Abstract
By the use of surface plasmon resonance spectroscopy, immunoglobulin G (IgG) subclass and IgM antibodies against three schistosome-derived carbohydrate structures, FLDN (Fucα1-3GalNAcβ1-4GlcNAcβ1-3Galα1), LDN-DF [GalNAcβ1-4(Fucα1-2Fucα1-3)GlcNAcβ1], and LDNF [GalNAcβ1-4(Fucα1-3)GlcNAcβ1-3Galα1], were measured in 184 previously unexposed Kenyan immigrants who moved into the Masongaleni area, where Schistosoma mansoni is endemic. They were sampled within their first year of exposure and again 2 years later. A cohort selected out of the original residents of the area, who had been exposed for many years, served as controls. Associations with responses to S. mansoni worm, egg (SEA), and cercarial (CERC) antigens were examined. In addition, we measured responses to keyhole limpet hemocyanin, a glycoprotein which carries glycan epitopes that are also expressed by schistosomes. Specific IgG1 responses were most pronounced against FLDN and LDN-DF and strongly associated with those previously measured to SEA and CERC. Similarly to previously published age profiles of IgG1 and IgG2 responses to SEA, levels of IgG1 against LDN-DF decreased with age. In contrast, specific IgM responses against the three schistosome-derived carbohydrate structures were most marked against LDNF. Our results indicate that, of the three glycan structures tested, the acute response against schistosome glycoconjugate antigens in young children is mainly directed against the LDN-DF epitope. The response to LDN-DF in older individuals and the responses to the two other epitopes were similar in the two cohorts, suggesting that these antigens are recognized in the early stages of infection and that the immune response persists. The biological significance of these observations needs further elucidation.
Many different glycoconjugates and polysaccharides are expressed in different stages of the schistosome life cycle, and they play a major role in the parasite's interaction with its environment, which changes from water, where it lives freely, to the bloodstream of its human host (6). It is therefore not surprising that carbohydrate antigens are increasingly investigated in regard to the diagnosis and immunology of schistosomiasis.
Advances in technology have led to the identification of structures of a large number of schistosome glycans (9). Some of these glycan elements, such as LewisX, GalNAcβ1-4GlcNAc (LDN), and GalNAcβ1-4(Fucα1-3)GlcNAc (LDNF), are similar to those sometimes found on mammalian molecules. However, other schistosome-derived carbohydrates, including Fucα1-3GalNAcβ1-4GlcNAc (FLDN) and GalNAcβ1-4(Fucα1-2Fucα1-3)GlcNAc (LDN-DF), display unusual and unique nonmammalian features such as the abundant presence of fucosylated residues.
In the present study, we have focused on FLDN-, LDN-DF-, and LDNF-containing carbohydrate structures. These structures are predominantly expressed on the surface of cercariae and in eggs, but they also appear in other stages of the schistosome life cycle (10, 24). LDNF and its modified versions are also present on some vertebrate glycoproteins including glycodelin A (5, 14). Furthermore, the presence of LDNF motifs has also been demonstrated on a biantennary N-linked oligosaccharide which is a potent inhibitor of E-selectin-mediated adhesion (8).
It has been known for many years that glycosylated antigens are involved in the induction of the humoral immune response during schistosomiasis (15, 17). In one of the first longitudinal human population studies, Butterworth and colleagues found that high levels of immunoglobulin M (IgM) and IgG2 anti-egg polysaccharide antibodies were predictive of future susceptibility to reinfection (4). Others have suggested that IgE directed towards schistosome worm glycolipids could play an important role in resistance to reinfection (22). LDNF and LDN-DF are both immunogenic for the human host; however, the immune response to LDNF seems to be mainly humoral, while LDN-DF is able to stimulate innate cellular immune responses also (23, 25). The immunogenicity of FLDN has not been studied in great detail for the human host; infected chimpanzees do produce specific antibodies to this epitope (A. van Remoortere, unpublished data).
Interestingly, it has been suggested that Fucα1-3GalNAcβ4, which is part of the FLDN structure, is the major antigenic motif responsible for the cross-reactivity between Schistosoma mansoni glycolipids and keyhole limpet hemocyanin (KLH) (10). Because of its commercial availability, KLH is currently being examined for use in the diagnosis of and vaccination against schistosomiasis, with various results (11, 20, 27, 29).
Surface plasmon resonance (SPR) spectroscopy is a valuable technique for monitoring antiglycan antibody levels in serum which has the considerable advantage over enzyme-linked immunosorbent assay (ELISA) that it allows study of minute amounts of antigen, necessary in the case of synthetic oligosaccharides (25). By this technique IgG subclass and IgM antibodies to FLDN, LDN-DF, and LDNF were measured in a cohort of previously unexposed Kenyan immigrants who moved into the Masongaleni area, where S. mansoni is endemic (18). A cohort selected out of the original residents of the area, who had been exposed for many years, served as controls. Previous work described the influence of infection duration, infection intensity, and age on the development of specific antibody responses to S. mansoni adult worm antigen (SWA) and soluble egg antigen (SEA) in these cohorts (16). In this study, the developments of specific antibody responses to a carbohydrate structure shared between schistosomes and mammals (LDNF) and to two nonmammalian schistosome-derived structures (FLDN and LDN-DF) were compared. In addition, associations with responses to worm, egg, and cercarial antigens as well as KLH were examined.
MATERIALS AND METHODS
Sera.
Two Kenyan cohorts, from the Masongaleni area, where S. mansoni is endemic at a low intensity, were studied (16, 18). From March 1992 on, an area adjacent to an established settlement was allocated to several thousand displaced immigrants who were members of the same Kamba ethnic group. The immigrants had previously inhabited the Chuylu Hills, an area that is free of schistosomiasis. Random, age-stratified cohorts of both communities were selected for the study. Blood samples were taken from the immigrant cohort (n = 184, ages 5 to 59 years, 93 females and 91 males) within 1 year of their arrival and again approximately 2 years later. From the cohort selected from the established resident community (n = 235, ages 5 to 59 years, 148 females and 87 males), blood was sampled once. Positive controls consisted of sera from S. mansoni-infected individuals from the WHO/TDR Reference Serum Bank for African Schistosomiasis. As negative controls, we selected 10 sera from Dutch blood donors with no history of schistosomiasis.
Determination of specific antibody responses to LDNF, FLDN, and LDN-DF.
SPR analysis was carried out using a BIAcore 3000 instrument (Biacore AB, Uppsala, Sweden) as previously described by van Remoortere and colleagues (25). FLDN [Fucα1-3GalNAcβ1-4GlcNAcβ1-3Galα1-(CH2)5NH2], LDN-DF [GalNAcβ1-4(Fucα1-2Fucα1-3)GlcNAcβ1-(CH2)8COOH], and LDNF [GalNAcβ1-4(Fucα1-3)GlcNAcβ1-3Galα1-(CH2)5NH2] were synthesized and coupled to bovine serum albumin (BSA) as described elsewhere (1, 24). The number of oligosaccharide molecules per molecule of BSA was 5 for LDNF and FLDN and 11 for LDN-DF. The neoglycoproteins were immobilized at a flow rate of 5 μl/min in 10 mM sodium acetate (pH 4.0) onto a carboxymethylated dextran CM5 sensor chip by covalent amine coupling according to the instructions of the manufacturer (Biacore AB) until an increase of approximately 10,000 response units (RU) was observed. All analyses were performed at a flow rate of 2 μl/min with HEPES-buffered saline as an eluent. Sera were diluted 1:40 in running buffer and injected for 10 min followed by 3.5 min of buffer injection to allow dissociation. The IgG subclass and IgM isotype of the antibodies were determined by successive 10-min injections with mouse anti-human IgG1, IgG2, IgG3, and IgG4 (CLB, Amsterdam, The Netherlands) and swine anti-human IgM (Nordic, Tilburg, The Netherlands). All conjugates were diluted at 1:100 in running buffer, and each injection was followed by 3.5 min of dissociation time. Regeneration was performed using a 4-min pulse of 20 mM HCl at a flow rate of 5 μl/min. We were able to use the same sensor chip for all measurements. To monitor the viability of the chip, the same positive control was run after every 15th sample. Furthermore, a subset of 85 samples was examined at the beginning and the end of the series of measurements and showed similar results (paired sample correlations ranged from 0.674 to 0.898 on log-transformed data).
SPR data were corrected for nonspecific binding by using the BSA channel as a blank.
Determination of specific antibody levels against KLH.
Specific IgG1 and IgM responses to KLH (Sigma Aldrich, Zwijndrecht, The Netherlands) were measured by ELISA by methods similar to those described previously (16). All assays testing for one particular isotype response were carried out at the same time. Briefly, KLH was applied as a coating overnight at a concentration of 2.5 μg/ml for IgG1 and 5 μg/ml for IgM. The plates were washed six times between each incubation step. Following the blocking step (1% BSA, 1 h of incubation at room temperature [RT]), sera (diluted at 1/500 and incubated for 1 h at RT) were randomly distributed into the wells of microtiter plates (Nunc Maxisorb flat-bottomed plates). These sera, which were tested in duplicate on different plates, had previously been treated to prevent viral contamination (19). As the detecting antibody we used mouse anti-human IgG1-peroxidase (1/1,000,1 h at RT) or goat anti-human IgM-peroxidase (1/1,000, overnight at 4°C) (reagents from CLB and Sigma, respectively). Specific antibody responses were detected with tetramethylbenzidine solution, stopped with 0.5 M sulfuric acid, and expressed as the mean optical density of the triplicate samples.
Data analysis.
Spearman rank correlation coefficients for log-transformed data [log (x + 1) for the neoglycoprotein responses and log (x + 0.1) for the crude antigens] were used to express associations between specific antibody responses to the neoglycoproteins carrying FLDN, LDN-DF, and LDNF and those against KLH, as well as SWA, SEA, and crude cercarial antigen (CERC). The percentages of neoglycoprotein IgG1 and IgM responders were determined in the three sets of sera with use of the mean and 3 standard deviations of the negative-control sera as a cutoff. The cohorts were divided into four age groups. For the immigrants first sampled these were as follows: group 1, n = 45 (5 to 13 years); 2, n = 47 (14 to 27 years); 3, n = 47 (28 to 40 years), and 4, n = 45 (41 to 59 years). The groups for the second sampling contained the same individuals, who were now 2 years older. The residents were divided into the following age groups: group 1, n = 63 (6 to 14 years); 2, n = 55 (15 to 24 years); 3, n = 60 (25 to 41 years); and 4, n = 58 (42 to 60 years). Responses of the various age groups by cohort as well as differences in responses by age group between the immigrants and residents were compared by the Mann-Whitney test, while differences between the two immigrant samplings were examined by the Wilcoxon signed rank test. For these comparisons the nonresponders were excluded. All statistical analysis was performed using SPSS software (SPSS Inc., Chicago, Ill.).
RESULTS
By the use of SPR spectroscopy specific IgG subclass and IgM responses against FLDN, LDN-DF, and LDNF were measured in a cohort of Kenyan immigrants who had been newly exposed to S. mansoni. They were sampled within their first year of moving into the area of endemicity and again 2 years later after the majority had acquired the infection. Their responses were compared to those of a resident cohort that had lived in the same area of endemicity all their lives.
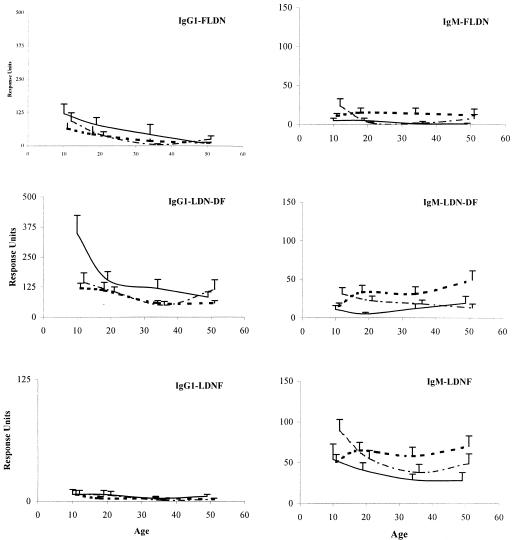
The focus of this report is on specific IgG1 and IgM responses because, apart from IgG2 responses against LDN-DF (132 responders out of 603 examined samples), specific IgG2, IgG3, and IgG4 responses against the three structures were scarce. Figure 1 shows the age profiles of the geometric mean IgG1 and IgM responses to the three glycoconjugates in the resident cohort as well as the newly exposed immigrants, who were sampled at two different time points. Before determination of the geometric mean the various cutoff values were subtracted from the RU measured for each sample.
FIG. 1.
Specific IgG1 and IgM responses against FLDN, LDN-DF, and LDNF versus age. Results are expressed as the geometric mean RU which were detected by SPR spectroscopy in the Kenyan resident cohort sampled in March 1994 (thick dotted line) and the immigrant cohort sampled between May and November 1993 (black line) and between August and September 1995 (dashed line). Error bars represent the 95% confidence intervals.
Specific IgG1 responses against FLDN and LDN-DF decreased with age; this was most pronounced in the first immigrant sampling. The difference in RU between the youngest children (age group 1) and the older cohort members (age groups 2, 3, and 4) was significant in the first immigrant sampling only (Z = −2.588 and P = 0.01 for FLDN and Z = −3.117 and P = 0.002 for LDN-DF). IgG1 responses to the LDNF epitope were low in all groups. The geometric mean IgG1 responses by age group to all epitopes were comparable in the immigrant (both time points) and resident sera, apart from a higher IgG1 response to LDN-DF in the youngest immigrant children within their first year of exposure. This difference was significant compared to the responses measured in the same children 2 years later (Z = −2.103, P = 0.035) and compared to the responses of the resident children (Z = −2.982, P = 0.003).
In the resident cohort, IgM responses to FLDN were low in all age groups, while IgM responses to LDN-DF increased with age. The age profile of IgM responses against LDNF showed a decrease with age in the immigrant samplings. Neither age trend was statistically significant. IgM responses had slightly increased with age in the immigrant children between the first and second time points (Z = −2.890, P = 0.004). In contrast with the IgG1 responses, IgM responses to FLDN and LDN-DF were low at the time of the first immigrant serum collection; 2 years later the geometric mean responses had increased in the youngest children only (Z = −2.578 and P = 0.01 for FLDN and Z = −2.286 and P = 0.022 for LDN-DF). Again, with the exception of the youngest immigrant children at the second time point, the intensities of IgM responses in the immigrants and residents were similar.
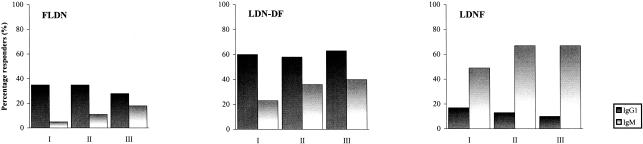
To compare IgM and IgG1 responses to the FLDN and LDN-DF epitopes with those to the LDNF structure that seems to occur also among vertebrate glycans, we determined the percentage of responders to the three neoglycoproteins by using the geometric mean response plus 3 standard deviations of 10 nonexposed Dutch blood donors as a cutoff (Fig. 2). These values were 14.7, 30.5, and 18.1 RU for IgG1 responses and 48.2, 42.2, and 32.8 RU for IgM responses to FLDN, LDN-DF, and LDNF, respectively. Interestingly, IgG1 was the most frequently observed specific antibody response to FLDN and LDN-DF in all samplings while IgM predominated in the response to LDNF.
FIG. 2.
Percentages of IgG1 and IgM responders against FLDN, LDN-DF, and LDNF of the Kenyan immigrant cohorts from the first (I) and second (II) samplings as well as of the resident cohort (III). Specific IgG1 and IgM responses were measured by SPR spectroscopy.
Associations between specific IgG1 and IgM responses to the neoglycoprotein epitopes and those against S. mansoni worm, egg, and cercarial antigens as well as KLH are described in Table 1. IgG1 responses to FLDN and LDN-DF were related to SEA- and CERC-specific IgG1 but not to anti-SWA IgG1 in the majority of the samples. IgG1-FLDN responses also correlated with IgG1 responses to KLH. A significant correlation between IgG1 responses to LDN-DF and those to KLH was observed only in the immigrant cohort at the first time point. IgM responses to FLDN did not correlate with those against any of the crude antigens, while IgM anti-LDN-DF responses were associated with IgM responses to SEA and CERC in the first immigrant serum collection and with IgM responses to SEA and KLH in the resident serum. No association between IgG1 anti-LDNF and IgG1 responses against any of the crude antigens was observed. LDNF-specific IgM responses were associated with SEA-specific IgM, while an association with IgM against CERC and KLH was observed in the resident cohort only.
TABLE 1.
Spearman rank correlation coefficients for log-transformed data describing the association between IgG1 or IgM responses to S. mansoni crude adult worm antigen (SWA), SEA, and CERC as well as KLH measured by ELISA and specific IgG1 or IgM responses against the neoglycan epitopes FLDN, LDN-DF, and LDNF measured by SPR
| Epitope | Response | Value for antigen and cohorta
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SWA
|
SEA
|
CERC
|
KLH
|
||||||||||
| I | II | III | I | II | III | I | II | III | I | II | III | ||
| FLDN | IgG1 | NS | NS | NS | 0.43*** | 0.36** | 0.31* | 0.53*** | 0.28* | NS | 0.73*** | 0.39** | 0.384** |
| IgM | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | |
| LDN-DF | IgG1 | NS | NS | NS | 0.52*** | 0.33** | 0.20* | 0.56*** | 0.26** | 0.17* | 0.56*** | NS | NS |
| IgM | NS | NS | NS | 0.36* | NS | 0.37*** | 0.48** | NS | NS | NS | NS | 0.412*** | |
| LDNF | IgG1 | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS |
| IgM | NS | NS | NS | 0.30*** | 0.25** | 0.35*** | NS | NS | 0.2* | NS | NS | 0.26** | |
Cohorts: I, immigrant cohort (n = 184) sampled within 1 year of first exposure to S. mansoni; II, same cohort sampled again 2 years later; III, resident cohort (n = 235). Significance: *, P < 0.05; **, 0.001 < P < 0.05; ***, P < 0.001; NS, not significant.
DISCUSSION
Continuing our previous studies of the humoral immune responses to defined carbohydrate epitopes that occur on schistosomes, we have examined the development of specific IgG subclass and IgM responses to FLDN, LDN-DF, and LDNF in a longitudinal human population study. It was previously shown that LDN-DF is highly immunogenic (25), and we wanted to compare responses to other fucosylated variants of the LDN backbone. Furthermore, we have selected these three structures because we were interested in the comparison of responses to carbohydrate structures that are shared between schistosomes and mammals with those to nonmammalian schistosome-derived structures. Finally, FLDN was of specific interest to us because part of this structure is thought to be the main epitope that is responsible for the cross-reactivity between schistosome antigens and KLH (10).
Specific IgG1 responses were most pronounced against FLDN and LDN-DF (Fig. 2), and they were strongly associated with those previously measured to SEA and CERC (Table 1). Similarly to previously published age profiles of IgG1 and IgG2 responses to SEA (16, 21, 28), levels of IgG1 against LDN-DF decreased with age (Fig. 1). Longitudinally, they were higher in the youngest immigrant children within their first year of exposure and decreased again to levels similar to those measured in the resident children 2 years later. A similar observation was made for IgG2 responses against LDN-DF (results not shown). The longitudinal development of IgG1 and IgG2 responses to LDN-DF is in contrast to the development of specific IgG1 and IgG2 responses to S. mansoni egg (16) and cercarial (C. W. A. Naus, unpublished results) antigens, which were similar in immigrant and resident children at the time of the first immigrant sampling and increased 2 years later. It was shown previously that responses against peptides became prominent only as the antiglycan response decreased (7).
Another interesting observation is of course that, in contrast to specific IgG1 responses to crude parasitic antigens (16) and with the exception of the acute response of the youngest immigrant children, the intensities of responses to the neoglycoconjugates were remarkably similar in the immigrants and the residents.
The most interesting observation about the specific IgM responses against the three schistosome-derived carbohydrate structures was that they were most marked against LDNF (Fig. 2). Furthermore, in contrast to IgG1 responses, the intensity of the IgM response was higher in the immigrant children at the second time point (Fig. 1). Studies with monoclonal antibodies demonstrated that LDNF is present on the surface of eggs (24). In agreement with this observation, we found that the IgM response to LDNF was strongly associated with the IgM response to SEA (Table 1). Besides their expression on schistosome-derived molecules, LDNF motifs are also present on human glycodelin A (5, 14) and on a human oligosaccharide, expressed on protein C, which is a potent inhibitor of E-selectin-mediated adhesion (8). We do not know whether the IgM antibodies, which recognize schistosome-derived LDNF, are able to bind to the host's native LDNF structures or whether the schistosome LDNF motif is any way directly involved in interactions with host molecules. Studies of LewisX-related structures suggest that the expression of host antigens by the parasite may be important in the biological balance between the parasite and its host (23, 26). Interestingly, with respect to LDNF, it has been suggested that the potent immunosuppressive activities of glycodelin A are linked to the presence of fucosylated LDN antennae (5).
Our results indicate that the acute response against schistosome glycoconjugate antigens in young children is strongly directed against the LDN-DF epitope. This response is of the IgG subclass. As the infection progresses, the specific antibody response against LDN-DF does persist; however, the IgG1 response decreases and the IgM response increases in the youngest children. We do not know whether this acute IgG1 response is directed against LDN-DF present on the cercarial glycocalyx or the eggshell or on antigens secreted by eggs. Similarly, in a study of S. mansoni infection in chimpanzees it was observed that the IgG response to LDN-DF was high during the acute stages of infection and decreased as the infection progressed to the chronic stage (van Remoortere, submitted). It is of course unclear why IgG1 responses decrease and IgM responses increase as infection progresses. The fact that we observe this effect only in children and not in adults who have a similar history of exposure indicates a strong age-related influence. Further studies are needed to provide more insight into the biological relevance of immune responses to glycoconjugates which are shared between schistosomes and their hosts.
It has been known for many years that KLH cross-reacts with schistosome antigens. Some have suggested that detection of antibodies to KLH can be used to distinguish between the acute and chronic stages of a schistosome infection (2, 11, 12). However, others have reported that the KLH-ELISA was not useful for serodiagnosis of travelers and for mild schistosome infections (27) and that the anti-KLH responses were similar in acutely and in chronically infected patients (13). Vaccination with KLH resulted in partial protection of calves against infection with S. bovis and sheep against infection with S. japonicum (3, 20). Kantelhardt and colleagues suggested recently that the part of the FLDN structure (Fucα1-3GalNAc) that is shared by schistosome glycans and KLH provides a basis for the potential of KLH in schistosomiasis vaccination and serodiagnosis (10).
In this study, the human humoral immune response to FLDN was characterized and compared to the response to total KLH. Our results corroborate the cross-reactivity, since we found an association between IgG1 responses to FLDN and those to KLH. Because the IgM response to FLDN was negligible, we did not observe a similar association for this isotype (Table 1). Specific IgG1 antibodies to FLDN were found in fewer than half of the examined individuals (Fig. 2), while the percentage of positive responders to KLH was practically 100% (results not shown). It therefore is unlikely that the measurement of specific antibody responses to FLDN would contribute to the serodiagnosis of schistosomiasis. An association between IgM responses to KLH and those to LDNF as well as to LDN-DF was observed only in the resident cohort. If these epitopes were also present on KLH, we would expect to find a correlation in the immigrants as well. Our results therefore suggest that FLDN is not the only cross-reactive epitope; however, we did not find any indication that LDNF or LDN-DF is cross-reactive with KLH. Further studies of cross-reactive carbohydrate structures of KLH and schistosomes are needed to elucidate the interesting and perhaps biologically beneficial association between the immune responses of a schistosome-infected host and the immune response against KLH.
To summarize our main findings, we have shown that specific antibody responses of individuals infected with S. mansoni against two nonmammalian glycoconjugates were predominantly of the IgG subclass whereas responses against a structure shared between mammals and schistosomes were dominated by IgM. The response against LDN-DF in young children in their first year of exposure was characterized by high IgG1 levels which decreased to levels similar to those of residents 2 years later. In contrast IgM responses against all examined epitopes increased with duration of exposure in the same children.
Acknowledgments
We thank K. Ágoston and J. Kérèkgyarto for synthesizing the FLDN and LDNF structures. We also thank the fieldworkers for their hard work and the inhabitants of Masongaleni for their participation.
Editor: W. A. Petri, Jr.
REFERENCES
- 1.Ágoston, K., J. Kérékgyarto, J. Hajko, G. Batta, D. J. Lefeber, J. P. Kamerling, and J. F. Vliegenthart. 2002. Synthesis of fragments of the glycocalyx glycan of the parasite Schistosoma mansoni. Chemistry 8:151-161. [DOI] [PubMed] [Google Scholar]
- 2.Alves-Brito, C. F., A. J. Simpson, L. M. Bahia-Oliverira, A. L. Rabello, R. S. Rocha, J. R. Lambertucci, G. Gazzinelli, N. Katz, and R. Correa-Oliveira. 1992. Analysis of anti-keyhole limpet haemocyanin antibody in Brazilians supports its use for the diagnosis of acute schistosomiasis mansoni. Trans. R. Soc. Trop. Med. Hyg. 86:53-56. [DOI] [PubMed] [Google Scholar]
- 3.Bushara, H. O., M. E. Bashir, K. H. Malik, M. M. Mukhtar, F. Trottein, A. Capron, and M. G. Taylor. 1993. Suppression of Schistosoma bovis egg production in cattle by vaccination with either glutathione S-transferase or keyhole limpet haemocyanin. Parasite Immunol. 15:383-390. [DOI] [PubMed] [Google Scholar]
- 4.Butterworth, A., D. Dunne, A. Fulford, M. Capron, J. Khalife, A. Capron, D. Koech, J. Ouma, and R. Sturrock. 1988. Immunity in human schistosomiasis mansoni: cross-reactive IgM and IgG2 anti-carbohydrate antibodies block the expression of immunity. Biochimie 70:1053-1063. [DOI] [PubMed] [Google Scholar]
- 5.Dell, A., H. R. Morris, R. L. Easton, M. Panico, M. Patankar, S. Oehniger, R. Koistinen, H. Koistinen, M. Seppala, and G. F. Clark. 1995. Structural analysis of the oligosaccharides derived from glycodelin, a human glycoprotein with potent immunosuppressive and contraceptive activities. J. Biol. Chem. 270:24116-24126. [DOI] [PubMed] [Google Scholar]
- 6.Dunne, D. W. 1990. Schistosome carbohydrates. Parasitol. Today 6:45-48. [DOI] [PubMed] [Google Scholar]
- 7.Eberl, M., J. A. Langermans, R. A. Vervenne, A. K. Nyame, R. D. Cummings, A. W. Thomas, P. S. Coulson, and R. A. Wilson. 2001. Antibodies to glycans dominate the host response to schistosome larvae and eggs: is their role protective or subversive? J. Infect. Dis. 183:1238-1247. [DOI] [PubMed] [Google Scholar]
- 8.Grinnell, B. W., R. B. Hermann, and S. B. Yan. 1994. Human protein C inhibits selectin-mediated cell adhesion: role of unique fucosylated oligosaccharide. Glycobiology 4:221-225. [DOI] [PubMed] [Google Scholar]
- 9.Hokke, C. H., and A. M. Deelder. 2001. Schistosome glycoconjugates in host-parasite interplay. Glycoconj. J. 18:573-587. [DOI] [PubMed] [Google Scholar]
- 10.Kantelhardt, S. R., M. Wuhrer, R. D. Dennis, M. J. Doenhoff, Q. Bickle, and R. Geyer. 2002. Fuc(α1→3)GalNAc-: major antigenic motif of Schistosoma mansoni glycolipids implicated in infection sera and keyhole limpet hemocyanin cross-reactivity. Biochem. J. 366:217-223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li, Y., A. L. Rabello, A. J. Simpson, and N. Katz. 1994. The serological differentiation of acute and chronic Schistosoma japonicum infection by ELISA using keyhole limpet haemocyanin as antigen. Trans. R. Soc. Trop. Med. Hyg. 88:249-251. [DOI] [PubMed] [Google Scholar]
- 12.Mansour, M. M., P. O. Ali, Z. Farid, A. J. Simpson, and J. W. Woody. 1989. Serological differentiation of acute and chronic schistosomiasis mansoni by antibody responses to keyhole limpet hemocyanin. Am. J. Trop. Med. Hyg. 41:338-344. [PubMed] [Google Scholar]
- 13.Markl, J., E. D. Nour, S. Winter-Simanowski, and U. A. Simanowski. 1991. Specific IgG activity of sera from Egyptian schistosomiasis patients to keyhole limpet hemocyanin (KLH). Naturwissenschaften 78:30-31. [DOI] [PubMed] [Google Scholar]
- 14.Morris, H. R., A. Dell, R. L. Easton, M. Panico, H. Koistinen, R. Koistinen, S. Oehninger, M. S. Patankar, M. Seppala, and G. F. Clark. 1996. Gender-specific glycosylation of human glycodelin affects its contraceptive activity. J. Biol. Chem. 271:32159-32167. [DOI] [PubMed] [Google Scholar]
- 15.Nash, T. E., M. N. Lunde, and A. W. Cheever. 1981. Analysis and antigenic activity of a carbohydrate fraction derived from adult Schistosoma mansoni. J. Immunol. 126:805-810. [PubMed] [Google Scholar]
- 16.Naus, C. W., G. Kimani, J. H. Ouma, A. J. Fulford, M. Webster, G. J. van Dam, A. M. Deelder, A. E. Butterworth, and D. W. Dunne. 1999. Development of antibody isotype responses to Schistosoma mansoni in an immunologically naive immigrant population: influence of infection duration, infection intensity, and host age. Infect. Immun. 67:3444-3451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Omer Ali, P., M. Mansour, J. N. Woody, S. R. Smithers, and A. J. Simpson. 1989. Antibody to carbohydrate and polypeptide epitopes on the surface of schistosomula of Schistosoma mansoni in Egyptian patients with acute and chronic schistosomiasis. Parasitology 98:417-424. [DOI] [PubMed] [Google Scholar]
- 18.Ouma, J. H., A. J. Fulford, H. C. Kariuki, G. Kimani, R. F. Sturrock, G. Muchemi, A. E. Butterworth, and D. W. Dunne. 1998. The development of schistosomiasis mansoni in an immunologically naive immigrant population in Masongaleni, Kenya. Parasitology 117:123-132. [DOI] [PubMed] [Google Scholar]
- 19.Poulsen, L. K., and T. B. Sorensen. 1993. Elimination of viral infection risk from blood samples for allergy testing. Allergy 48:207-208. [DOI] [PubMed] [Google Scholar]
- 20.Taylor, M. G., M. C. Huggins, F. Shi, J. Lin, E. Tian, P. Ye, W. Shen, C. G. Qian, B. F. Lin, and Q. D. Bickle. 1998. Production and testing of Schistosoma japonicum candidate vaccine antigens in the natural ovine host. Vaccine 16:1290-1298. [DOI] [PubMed] [Google Scholar]
- 21.Van Dam, G. J., F. F. Stelma, B. Gryseels, S. T. Falcao Ferreira, I. Talla, M. Niang, J. P. Rotmans, and A. M. Deelder. 1996. Antibody response patterns against Schistosoma mansoni in a recently exposed community in Senegal. J. Infect. Dis. 173:1232-1241. [DOI] [PubMed] [Google Scholar]
- 22.Van der Kleij, D., A. G. Tielens, and M. Yazdanbakhsh. 1999. Recognition of schistosome glycolipids by immunoglobulin E: possible role in immunity. Infect. Immun. 67:5946-5950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Van der Kleij, D., A. Van Remoortere, J. H. Schuitemaker, M. L. Kapsenberg, A. M. Deelder, A. G. Tielens, C. H. Hokke, and M. Yazdanbakhsh. 2002. Triggering of innate immune responses by schistosome egg glycolipids and their carbohydrate epitope GalNAc β1-4(Fucα1-2Fucα1-3)GlcNAc. J. Infect. Dis. 185:531-539. [DOI] [PubMed] [Google Scholar]
- 24.van Remoortere, A., C. H. Hokke, G. J. van Dam, I. van Die, A. M. Deelder, and D. H. van den Eijnden. 2000. Various stages of schistosoma express Lewis(x), LacdiNAc, GalNAcβ1-4 (Fucα1-3)GlcNAc and GalNAcβ1-4(Fucα1-2Fucα1-3)GlcNAc carbohydrate epitopes: detection with monoclonal antibodies that are characterized by enzymatically synthesized neoglycoproteins. Glycobiology 10:601-609. [DOI] [PubMed] [Google Scholar]
- 25.van Remoortere, A., G. J. van Dam, C. H. Hokke, D. van den Eijnden, I. van Die, and A. M. Deelder. 2001. Profiles of immunoglobulin M (IgM) and IgG antibodies against defined carbohydrate epitopes in sera of Schistosoma-infected individuals determined by surface plasmon resonance. Infect. Immun. 69:2396-2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Velupillai, P., E. A. dos Reis, M. G. dos Reis, and D. A. Harn. 2000. Lewis(x)-containing oligosaccharide attenuates schistosome egg antigen-induced immune depression in human schistosomiasis. Hum. Immunol. 61:225-232. [DOI] [PubMed] [Google Scholar]
- 27.Verweij, J. J., A. M. Polderman, L. G. Visser, and A. M. Deelder. 1995. Measurement of antibody response to keyhole limpet haemocyanin was not adequate for early diagnosis of schistosomiasis in a group of Dutch visitors to Mali. Trans. R. Soc. Trop. Med. Hyg. 89:48-50. [DOI] [PubMed] [Google Scholar]
- 28.Webster, M., B. D. Libranda-Ramirez, G. D. Aligui, R. M. Olveda, J. H. Ouma, H. C. Kariuki, G. Kimani, G. R. Olds, A. J. Fulford, A. E. Butterworth, and D. W. Dunne. 1997. The influence of sex and age on antibody isotype responses to Schistosoma mansoni and Schistosoma japonicum in human populations in Kenya and the Philippines. Parasitology 114:383-393. [DOI] [PubMed] [Google Scholar]
- 29.Xue, C. G., M. G. Taylor, Q. D. Bickle, L. Savioli, and E. A. Renganathan. 1993. Diagnosis of Schistosoma haematobium infection: evaluation of ELISA using keyhole limpet haemocyanin or soluble egg antigen in comparison with detection of eggs or haematuria. Trans. R. Soc. Trop. Med. Hyg. 87:654-658. [DOI] [PubMed] [Google Scholar]