T-cell recognition of a particular peptide after peptide immunization and protection after parasite challenge. This is in keeping with the previous observation of Tian et al. (30) that vaccination of mice with defined T-cell epitopes of MSP119 would not protect them from parasite challenge. In the analysis of this experiment, we cannot exclude the possibility that one or more H-2-restricted epitopes were not represented by the series of overlapping peptides.
A successful vaccine against malaria will be able to immunize across a wide range of MHC class II haplotypes and elicit high levels of protection in its recipients. MHC-restricted responses are a concern, particularly in subunit vaccines where the necessary T-cell epitope(s) may not be incorporated. Vaccines containing MSP119 will also be restricted by the ability of T cells to recognize and respond to the T-cell epitopes within the protein. Studies performed with P. falciparum MSP119 demonstrated that T-cell responses to the protein were found in only 26% of naturally infected individuals tested and suggested that the complex structure of the native protein may be inhibiting antigen processing or presentation (9). This illustrates the need to develop strategies to overcome nonresponsiveness. The present study linked DT, a source of T helper epitopes, to yMSP119 in an attempt to overcome the MHC-linked nonresponsiveness observed in H-2s mice. The carrier, DT, primed T helper cells for a secondary response to yMSP119. This phenomenon of carrier priming has previously been reported for different conjugates (23, 29). This antigenic modification of yMSP119 resulted in an immunogenic conjugate which enabled H-2s mice to respond without eliminating the responses observed in another mouse haplotype (H-2b).
Although both mouse strains immunized with the conjugates controlled parasitemia, the mice exhibited different levels of protection, with the B10.S mice having higher levels of parasitemia (Fig. 7). The higher peak parasitemia in the B10.S mice compared to the C57BL/10 mice is most likely a reflection of the lower antibody titers in this mouse strain.
The C57BL/10 mice that received yMSP119 alone did not develop patent parasitemia, yet the mice immunized with the conjugates developed low-level, controlled parasitemia (Fig. 7). The mice immunized with the conjugates also had a lower mean yMSP119-specific antibody titer than the mice immunized with yMSP119 alone. The course of parasitemia was in part influenced by the mouse strain and the amount of antibody present at the time of challenge, which is related to the total amount of yMSP119 received in the immunizations. The C57BL/10 mice immunized with yMSP119 received 20 μg of the recombinant protein, while the mice immunized with the conjugate received 20 μg of total protein, which included both yMSP119 and DT. A great amount of the antibody produced by the mice receiving the conjugate was specific for DT (data not shown) and may have diminished the MSP119-specific response. Increasing the total amount of conjugate given to the mice may result in a greater antibody response to yMSP119.
The rationale for the addition of T-cell epitopes to subunit vaccines is to increase the percentage of the population that can respond. After vaccination with an antigen coupled to an unrelated T-cell epitope, the level of boosting that would result from natural infection is unclear; however, in individuals who could not respond to the antigen alone, boosting would not be predicted. The most likely outcome of vaccination with an MSP119 conjugate would be the production of a high titer of antibody that would suppress the parasitemia to a low level. This reduction in parasite burden would allow the recipient of the vaccine to survive the infection and give the recipient time to develop additional parasite-specific immune responses independent of the vaccine (14). The results presented here expand upon a previous study that expressed a GST fusion protein containing MSP119 with a defined T-cell epitope inserted between GST and MSP119 (1).
While the phenomenon of epitope-specific suppression (11, 27) should be addressed when using proteins, such as DT and tetanus toxoid, as a carrier for haptens, the carrier priming experiment in this study showed that the presence of an existing immune response to DT enhanced rather than suppressed a subsequent immune response to yMSP119 when yMSP119 was delivered as a DT conjugate (Fig. 10). It is probable that the initial immunization with the carrier, DT, resulted in the expansion of a population of T helper cells that upon subsequent immunization with the yMSP119-DT conjugate, facilitated a more rapid yMSP119-specific antibody response. These observations suggest that the presence of a DT-specific immune response in humans vaccinated by the normal vaccination regimen may in fact assist the development of an immune response to yMSP119 when delivered in the form of a DT conjugate. Further studies will be conducted to ascertain whether an immunization regimen consisting of a carrier preimmunization followed by multiple conjugate immunizations can induce a protective immune response.
When considering the use of vaccines in humans, it is necessary to address safety issues. Human studies have been undertaken to examine the side effect profile of immunizations containing carrier proteins, such as DT and tetanus toxoid (16, 24). Both studies observed that hypersensitivity reactions tended to occur after the third immunization with the conjugate and proposed that changes to formulation and immunization schedule (specifically the time between doses) may improve the safety profile. The immunization strategy outlined in the present study was a proof-of-principle study in mice, addressing issues, such as immunogenicity and epitopic suppression versus carrier priming. If further studies were to demonstrate that these conjugates were immunogenic in humans, a possible strategy to overcome hypersensitivity reactions induced by multiple doses would be to use MSP119-DT in place of DT in the normal vaccine schedule. This could simultaneously limit the number of additional immunizations required and exploit any preexisting immune response to the carrier, further enhancing the conjugate's immunogenicity.
In conclusion, it was possible to overcome the genetic restriction seen in B10.S mice when immunizing with yMSP119. The addition of helper T epitopes present in DT to yMSP119 resulted in an immunogen that was able to induce antibodies and protective immunity in previously totally nonresponsive, P. yoelii-susceptible mice. DT may be a preferred conjugate partner for MSP119, as prior exposure to DT subsequently enhances the immune response to the conjugate. These results may have an important application when considering ways to enhance the immunogenicity of P. falciparum yMSP119 vaccines for use in the human population.
Acknowledgments
This work was supported in part by the National Health and Medical Research Council of Australia and by the United National Development Program/World Bank/World Health Organization Special Programme for Research and Training in Tropical Diseases.
We thank Carole Long for providing MAb 302 and Michelle Gatton for advice on statistical analysis.
Editor: S. H. E. Kaufmann
REFERENCES
- 1.Ahlborg, N., I. T. Ling, A. A. Holder, and E. M. Riley. 2000. Linkage of exogenous T-cell epitopes to the 19-kilodalton region of Plasmodium yoelii merozoite surface protein 1 (MSP119) can enhance protective immunity against malaria and modulate the immunoglobulin subclass response to MSP119. Infect. Immun. 68:2102-2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blackman, M. J., H. G. Heidrich, S. Donachie, J. S. McBride, and A. A. Holder. 1990. A single fragment of a malaria merozoite surface protein remains on the parasite during red cell invasion and is the target of invasion-inhibiting antibodies. J. Exp. Med. 172:379-382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blackman, M. J., and A. A. Holder. 1992. Secondary processing of the Plasmodium falciparum merozoite surface protein-1 (MSP1) by a calcium-dependent membrane-bound serine protease: shedding of MSP133 as a noncovalently associated complex with other fragments of the MSP1. Mol. Biochem. Parasitol. 50:307-315. [DOI] [PubMed] [Google Scholar]
- 4.Blackman, M. J., H. Whittle, and A. A. Holder. 1991. Processing of the Plasmodium falciparum major merozoite surface protein-1: identification of a 33-kilodalton secondary processing product which is shed prior to erythrocyte invasion. Mol. Biochem. Parasitol. 49:35-44. [DOI] [PubMed] [Google Scholar]
- 5.Burns, J. M., Jr., W. R. Majarian, J. F. Young, T. M. Daly, and C. A. Long. 1989. A protective monoclonal antibody recognizes an epitope in the carboxyl-terminal cysteine-rich domain in the precursor of the major merozoite surface antigen of the rodent malarial parasite, Plasmodium yoelii. J. Immunol. 143:2670-2676. [PubMed] [Google Scholar]
- 6.Chang, S. P., S. E. Case, W. L. Gosnell, A. Hashimoto, K. J. Kramer, L. Q. Tam, C. Q. Hashiro, C. M. Nikaido, H. L. Gibson, C. T. Lee-Ng, P. J. Barr, B. T. Yokota, and G. S. Hut. 1996. A recombinant baculovirus 42-kilodalton C-terminal fragment of Plasmodium falciparum merozoite surface protein 1 protects Aotus monkeys against malaria. Infect. Immun. 64:253-261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Daly, T. M., and C. A. Long. 1993. A recombinant 15-kilodalton carboxyl-terminal fragment of Plasmodium yoelii yoelii 17XL merozoite surface protein 1 induces a protective immune response in mice. Infect. Immun. 61:2462-2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Di John, D., S. S. Wasserman, J. R. Torres, M. J. Cortesia, J. Murillo, G. A. Losonsky, D. A. Herrington, D. Sturcher, and M. M. Levine. 1989. Effect of priming with carrier on response to conjugate vaccine. Lancet 2:1415-1418. [DOI] [PubMed] [Google Scholar]
- 9.Egan, A., M. Waterfall, M. Pinder, A. Holder, and E. Riley. 1997. Characterization of human T- and B-cell epitopes in the C terminus of Plasmodium falciparum merozoite surface protein 1: evidence for poor T-cell recognition of polypeptides with numerous disulfide bonds. Infect. Immun. 65:3024-3031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Good, M., W. Maloy, M. Lunde, H. Margalit, J. Cornette, G. Smith, B. Moss, L. Miller, and J. Berzofsky. 1987. Construction of synthetic immunogen: use of new T-helper epitope on malaria circumsporozoite protein. Science 235:1059-1062. [DOI] [PubMed] [Google Scholar]
- 11.Herzenberg, L. A., and T. Tokuhisa. 1980. Carrier-priming leads to hapten-specific suppression. Nature 285:664-667. [DOI] [PubMed] [Google Scholar]
- 12.Hirunpetcharat, C., D. Stanisic, X. Q. Liu, J. Vadolas, R. A. Strugnell, R. Lee, L. H. Miller, D. C. Kaslow, and M. F. Good. 1998. Intranasal immunization with yeast-expressed 19 kD carboxyl-terminal fragment of Plasmodium yoelii merozoite surface protein-1 (yMSP119) induces protective immunity to blood stage malaria infection in mice. Parasite Immunol. 20:413-420. [DOI] [PubMed] [Google Scholar]
- 13.Hirunpetcharat, C., J. H. Tian, D. C. Kaslow, N. van Rooijen, S. Kumar, J. A. Berzofsky, L. H. Miller, and M. F. Good. 1997. Complete protective immunity induced in mice by immunization with the 19-kilodalton carboxyl-terminal fragment of the merozoite surface protein-1 (MSP119) of Plasmodium yoelii expressed in Saccharomyces cerevisiae: correlation of protection with antigen-specific antibody titer, but not with effector CD4+ T cells. J. Immunol. 159:3400-3411. [PubMed] [Google Scholar]
- 14.Hirunpetcharat, C., P. Vukovic, X. Q. Liu, D. C. Kaslow, L. H. Miller, and M. F. Good. 1999. Absolute requirement for an active immune response involving B cells and Th cells in immunity to Plasmodium yoelii passively acquired with antibodies to the 19-kDa carboxyl-terminal fragment of merozoite surface protein-1. J. Immunol. 162:7309-7314. [PubMed] [Google Scholar]
- 15.Hui, G. S., W. L. Gosnell, S. E. Case, C. Hashiro, C. Nikaido, A. Hashimoto, and D. C. Kaslow. 1994. Immunogenicity of the C-terminal 19-kDa fragment of the Plasmodium falciparum merozoite surface protein 1 (MSP1), yMSP119 expressed in S. cerevisiae. J. Immunol. 153:2544-2553. [PubMed] [Google Scholar]
- 16.Keitel, W. A., K. E. Kester, R. L. Atmar, A. C. White, N. H. Bond, C. A. Holland, U. Krzych, D. R. Palmer, A. Egan, C. Diggs, W. R. Ballou, B. F. Hall, and D. Kaslow. 1999. Phase I trial of two recombinant vaccines containing the 19kd carboxy terminal fragment of Plasmodium falciparum merozoite surface protein 1 (MSP-119) and T helper epitopes of tetanus toxoid. Vaccine 18:531-539. [DOI] [PubMed] [Google Scholar]
- 17.Kumar, A., R. Arora, P. Kaur, V. S. Chauhan, and P. Sharma. 1992. “Universal” T helper cell determinants enhance immunogenicity of a Plasmodium falciparum merozoite surface antigen peptide. J. Immunol. 148:1499-1505. [PubMed] [Google Scholar]
- 18.Kumar, S., A. Yadava, D. B. Keister, J. H. Tian, M. Ohl, K. A. Perdue-Greenfield, L. H. Miller, and D. C. Kaslow. 1995. Immunogenicity and in vivo efficacy of recombinant Plasmodium falciparum merozoite surface protein-1 in Aotus monkeys. Mol. Med. 1:325-332. [PMC free article] [PubMed] [Google Scholar]
- 19.Ling, I. T., S. A. Ogun, and A. A. Holder. 1994. Immunization against malaria with a recombinant protein. Parasite Immunol. 16:63-67. [DOI] [PubMed] [Google Scholar]
- 20.Majarian, W. R., T. M. Daly, W. P. Weidanz, and C. A. Long. 1984. Passive immunization against murine malaria with an IgG3 monoclonal antibody. J. Immunol. 132:3131-3137. [PubMed] [Google Scholar]
- 21.Miller, J. F., and G. F. Mitchell. 1967. The thymus and the precursors of antigen reactive cells. Nature 216:659-663. [DOI] [PubMed] [Google Scholar]
- 22.Pan, W., E. Ravot, R. Tolle, R. Frank, R. Mosbach, I. Turbachova, and H. Bujard. 1999. Vaccine candidate MSP-1 from Plasmodium falciparum: a redesigned 4917 bp polynucleotide enables synthesis and isolation of full-length protein from Escherichia coli and mammalian cells. Nucleic Acids Res. 27:1094-1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Peeters, C. C., A. M. Tenbergen-Meekes, J. T. Poolman, M. Beurret, B. J. Zegers, and G. T. Rijkers. 1991. Effect of carrier priming on immunogenicity of saccharide-protein conjugate vaccines. Infect. Immun. 59:3504-3510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ramasamy, R., D. A. Wijesundere, K. Nagendran, and M. S. Ramasamy. 1995. Antibody and clinical responses in volunteers to immunization with malaria peptide-diphtheria toxoid conjugates. Clin. Exp. Immunol. 99:168-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Riley, E. M. 1996. The role of MHC- and non-MHC-associated genes in determining the human immune response to malaria antigens. Parasitology 112(Suppl.):S39-S51. [PubMed]
- 26.Schutze, M. P., E. Deriaud, G. Przewlocki, and C. LeClerc. 1989. Carrier-induced epitopic suppression is initiated through clonal dominance. J. Immunol. 142:2635-2640. [PubMed] [Google Scholar]
- 27.Schutze, M. P., C. Leclerc, M. Jolivet, F. Audibert, and L. Chedid. 1985. Carrier-induced epitopic suppression, a major issue for future synthetic vaccines. J. Immunol. 135:2319-2322. [PubMed] [Google Scholar]
- 28.Schutze, M. P., C. Leclerc, F. R. Vogel, and L. Chedid. 1987. Epitopic suppression in synthetic vaccine models: analysis of the effector mechanisms. Cell. Immunol. 104:79-90. [DOI] [PubMed] [Google Scholar]
- 29.Shah, S., R. Raghupathy, O. Singh, G. P. Talwar, and A. Sodhi. 1999. Prior immunity to a carrier enhances antibody responses to hCG in recipients of an hCG-carrier conjugate vaccine. Vaccine 17:3116-3123. [DOI] [PubMed] [Google Scholar]
- 30.Tian, J. H., M. F. Good, C. Hirunpetcharat, S. Kumar, I. T. Ling, D. Jackson, J. Cooper, J. Lukszo, J. Coligan, J. Ahlers, A. Saul, J. A. Berzofsky, A. A. Holder, L. H. Miller, and D. C. Kaslow. 1998. Definition of T cell epitopes within the 19 kDa carboxyl terminal fragment of Plasmodium yoelii merozoite surface protein 1 (MSP119) and their role in immunity to malaria. Parasite Immunol. 20:263-278. [DOI] [PubMed] [Google Scholar]
- 31.Tian, J. H., L. H. Miller, D. C. Kaslow, J. Ahlers, M. F. Good, D. W. Alling, J. A. Berzofsky, and S. Kumar. 1996. Genetic regulation of protective immune response in congenic strains of mice vaccinated with a subunit malaria vaccine. J. Immunol. 157:1176-1183. [PubMed] [Google Scholar]
- 32.Wipasa, J., H. Xu, M. Makobongo, M. Gatton, A. Stowers, and M. F. Good. 2002. Nature and specificity of the required protective immune response that develops postchallenge in mice vaccinated with the 19-kilodalton fragment of Plasmodium yoelii merozoite surface protein 1. Infect Immun. 70:6013-6020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.World Health Organization. 1999. Rolling back malaria, p. 49-63. In The world health report, 1999: making a difference. World Health Organization, Geneva, Switzerland.