Abstract
Neisseria meningitidis serogroup B is a major cause of life-threatening meningitis and septicemia worldwide, and no effective vaccine is available. Initiation of innate and acquired immune responses to N. meningitidis is likely to be dependent on cellular responses of dendritic cells (DC) to antigens present in the outer membrane (OM) of the meningococcus. In this study, the responses of human monocyte-derived DC (mo-DC) to OM isolated from parent (lipopolysaccharide [LPS]-replete) meningococci and from a mutant deficient in LPS were investigated. Parent OM selectively up-regulated Toll-like receptor 4 (TLR4) mRNA expression and induced mo-DC maturation, as reflected by increased production of chemokines, proinflammatory cytokines, and CD83, CD80, CD86, CD40, and major histocompatibility complex (MHC) class II molecules. In contrast, LPS-deficient OM selectively up-regulated TLR2 mRNA expression and induced moderate increases in both cytokine production and expression of CD86 and MHC class II molecules. Preexposure to OM, with or without LPS, augmented the allostimulatory properties of mo-DC, which induced proliferation of naive CD4+ CD45RA+ T cells. In addition, LPS-replete OM induced a greater gamma interferon/interleukin-13 ratio in naive T cells, whereas LPS-deficient OM induced the reverse profile. These data demonstrate that components of the OM, other than LPS, are also likely to be involved in determining the levels of DC activation and the nature of the T-helper immune response.
Infection caused by Neisseria meningitidis is characterized by life-threatening meningitis and septicemia (54). Meningococci initially colonize the nasopharyngeal mucosa, and the most likely route by which the bacteria enter the blood from the pharynx is via penetration of the mucosal epithelium and drainage to regional lymph nodes before they enter the circulation. During the course of infection, the growth and lysis of meningococci release outer membrane vesicles (OMV), which disseminate lipopolysaccharide (LPS) throughout the circulation (4). The formation of OMV has also been reported to occur in other gram-negative bacteria (2), and their role in pathogenesis is increasingly recognized. The relevance of OMV in the pathogenesis of meningococcal disease has been clearly demonstrated in several clinical studies. These structures have been observed in the blood and cerebrospinal fluid of patients with meningococcal infections (48). In addition, in a few patients with systemic meningococcal disease, the plasma LPS levels by far exceeded the expected level of LPS as judged by the number of live bacteria (7). Moreover, the LPS activity in patients' plasma was found in association with large LPS-containing structures (5). The severity of the disease has been shown to correlate with increasing concentrations of OMV-bound LPS (8) and the presentation of acute, compartmentalized intravascular and intracranial inflammatory responses, which are characterized by the production of tumor necrosis factor alpha (TNF-α) and interleukin-1β (IL-1β), IL-6, and IL-8 (6, 8). In addition, the pathophysiological effects of OMV-bound LPS have also been shown in both experimental animals (20) and an in vitro human whole-blood model (3).
Both innate and specifically acquired immune mechanisms are important during infection with meningococci, and immunity is generally accepted to be dependent on the presence of serum antibodies capable of inducing complement-mediated bactericidal activity (17, 18). These immune mechanisms are believed to involve recognition of specific microbial antigens defined as pathogen-associated molecular patterns, such as LPS, and specific pattern recognition receptors, which include the family of Toll-like receptors (TLRs) (30, 36). It is likely that within the nasopharyngeal mucosa, whole meningococci and released OMV interact with specialized antigen-presenting cells called dendritic cells (DC). Immature DC express TLRs and reside in various organs, including the respiratory tract (49). Following interaction with microbial constituents, activated and mature DC function to take up and process antigens and express them in the context of major histocompatibility complex (MHC) molecules for presentation to, and subsequent activation of, naive T cells.
Increasing evidence suggests that the nature of the stimulus received by the DC from foreign agents might condition or modulate their interaction with T cells and hence determine the type of T-helper response (Th1 or Th2) induced. The Th1-promoting capacity of DC correlates with their ability to produce IL-12, and pathogen-associated molecular pattern molecules known to induce IL-12 production by these cells include LPS, CpG DNA, and poly(I) · poly(C) (9, 12, 43). In contrast, cholera exotoxin, hyphae of Candida albicans, and filarial nematodes have been reported to modulate DC to promote Th2 polarization (14, 16, 56). The nature of microbe-derived stimuli received by the DC also determines the patterns of expression of MHC and costimulatory molecules that are critical for influencing Th1 or Th2 differentiation (16, 28, 39). Thus, following presentation of antigen by DC to T cells, costimulatory signals between these cells and the net effect of proinflammatory and inhibitory cytokines have been reported to direct the development of primary T-cell responses (27).
It is possible that the cellular responses of DC to antigens of meningococcal outer membranes (OM) are necessary for the initiation of a specific antibody-dependent immune response that is regulated by Th2 lymphocytes. Thus, knowledge of the biological response(s) of DC to stimulation with OM is likely to be important not only for understanding the disease process but also for the development of effective vaccines. In the present study, we have investigated the effects of OM, isolated from parent LPS-replete N. meningitidis and from an LPS-deficient mutant, on the biological activities of human monocyte-derived DC (mo-DC).
MATERIALS AND METHODS
Bacterial strains, growth conditions, and preparation of OM and pure LPS.
N. meningitidis strain H44/76 [B:15:P1.7,16: Cap+ Pil+ LPS+ (L3) Opa+ Opc+] is the subtype P1.7,16 reference strain (15). The LPS-deficient mutant N. meningitidis strain H44/76 pLAK33 (Cap+ Pil+ Opa+ Opc+ LPS−) was isolated at the National Institute of Public Health, Bilthoven, The Netherlands. It was derived from the parent strain by targeted disruption of the lpxA gene as described by Steeghs et al. (46). The phenotypes of both strains were defined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and immunoblotting with a specific monoclonal antibody (MAb) to class I and II pili and Opa and Opc proteins as described previously (19). In addition, no difference in pilin expression was found for the LPS-deficient mutant compared to the wild type as measured by a dot blot assay with the pilin-specific MAb SM1. The presence of capsule was confirmed by slide agglutination with antisera to specific meningococcal serogroups (Difco). The absence of LPS in the mutant strain was confirmed by low-Mr SDS-PAGE performed by the method of Schagger and von Jagow (40) with silver staining (21).
Meningococcal strains were grown on proteose-peptone agar at 37°C for 18 h in an atmosphere of 5% (vol/vol) CO2. OM were prepared from both strains by extraction of whole cells with lithium acetate as described previously (51). Analysis of OM preparations by SDS-PAGE and immunoblotting with a MAb specific to major OM proteins (25) demonstrated that the expression of PorA, PorB, Opa, Opc, and RmpM (class IV) proteins was similar for the parent and LPS-deficient mutant bacteria (data not shown), confirming the results of a previous study by Steeghs et al. (45). However, it was reported that expression of the iron limitation-inducible, cell surface-exposed lipoproteins LbpB and TbpB was reduced (45). LPS was purified from N. meningitidis strain MC58 [B:15:P1.7,16b: Cap+ Pil+ LPS+ (L3) Opa+ Opc+] by extraction with hot phenol as described previously (31); the preparation was also treated with DNase and RNase enzymes, and no protein was present. Strains MC58 and H44/76 have an identical LPS immunotype, L3 (B. Kuipers [RIVM, Bilthoven, The Netherlands], personal communication).
Cell preparations and culture conditions.
Peripheral blood (200 ml) from healthy volunteers was collected into Vacutainer tubes coated with K3 EDTA. Peripheral blood mononuclear cells (PBMCs) were isolated following density gradient centrifugation over Lymphoprep (1.077 g/ml; Nycomed Pharma, Oslo, Norway) at 900 × g for 30 min. Separate monocyte and T-cell fractions were obtained from PBMCs by countercurrent elutriation (Beckman J2-21 centrifuge with a JE-6B countercurrent elutriation rotor). Residual T and B cells were removed from the fractions with CD3 and CD19 MACs beads used according to the protocols of the manufacturer (Miltenyi Biotech), resulting in CD14+ monocyte populations of >95% purity (determined by flow cytometry). The T-cell fractions obtained after countercurrent elutriation were enriched for CD4+ T-helper cells by using the CD4 isolation kit, comprising a cocktail of hapten-conjugated MAbs against CD8, CD11b, CD16, CD19, CD36, and CD56 and antihapten magnetic microbeads (Miltenyi-Biotech). Naive CD4+ CD45RA+ T cells were purified from CD4+ cells by depletion of CD45RO+ cells with specific magnetic beads (Miltenyi Biotech). Purities of CD4+ (>98%) and naive (>95%) T-cell fractions were assessed by flow cytometry.
For the generation of mo-DC for phenotypic analysis, purified monocytes were resuspended at a density of 106 cells/ml in phenol red-free RPMI 1640 medium (Life Technologies) supplemented with 10% (vol/vol) low-endotoxin fetal calf serum (FCS) (HyClone, Pierce & Warriner, Cheshire, United Kingdom), 2 mM glutamine (Life Technologies), 100 U of penicillin (Life Technologies) per ml, and 100 μg of streptomycin (Life Technologies) per ml. At the start of culture, purified recombinant human IL-4 (R&D Systems) and recombinant human granulocyte-macrophage colony-stimulating factor (Leucomax; Sandoz Pharmaceuticals) were included, each at a final concentration of 1,000 U/ml. Every 2 days, half of the medium was replaced with fresh medium containing recombinant human IL-4 and recombinant human granulocyte-macrophage colony-stimulating factor at final concentrations of 500 and 1,000 U/ml, respectively. After 5 days of culture, CD1a+ CD14− mo-DC (>95% CD1a+ purity) were harvested for use. To obtain mo-DC for subsequent functional assays with T cells, the cells were differentiated from monocytes for 5 days in supplemented medium containing 2% (vol/vol) pooled human AB serum (Sigma) to minimize high background proliferation. Purified CD4+ or naive CD4+ CD45RA+ T cells were used in allogeneic mixed leukocyte reaction experiments. They were resuspended in phenol red-free RPMI 1640 medium supplemented with 5% (vol/vol) pooled human AB serum, 2 mM glutamine, 100 U of penicillin per ml, 100 μg of streptomycin per ml, 1% (vol/vol) sodium pyruvate, 0.05 M 2-mercaptoethanol, and 2 mM HEPES. All cell cultures were maintained at 37°C in a humidified atmosphere containing 5% (vol/vol) CO2.
Treatment of human mo-DC with OM and pure LPS.
Human mo-DC (CD1a+ CD14−), cultured in 24-well tissue culture plates in phenol red-free RPMI 1640 medium containing 10% (vol/vol) FCS and 2 mM glutamine but without antibiotics, were incubated with various concentrations (0.0001 to 10 μg/ml) of OM prepared from the parent strain H44/76 (OM) or from the LPS-deficient mutant (pLAK-OM) or with pure LPS. At each concentration tested, the relative amounts of LPS in all preparations were similar; thus, an OM preparation of 1 μg/ml based on protein content contained an equivalent concentration of LPS, which would be equivalent to an infecting dose of approximately 106 CFU of live bacteria (10). At various time points, supernatants were harvested and stored at −80°C for cytokine immunoassay. The viability of mo-DC during the experiments was investigated by using a cell viability assay (LIVE/DEAD; Molecular Probes) and confocal microscopy and was >95% following prolonged treatment (≥24 h) with any of the preparations. In addition, the mo-DC were prepared for analysis of cell surface markers by flow cytometry or subsequent coculture with allogeneic T cells.
Flow cytometry.
The expression of surface antigens on treated or control mo-DC was analyzed by flow cytometry. Cells were harvested and washed twice in ice-cold fluorescence-activated cell sorter (FACS) buffer (phosphate-buffered saline containing 10% [wt/vol] bovine serum albumin fraction V [Sigma] and 0.1% [wt/vol] sodium azide). Fc receptors were blocked with human Fcγ fragments prior to staining with fluorescent conjugated MAbs. Fluorescein isothiocyanate (FITC)- and phycoerythrin (PE)-conjugated MAbs were added at saturating concentrations and left for 30 min at 4°C in the dark, followed by two additional washes in FACS buffer. FITC- or PE-conjugated MAbs specific for CD1a (NA1/34; Dako), CD4 (B-F5; Diaclone), CD14 (MφP9; Becton Dickinson), CD40 (mAB89; Immunotech), CD45RO (UCHL1; Diaclone), CD45RA (B-C15; Diaclone), CD80 (MAB104; PharMingen), CD83 (HB15A; Immunotech), CD86 (FUN-1; PharMingen), and HLA-DR (L243; Becton Dickinson) were used to label the cells. TLR2 (clone TL2.1) and TLR4 (clone HTA125) were purchased from eBioscience (San Diego, Calif.). Relevant FITC- or PE-conjugated isotype control antibodies were also used. Expression of cell surface molecules was evaluated by single- or double-immunofluorescence staining, and analysis was performed with a FACScan flow cytometer (Becton Dickinson) and Cell Quest (Becton Dickinson) or WinMDI 2.7 software.
Quantification of endocytosis.
Stimulated or control mo-DC were washed by centrifugation in sterile phosphate-buffered saline and then resuspended in phenol red-free RPMI 1640 medium containing 10% (vol/vol) FCS, 2 mM glutamine, 100 U of penicillin per ml, 100 μg of streptomycin per ml, and 250 μg of FITC-conjugated dextran (Sigma) per ml. After 1 h of incubation at 37 or 4°C, antigen uptake was stopped by harvesting the cells, followed by three washes in ice-cold FACS buffer. Analysis of receptor-mediated endocytosis of FITC-dextran was performed with a FACScan. Surface binding values obtained by incubating cells at 4°C were subtracted from values measured at 37°C.
Analysis of cytokine levels in culture supernatants.
Supernatants were analyzed for the presence of cytokines or chemokines by specific immunoassay. Levels of IL-1β, IL-10 (Endogen), IL-6 (Diaclone Research), IL-12p40 (Biosource), IL-12p70 (Diaclone Research), IL-13 (Diaclone Research), and gamma interferon (IFN-γ) (Diaclone Research) were determined by using matched paired MAbs according to the manufacturers' instructions. For all cytokine enzyme-linked immunosorbent assay systems, tetramethylbenzidine-free base substrate (Sigma) was used to detect the horseradish peroxidase reaction. The color reaction was stopped with sulfuric acid (0.18 to 2 M), and the plates were read at a wavelength of 450 nm on a spectrophotometer (SpectraMAX340pc; Molecular Devices). The levels of chemokine proteins produced on incubation of mo-DC with OM preparations were quantified by sandwich immunoassays with matched pairs of specific antibodies (R&D) as described previously (11). The levels of IL-8, MIP-1α, MIP-1β, and RANTES were detected with a Delfia time-resolved fluorometry system (Wallac) as described previously (11). The concentration of each chemokine was determined by comparison with standard solutions of the corresponding purified recombinant protein (PeproTech EC) similarly treated.
Allogeneic mixed leukocyte reaction and naive T-cell proliferation.
Allogeneic mixed leukocyte reaction was performed with irradiated (1,000 rads) mo-DC and purified allogeneic T cells. The mo-DC were washed extensively prior to coculture with allogeneic T cells. Graded numbers of mo-DC per well were cultured with allogeneic purified CD4+ or CD4+ CD45RA+ T cells (105/well) for 5 days in 96-well U-bottom microtiter plates (Nunc). [3H]thymidine (0.5 μCi/well; Amersham Pharmacia Biotech) was added for the last 18 h; cells were harvested onto filter paper (Camo Ltd.) with a semiautomatic cell harvester (Skatron Instruments). [3H]thymidine incorporation was measured by scintillation counting (2500 TR liquid scintillation counter; Packard-Canberra), and results were expressed as average counts per minute for triplicate wells ± standard error (SE). To determine the priming ability of mo-DC, following a 4-day coculture of naive T cells and mo-DC, cells were harvested and restimulated with plate-bound anti-CD3 (1 μg/ml; clone OKT3) for a further 24 h. Cytokines in supernatants from these cultures were detected by use of specific immunoassays.
Isolation and quantification of mRNA for determination of TLR2 and TLR4 status.
mo-DC were stimulated with OM from the parent H44/74 strain, the LPS-deficient mutant strain (pLAK-OM), or pure LPS for 24 h. Treated and untreated cells were pelleted and used for RNA isolation. Total RNA was extracted by using the RNeasy kit (Qiagen) and treated with DNase I (RNase-free DNase; Promega) according to the manufacturer's instructions. RNA was quantified by using the RiboGreen RNA kit (Molecular Probes) (used as directed) with a fluorescence spectrophotometer and CytofluorII software. cDNA was prepared from 400 ng of total RNA by using the Omniscript reverse transcriptase preamplification system (Promega) with random hexamer primers (Promega). The cDNA levels of TLR2 and TLR4 were quantified by TaqMan PCR with an ABI prism 7700 sequence detector according to the instructions of the manufacturer (Applied Biosystems). The cDNA levels during the linear phase of amplification were normalized against 18s rRNA (predeveloped assay reagent; PE Applied Biosystems). Relative RNA concentrations were extrapolated by using a standard curve generated from human PBMC RNA. The following primers (Sigma-Genosys Ltd.) and probes (Biosource International) were used: TLR2 sense, 5′-CTACTGGGTGGAGAACCTTATGGT-3′; TLR2 antisense, 5′-CCGCTTATGAAGACACAACTTGA-3′; TLR2 probe, 5′-FAM-AGGAGCTGGAGAACTTCAATCCCCCC-TAMRA-3′; TLR4 sense, 5′-TCCATGAAGGTTTCCATAAAAGC-3′; TLR4 antisense, 5′-CTGCCAGGTCTGAGCAATCTC, and TLR4 probe, 5′-FAM-ATTGTTGTGGTGTCCCAGCACTTCATCC-TAMRA-3′.
Statistical analysis.
Statistical analysis was performed with Student's t test (two tailed) or the nonparametric Wilcoxon signed rank test, when appropriate, to compare responses within a subject group (ARCUS QuickStat for Windows; Research Solutions); P values of <0.05 were considered significant.
RESULTS
OM of N. meningitidis induce activation of mo-DC.
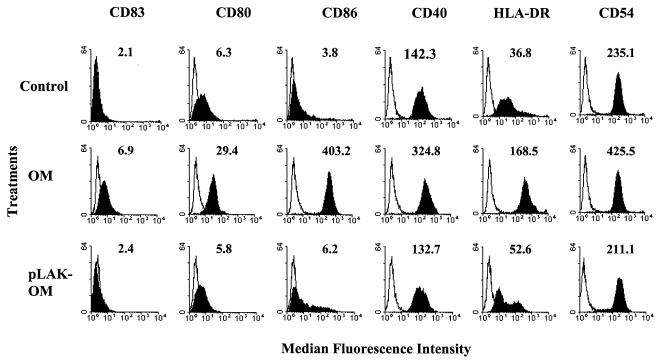
mo-DC were stimulated with various concentrations (0.01 to 10 μg/ml) of OM from the parent H44/76 strain (OM) or from the LPS-deficient mutant (pLAK-OM), as well as with pure LPS. After 24 h, treated and untreated mo-DC were collected and analyzed by flow cytometry for expression of cell surface molecules involved in T-helper cell activation (Fig. 1). Phase-contrast microscopy of mo-DC treated with parent H44/76 OM or pure LPS demonstrated the acquisition of distinct morphological changes, notably pronounced dendrite formation and increased cellular clumping, which reflected an activated phenotype compared to control, untreated cells. By contrast, the morphologies of mo-DC treated with pLAK-OM and control cells were similar, although the former contained a greater proportion of veiled dendrites only at the higher concentrations (≥1.0 μg/ml) tested (data not shown). Untreated immature mo-DC constitutively expressed moderate to high levels of MHC class II molecules (HLA-DR), the T-cell activating molecule CD40, and the adhesion molecule CD54, with low levels of the costimulatory molecules CD80 and CD86 and the DC maturation marker CD83. Stimulation of mo-DC with parent OM resulted in marked elevation of the expression of all of these molecules. Significant expression of cell surface molecules above control levels was observed when parent OM concentrations of as low as 0.1 μg/ml were used, with maximal expression achieved at concentrations of 1 μg/ml (Fig. 1) and above. By contrast, expression of cell surface molecules induced by pLAK-OM was observed only when concentrations of ≥1.0 μg/ml were tested (Fig. 1) and was limited to minor up-regulation of CD86 and MHC class II molecules. Purified meningococcal LPS induced maturation of the mo-DC phenotype similarly to parent OM (data not shown).
FIG. 1.
Phenotypic analysis of mo-DC activated by N. meningitidis OM. Data are shown for cell surface expression of CD83, CD80, CD86, CD40, HLA-DR, and CD54 on control untreated mo-DC or cells that had been stimulated for 24 h with a 1-μg/ml concentration of OM from the parent H44/76 strain (OM) or from the LPS-deficient mutant (pLAK-OM). Expression of the indicated markers is shown by the solid histograms, whereas cells stained with relevant isotype MAb are indicated by the open histograms. The numbers on each histogram correspond to the median fluorescence intensity of MAb staining. The results shown are from one donor and are representative of similar data obtained from experiments carried out with mo-DC from eight different donors.
OM of N. meningitidis augment chemokine and proinflammatory cytokine production by mo-DC.
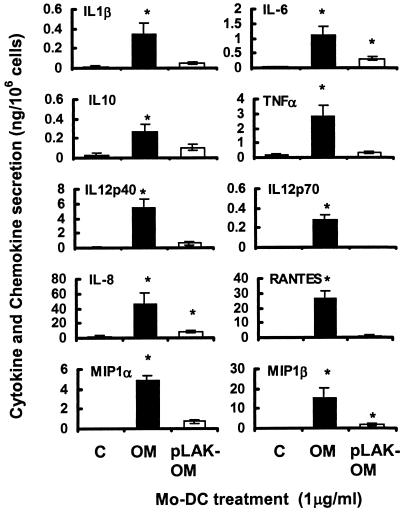
The ability of mo-DC to secrete proinflammatory cytokines and chemokines over time in response to stimulation with various concentrations (0.0001 to 1 μg/ml) of parental H44/76 strain OM and LPS-deficient mutant pLAK-OM and with pure LPS was investigated. Dose-dependent increases in the production of cytokines and chemokines by mo-DC were observed following treatment with parent OM. Doses of as low as 0.01 μg of parent OM per ml induced significant cytokine and chemokine production, and maximal secretion was observed at 24 h with 1 μg/ml, the highest concentration tested (Fig. 2). Parent OM induced significant (P < 0.05) secretion of the cytokines IL-1β, IL-6, IL-10, TNF-α, IL-12p40, and IL-12p70 and the chemokines IL-8, MIP-1α, MIP-1β, and RANTES (Fig. 2). By contrast, even when used at a concentration of 1 μg/ml, pLAK-OM failed to induce cytokine secretion, except for low but significant (P < 0.05) levels of IL-6 and IL-10 (Fig. 2). In addition, low but significant (P < 0.05) levels of chemokines IL-8 and MIP-1β were secreted after stimulation for 24 h with pLAK-OM. However, although there was a trend towards increased secretion of MIP-1α and RANTES from mo-DC stimulated with pLAK-OM, this was not significant compared with control cells (P > 0.05). In general, pure LPS at concentrations of up to 1 μg/ml induced amounts of cytokines comparable to those induced by native OM, including secretion of IL-6, IL-10, IL-12p40, and TNF-α (data not shown).
FIG. 2.
Effects of N. meningitidis OM on chemokine and cytokine production by mo-DC. Cells were stimulated for 24 h with various concentrations of OM from the parent H44/76 strain (OM) or from the LPS-deficient mutant (pLAK-OM) and compared with control, unstimulated cells. The data shown are the maximal levels of secretion induced with a 1-μg/ml concentration of preparation(s). Results are presented as means ± SEs of data from five donors (IL-1β, RANTES, and MIP-1α), six donors (IL-8 and MIP-1β), or eight donors (IL-6, IL-10, IL-12p40, IL-12p70, and TNF-α). *, P < 0.05 (t test) compared to control cells.
Effects of OM from N. meningitidis on receptor-mediated endocytosis by mo-DC.
An early event during the maturation of DC is a reduction in the ability to capture exogenous antigens, which is reflected by diminished receptor-mediated endocytosis (33). In individual experiments with mo-DC from five different donors, the cells were treated with various concentrations (0.01 to 1 μg/ml) of parent OM, pLAK-OM, or pure LPS and also left untreated as controls. Flow cytometric analyses revealed that immature mo-DC were capable of taking up FITC-labeled dextran at 37°C (mean fluorescence intensity ± standard error [SE], 41.0 ± 5.2; n = 5). This uptake was markedly inhibited following 24 h of stimulation of mo-DC with parent OM (mean fluorescence intensity ± SE, 2.3 ± 1.2; P < 0.05; n = 5) or pure LPS (mean fluorescence intensity ± SE, 3.4 ± 0.5; P < 0.05; n = 5); with both of these preparations, inhibition was maximal at the highest concentration of each tested (1 μg/ml). By contrast, FITC-dextran uptake was partially reduced in mo-DC stimulated with pLAK-OM at the highest concentration tested (mean fluorescence intensity ± SE, 15.2 ± 2.3; P < 0.05; n = 5).
Effects of N. meningitidis OM on mo-DC driven T-cell proliferation.
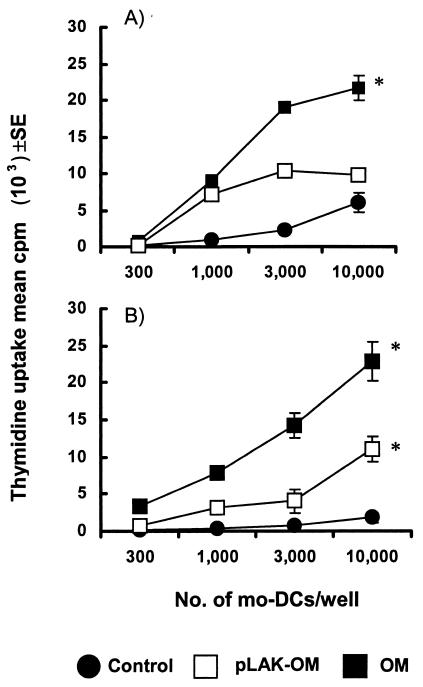
The observations described above demonstrate that OM from N. meningitidis activate mo-DC. The possible functional consequences of these effects were examined by assessing the capacity of OM-treated mo-DC to stimulate allogeneic T-cell proliferation. In the absence of any activation, immature mo-DC induced only modest proliferation of allogeneic CD4+ T cells, which was observed at high mo-DC/T-cell ratios (Fig. 3A). mo-DC stimulated with parent OM induced strong CD4+ proliferation (Fig. 3A). In addition, mo-DC pretreated with LPS-deficient OM also stimulated allogeneic CD4+ proliferation at high mo-DC/T-cell ratios; however, this was at a lower level than for mo-DC stimulated with parent OM (Fig. 3A). The ability of mo-DC to activate primary T-cell responses was also investigated (Fig. 3B). mo-DC pulsed with parent OM or LPS-deficient pLAK-OM induced significant (P < 0.05) stimulation of naive CD4+ CD45RA+ T-cell proliferation compared with control mo-DC.
FIG. 3.
Effect of N. meningitidis OM on mo-DC-driven T-cell proliferation. Immature mo-DC were treated for 24 h with the optimal 1-μg/ml concentration of OM from the parent H44/76 strain (OM) or from the LPS-deficient mutant (pLAK-OM) and were also left untreated in culture as controls. Graded numbers of mo-DC were cocultured with 105 cells of purified CD4+ allogeneic T cells (A) or CD4+ CD45RA+ naive allogeneic T cells (B) per well. Results are for triplicate cultures from one donor and are representative of similar data obtained from experiments done with mo-DC from five different donors. *, P < 0.05 compared to control cells.
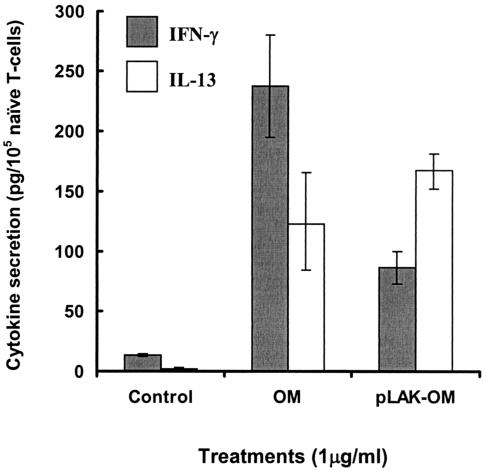
mo-DC preexposed to either parent OM, LPS-deficient pLAK-OM, or pure LPS also encouraged naive T-cell differentiation into effector cells producing both Th1 (IFN-γ)- and Th2 (IL-13)-type cytokines (Fig. 4). T-cell supernatants analyzed after 4 days of priming with mo-DC stimulated with parent OM contained significantly (P < 0.05) higher levels of IFN-γ than of IL-13. Pure LPS, tested at a concentration equivalent to that of parent OM, also induced significantly (P < 0.05) higher levels of IFN-γ than of IL-13 (data not shown). Conversely, mo-DC stimulated with LPS-deficient pLAK-OM encouraged the development of effector cells producing significantly (P < 0.05) higher levels of IL-13 than of IFN-γ.
FIG. 4.
Activation of mo-DC by N. meningitidis OM promotes cytokine production by primed naive T cells. Allogeneic CD4+ CD45+ naive T cells were cocultured with mo-DC preexposed for 24 h to the optimal 1-μg/ml concentration of OM from the parent H44/76 strain (OM) or from the LPS-deficient mutant (pLAK-OM). Results are presented as mean cytokine production ± SE from experiments done with mo-DC from three different donors.
OM from N. meningitidis induce changes in TLR expression by mo-DC.
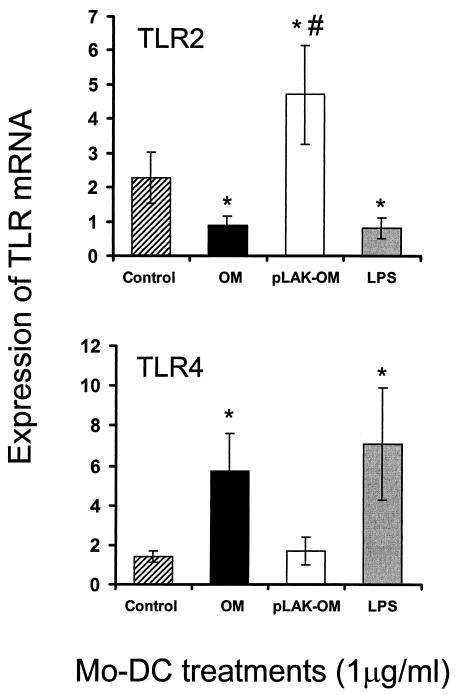
The ability of parent OM, LPS-deficient pLAK-OM, and purified meningococcal LPS to affect the expression of TLR2 and TLR4 mRNAs by mo-DC was examined by using semiquantitative TaqMan reverse transcription-PCR (Fig. 5). Compared with control, untreated mo-DC, stimulation of mo-DC for 24 h with either parent OM or pure LPS resulted in a significant twofold (P < 0.05) down-regulation of TLR2 mRNA expression, while a significant (P < 0.05) fivefold increase in TLR4 mRNA expression was also observed. These differential regulation patterns of TLR2 and TLR4 mRNA expression were also observed in mo-DC treated with Escherichia coli LPS (data not shown). By contrast, stimulation of mo-DC with LPS-deficient pLAK-OM had no effect on TLR4 mRNA expression (P > 0.05), whereas there was a significant (P < 0.05) twofold increase in TLR2 mRNA expression, compared to control, untreated cells.
FIG. 5.
Expression of TLR2 and TLR4 mRNAs by mo-DC treated with meningococcal OM. Cells were left untreated or exposed to the optimal 1-μg/ml concentration of OM from the parent H44/76 strain (OM) or from the LPS-deficient mutant (pLAK-OM), or to pure meningococcal LPS, for 24 h. Results show the averages ± SEs from experiments with mo-DC from five donors, except for TLR2 with pLAK-OM treatment, where four donors were used. *, P < 0.05 (t test). #, one additional donor (data not shown) did not show a change in TLR2 mRNA expression following treatment.
DISCUSSION
Following infection with N. meningitidis, LPS-replete OM vesicles shed by the bacteria constitute an important virulence mechanism. In the present study, expression of TLRs appeared to be differentially regulated by the presence of meningococcal LPS in OM. Expression of TLR4 mRNA was up-regulated following exposure of mo-DC to both LPS-rich parent OM and pure LPS, whereas expression of TLR2 mRNA was decreased. Conversely, mo-DC incubated with LPS-deficient OM showed increased TLR2 mRNA expression, while expression of TLR4 mRNA was unaffected. These findings with mo-DC extend the observations from other studies with monocytes and macrophages, which demonstrated that LPS-deficient meningococci activated responses in these cells through TLR2 (23, 37). Although the nature of the OM components that activate mo-DC responses is not well characterized, it has been reported that the meningococcal PorB protein mediated signals through TLR2 on B cells (35). The observed regulation of TLRs following the interaction of meningococcal OM with mo-DC suggests that these cells are likely to participate in the innate immune recognition of N. meningitidis. Significantly, a recent study has shown that only rare heterozygous missense mutations of TLR4 contributed to the development of systemic meningococcal disease in white human populations (42), whereas the more common (functional) polymorphism Asp299Gly of TLR4 did not (38).
Another important consequence of TLR signaling is the possible modulation of DC function in subsequent generation of the acquired immune response. Interaction of immature DC with many bacteria and/or their components leads to their activation, maturation, and acquisition of potent antigen-presenting properties by virtue of increased expression of surface MHC class II costimulatory molecules and production of critical regulatory cytokines (1). mo-DC exposed either to LPS-containing OM or to LPS-deficient OM showed significant reductions in receptor-mediated endocytosis, a response characteristic of DC maturation. This agrees with findings by Dixon et al. that paraformaldehyde-fixed whole N. meningitidis H44/76 and the LPS-deficient isogenic mutant induced maturation in mo-DC (13). The observations in the present study clearly demonstrate that components of the bacterial OM other than LPS can induce mo-DC maturation. Although the exact nature of these modulins remains to be confirmed, some components of pathogenic Neisseria spp., notably the OM porin proteins, have been shown to activate immune effector cells (34, 35, 41, 55).
The presentation by DC of MHC class II-antigen peptide complexes to CD4+ T-helper cells initiates the induction of acquired immunity to pathogens (22, 47). The subsequent differentiation of T-helper cells may be determined by the combination of signals through soluble cytokines and surface costimulatory molecules. LPS-containing OM induced strong expression of HLA-DR and CD86 on mo-DC together with moderate increases of CD40 and CD80, an effect that was largely reproduced by the LPS alone. Thus, LPS-containing OM activated mo-DC to a phenotype typical of more mature DC, which would be expected to have enhanced T-cell priming ability. However, mo-DC also exhibited responses to LPS-deficient OM, with moderate increases in the expression of MHC class II and CD86 costimulatory molecules.
Recent studies reported that after exposure to whole meningococci of strains H44/76 and MC58, mo-DC secreted high levels of TNF-α, IL-1β, IL-6, and IL-8 (29, 52) and that when PBMCs were treated with H44/76 OM complexes they produced TNF-α and IL-1β (44). In the present study, parent, LPS-replete OM induced secretion of the proinflammatory cytokines IL-1β, IL-6, IL-12p40, IL-12p70, and TNF-α; the chemokines IL-8, RANTES, MIP-1α, and MIP-1β; and the anti-inflammatory cytokine IL-10. Secretion of these cytokines was also detected from cultures of mo-DC infected with live H44/76 meningococci in this study (data not shown). By contrast, LPS-deficient OM did not induce significant cytokine and chemokine secretion by mo-DC, other than low levels of IL-6, IL-8, IL-10, and MIP-1β. These observations with isolated OM are in agreement with several studies describing attenuated cytokine production from human cells infected with whole LPS-deficient bacteria (13, 24, 44, 53). Hence, as expected, LPS in the OM is a major stimulus for the production of both proinflammatory cytokines and chemokines by human DC. However, the present data also suggest that components other than LPS in the OM are able to stimulate the production of inflammatory mediators by DC. The nature of these non-LPS modulins is not known, but they may include porins, other OM proteins and lipoproteins, and several components such as peptidoglycan and bacterial DNA, which may be found as possible contaminants of OM vesicles.
mo-DC preexposed to either parent LPS-replete OM or LPS-deficient OM were capable of inducing potent allogeneic CD4+ T-cell proliferation. Moreover, mo-DC activated by either OM preparation stimulated allogeneic primary naive T-cell responses. Although the levels of proliferation of CD4+ or naive CD4+ CD45RA+ T cells were greater when the stimulating mo-DC were pretreated with LPS-replete OM, mo-DC driven T-cell proliferation was also enhanced by LPS-deficient OM. In addition, mo-DC preexposed to parent OM acquired the capacity to prime naive T-helper cells into effector cells exhibiting a higher IFN-γ/IL-13 cytokine profile. In contrast, the reverse ratio of cytokine production (higher IL-13/IFN-γ ratio) was observed in naive T cells when they were activated with mo-DC treated with LPS-deficient OM. Thus, these findings clearly demonstrate the ability of meningococcal OM to modulate the antigen-presenting capacity of DC to initiate and promote differentiation of primary T-cell responses. Furthermore, these data also suggest that components of the OM other than LPS contribute significantly to these activities.
In summary, the present study has demonstrated that OM from N. meningitidis are immunomodulatory and exert distinct effects on human mo-DC. OM induced changes in mo-DC phenotype, which resulted in the DC acquiring potent T cell-activating properties. However, there is increasing evidence that different DC subsets (whether of myeloid or lymphoid origin) which express distinct pattern recognition receptors, in conjunction with both the levels and duration of antigen encountered and the presence of cytokines within the microenvironment, all influence the subsequent balance between Th1 and Th2 cells (26, 32, 49, 50). In addition, individual components of the meningococcal OM, other than LPS, may play roles in determining the levels of DC activation and the nature of the immune response. Thus, the appropriate response mounted against meningococcal OM in vivo is likely to involve a combination of all of these factors. Modulation of these biological activities suggests the possibility of inducing or manipulating the desired innate and/or adaptive immune responses with appropriately designed vaccines.
Acknowledgments
This work was supported by the Medical Research Council of Great Britain (studentship awarded to T.A.-B.), the University of Southampton Strategic Development Fund, the Meningitis Research Foundation, and the Asthma and Allergy Inflammation Research Charity, Southampton, United Kingdom.
We thank L. Steeghs and P. van der Ley (RIVM) for the LPS-deficient mutant N. meningitidis strain H44/76 pLAK33 and B. Kuipers (RIVM) for LPS immunotyping.
Editor: J. N. Weiser
REFERENCES
- 1.Banchereau, J., F. Briere, C. Caux, J. Davoust, S. Lebecque, Y. J. Liu, B. Pulendran, and K. Palucka. 2000. Immunobiology of dendritic cells. Annu. Rev. Immunol. 18:767-811. [DOI] [PubMed] [Google Scholar]
- 2.Beveridge, T. J. 1999. Structures of gram-negative cell walls and their derived membrane vesicles. J. Bacteriol. 181:4725-4733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bjerre, A., B. Brusletto, E. Rosenqvist, E. Namork, P. Kierulf, R. Ovstebo, G. B. Joo, and P. Brandtzaeg. 2000. Cellular activating properties and morphology of membrane-bound and purified meningococcal lipopolysaccharide. J. Endotoxin Res. 6:437-445. [PubMed] [Google Scholar]
- 4.Brandtzaeg, P., A. Bjerre, R. Ovstebo, B. Brusletto, G. B. Joo, and P. Kierulf. 2001. Neisseria meningitidis lipopolysaccharides in human pathology. J. Endotoxin Res. 7:401-420. [PubMed] [Google Scholar]
- 5.Brandtzaeg, P., K. Bryn, P. Kierulf, R. Ovstebo, E. Namork, B. Aase, and E. Jantzen. 1992. Meningococcal endotoxin in lethal septic shock plasma studied by gas chromatography, mass spectrometry, ultracentrifugation and electron microscopy. J. Clin. Investig. 89:816-823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brandtzaeg, P., A. Halstensen, P. Kierulf, T. Espevik, and A. Waage. 1992. Molecular mechanisms in the compartmentalized inflammatory response presenting as meningococcal meningitis or septic shock. Microb. Pathog. 13:423-431. [DOI] [PubMed] [Google Scholar]
- 7.Brandtzaeg, P., P. Kierulf, P. Gaustad, A. Skulberg, J. N. Bruun, S. Halvorsen, and E. Sorensen. 1989. Plasma endotoxin as a predictor of multiple organ failure and death in systemic meningococcal disease. J. Infect. Dis. 159:195-204. [DOI] [PubMed] [Google Scholar]
- 8.Brandtzaeg, P., R. Ovstebo, and P. Kierulf. 1992. Compartmentalization of lipopolysaccharide production correlates with clinical presentation in meningococcal disease. J. Infect. Dis. 166:650-652. [DOI] [PubMed] [Google Scholar]
- 9.Cella, M., M. Salio, Y. Sakakibara, H. Langen, I. Julkunen, and A. Lanzavecchia. 1999. Maturation, activation, and protection of dendritic cells induced by double-stranded RNA. J. Exp. Med. 189:821-829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Christodoulides, M., B. Makepeace, K. Partridge, D. Kaur, M. I. Fowler, R. O. Weller, and J. E. Heckels. 2002. Interaction of Neisseria meningitidis with human meningeal cells induces the secretion of a distinct group of chemotactic, proinflammatory, and growth factor cytokines. Infect. Immun. 70:4035-4044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Christodoulides, M., J. S. Everson, B. Liu, P. R. Lambden, P. J. Watt, E. J. Thomas, and J. E. Heckels. 2000. Interaction of primary human endometrial cells with Neisseria gonorrhoeae expressing green fluorescent protein. Mol. Microbiol. 35:32-43. [DOI] [PubMed] [Google Scholar]
- 12.de Jong, E. C., P. L. Vieira, P. Kalinski, J. H. Schuitemaker, Y. Tanaka, E. A. Wierenga, M. Yazdanbakhsh, and M. L. Kapsenberg. 2002. Microbial compounds selectively induce Th1 cell-promoting or Th2 cell-promoting dendritic cells in vitro with diverse Th cell-polarizing signals. J. Immunol. 168:1704-1709. [DOI] [PubMed] [Google Scholar]
- 13.Dixon, G. L. J., P. J. Newton, B. M. Chain, D. Katz, S. R. Andersen, S. Wong, P. van der Ley, N. Klein, and R. E. Callard. 2001. Dendritic cell activation and cytokine production induced by group B Neisseria meningitidis: interleukin-12 production depends on lipopolysaccharide expression in intact bacteria. Infect. Immun. 69:4351-4357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.d'Ostiani, C. F., G. Del Sero, A. Bacci, C. Montagnoli, A. Spreca, A. Mencacci, P. Ricciardi-Castagnoli, and L. Romani. 2000. Dendritic cells discriminate between yeasts and hyphae of the fungus Candida albicans. Implications for initiation of T helper cell immunity in vitro and in vivo. J. Exp. Med. 191:1661-1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Frasch, C. E., W. D. Zollinger, and J. T. Poolman. 1985. Serotype antigens of Neisseria meningitidis and a proposed scheme for designation of serotypes. Rev. Infect. Dis. 7:504-510. [DOI] [PubMed] [Google Scholar]
- 16.Gagliardi, M. C., F. Sallusto, M. Marinaro, A. Langenkamp, A. Lanzavecchia, and M. T. De Magistris. 2000. Cholera toxin induces maturation of human dendritic cells and licenses them for Th2 priming. Eur. J. Immunol. 30:2394-2403. [DOI] [PubMed] [Google Scholar]
- 17.Goldschneider, I., E. C. Gotschlich, and M. S. Artenstein. 1969. Human immunity to the meningococcus. I. The role of humoral antibodies. J. Exp. Med. 129:1307-1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goldschneider, I., E. C. Gotschlich, and M. S. Artenstein. 1969. Human immunity to the meningococcus. II. Development of natural immunity. J. Exp. Med. 129:1327-1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hardy, S. J., M. Christodoulides, R. O. Weller, and J. E. Heckels. 2000. Interactions of Neisseria meningitidis with cells of the human meninges. Mol. Microbiol. 36:817-829. [DOI] [PubMed] [Google Scholar]
- 20.Hazelzet, J. A., R. Stubenitsky, A. B. Petrov, G. W. van Wieringen, E. van der Voort, J. Hess, W. C. J. Hop, L. G. Thijs, D. J. Duncker, J. T. Poolman, and P. D. Verdouw. 1999. Cardiovascular aspects of experimental meningococcal sepsis in young and older awake piglets: age-related differences. Shock 12:145-154. [DOI] [PubMed] [Google Scholar]
- 21.Hitchcock, P. J., and T. M. Brown. 1983. Morphological heterogeneity among Salmonella lipopolysaccharide chemotypes in silver-stained polyacrylamide gels. J. Bacteriol. 154:269-277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Inaba, K., S. Turley, F. Yamaide, T. Iyoda, K. Mahnke, M. Inaba, M. Pack, M. Subklewe, B. Sauter, D. Sheff, M. Albert, N. Bhardwaj, I. Mellman, and R. M. Steinman. 1998. Efficient presentation of phagocytosed cellular fragments on the major histocompatibility complex class II products of dendritic cells. J. Exp. Med. 188:2163-2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ingalls, R. R., E. Lien, and D. T. Golenbock. 2000. Differential roles of TLR2 and TLR4 in the host response to Gram-negative bacteria: lessons from a lipopolysaccharide-deficient mutant of Neisseria meningitidis. J. Endotoxin Res. 6:411-415. [PubMed] [Google Scholar]
- 24.Ingalls, R. R., E. Lien, and D. T. Golenbock. 2001. Membrane-associated proteins of a lipopolysaccharide-deficient mutant of Neisseria meningitidis activate the inflammatory response through Toll-like receptor 2. Infect. Immun. 69:2230-2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jones, G. R., M. Christodoulides, J. L. Brooks, A. R. O. Miller, K. A. V. Cartwright, and J. E. Heckels. 1998. Dynamics of carriage of Neisseria meningitidis in a group of military recruits: subtype stability and specificity of the immune response following colonisation. J. Infect. Dis. 178:451-459. [DOI] [PubMed] [Google Scholar]
- 26.Kadowaki, N., S. Ho, S. Antonenko, R. W. Malefyt, R. A. Kastelein, F. Bazan, and Y. J. Liu. 2001. Subsets of human dendritic cell precursors express different Toll-like receptors and respond to different microbial antigens. J. Exp. Med. 194:863-869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kalinski, P., C. M. Hilkens, E. A. Wierenga, and M. L. Kapsenberg. 1999. T-cell priming by type-1 and type-2 polarized dendritic cells: the concept of a third signal. Immunol. Today 20:561-567. [DOI] [PubMed] [Google Scholar]
- 28.Kalinski, P., C. M. U. Hilkens, A. Snijders, F. G. M. Snijdewint, and M. L. Kapsenberg. 1997. IL-12-deficient dendritic cells, generated in the presence of prostaglandin E2, promote type 2 cytokine production in maturing human naive T helper cells. J. Immunol. 159:28-35. [PubMed] [Google Scholar]
- 29.Kolb-Maurer, A., A. Unkmeir, U. Kammerer, C. Hubner, T. Leimbach, A. Stade, E. Kampgen, M. Frosch, and G. Dietrich. 2001. Interaction of Neisseria meningitidis with human dendritic cells. Infect. Immun. 69:6912-6922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kopp, E. B., and R. Medzhitov. 1999. The Toll-receptor family and control of innate immunity. Curr. Opin. Immunol. 11:13-18. [DOI] [PubMed] [Google Scholar]
- 31.Lambden, P. R., and J. E. Heckels. 1982. Synthesis of immunogenic oligosaccharide-protein conjugates from the lipopolysaccharide of Neisseria gonorrhoeae p9. J. Immunol. Methods 48:233-240. [DOI] [PubMed] [Google Scholar]
- 32.Langenkamp, A., M. Messi, A. Lanzavecchia, and F. Sallusto. 2000. Kinetics of dendritic cell activation: impact on priming of Th1, Th2 and nonpolarized T-cells. Nat. Immunol. 1:311-316. [DOI] [PubMed] [Google Scholar]
- 33.Lanzavecchia, A. 1990. Receptor-mediated antigen uptake and its effect on antigen presentation to class II-restricted T lymphocytes. Annu. Rev. Immunol. 8:773-793. [DOI] [PubMed] [Google Scholar]
- 34.Mackinnon, F. G., Y. Ho, M. S. Blake, F. Michon, A. Chandraker, M. H. Sayegh, and L. M. Wetzler. 1999. The role of B/T costimulatory signals in the immunopotentiating activity of neisserial porin. J. Infect. Dis. 180:755-761. [DOI] [PubMed] [Google Scholar]
- 35.Massari, P., P. Henneke, Y. Ho, E. Latz, D. T. Golenbock, and L. M. Wetzler. 2002. Cutting edge: immune stimulation by neisserial porins is Toll-like receptor 2 and MyD88 dependent. J. Immunol. 168:1533-1537. [DOI] [PubMed] [Google Scholar]
- 36.Ozinsky, A., D. M. Underhill, J. D. Fontenot, A. M. Hajjar, K. D. Smith, C. B. Wilson, L. Schroeder, and A. Aderem. 2000. The repertoire for pattern recognition of pathogens by the innate immune system is defined by cooperation between Toll-like receptors. Proc. Natl. Acad. Sci. USA 97:13766-13771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pridmore, A. C., D. H. Wyllie, F. Abdillahi, L. Steeghs, P. van der Ley, S. K. Dower, and R. C. Read. 1901. A lipopolysaccharide-deficient mutant of Neisseria meningitidis elicits attenuated cytokine release by human macrophages and signals via Toll-like receptor (TLR) 2 but not via TLR4/MD2. J. Infect. Dis. 183:89-96. [DOI] [PubMed] [Google Scholar]
- 38.Read, R. C., J. Pullin, S. Gregory, R. Borrow, E. B. Kaczmarski, F. S. di Giovine, S. K. Dower, C. Cannings, and A. G. Wilson. 2001. A functional polymorphism of Toll-like receptor 4 is not associated with likelihood or severity of meningococcal disease. J. Infect. Dis. 184:640-642. [DOI] [PubMed] [Google Scholar]
- 39.Rescigno, M., M. Martino, C. L. Sutherland, M. R. Gold, and P. Ricciardi-Castagnoli. 1998. Dendritic cell survival and maturation are regulated by different signaling pathways. J. Exp. Med. 188:2175-2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schagger, H., and G. von Jagow. 1987. Tricine-sodium dodecyl sulphate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal. Biochem. 166:368-379. [DOI] [PubMed] [Google Scholar]
- 41.Simpson, S. D., Y. Ho, P. A. Rice, and L. M. Wetzler. 1999. T lymphocyte response to Neisseria gonorrhoeae porin in individuals with mucosal gonococcal infections. J. Infect. Dis. 180:762-773. [DOI] [PubMed] [Google Scholar]
- 42.Smirnova, I., N. Mann, A. Dols, H. H. Derkx, M. L. Hibberd, M. Levin, and B. Beutler. 2003. Assay of locus-specific genetic load implicates rare Toll-like receptor 4 mutations in meningococcal susceptibility. Proc. Natl. Acad. Sci. USA 100:6075-6080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sparwasser, T., E.-S. Koch, R. M. Vabulas, K. Heeg, G. B. Lipford, J. W. Ellwart, and H. Wagner. 1998. Bacterial DNA and immunostimulatory CpG oligonucleotides trigger maturation and activation of murine dendritic cells. Eur. J. Immunol. 28:2045-2054. [DOI] [PubMed] [Google Scholar]
- 44.Sprong, T., N. Stikkelbroeck, P. van der Ley, L. Steeghs, L. van Alphen, N. Klein, M. G. Netea, J. W. M. van der Meer, and M. van Deuren. 2001. Contributions of Neisseria meningitidis LPS and non-LPS to proinflammatory cytokine response. J. Leukoc. Biol. 70:283-288. [PubMed] [Google Scholar]
- 45.Steeghs, L., H. de Cock, E. Evers, B. Zomer, J. Tommassen, and P. van der Ley. 2001. Outer membrane composition of a lipopolysaccharide-deficient Neisseria meningitidis mutant. EMBO J. 20:6937-6945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Steeghs, L., R. den Hartog, A. den Boer, B. Zomer, P. Roholl, and P. van der Ley. 1998. Meningitis bacterium is viable without endotoxin. Nature 392:449-450. [DOI] [PubMed] [Google Scholar]
- 47.Steinman, R. M. 1991. The dendritic cell system and its role in immunogenicity. Annu. Rev. Immunol. 9:271-296. [DOI] [PubMed] [Google Scholar]
- 48.Stephens, D. S., K. M. Edwards, and F. M. G. Morris. 1982. Pili and outer membrane appendages on Neisseria meningitidis in the cerebrospinal fluid of an infant. J. Infect. Dis. 146:568. [DOI] [PubMed] [Google Scholar]
- 49.Stumbles, P. A., J. A. Thomas, C. L. Pimm, P. T. Lee, T. J. Venaille, S. Proksch, and P. G. Holt. 1998. Resting respiratory tract dendritic cells preferentially stimulate T helper cell type 2 (Th2) responses and require obligatory cytokine signals for induction of Th1 immunity. J. Exp. Med. 188:2019-2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tanaka, H., C. E. Demeure, M. Rubio, G. Delespesse, and M. Sarfati. 2000. Human monocyte-derived dendritic cells induce naive T cell differentiation into T helper cell type 2 (Th2) or Th1/Th2 effectors. Role of stimulator/responder ratio. J. Exp. Med. 192:405-412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tinsley, C. R., and J. E. Heckels. 1986. Variation in the expression of pili and outer membrane protein by Neisseria meningitidis during the course of meningococcal infection. J. Gen. Microbiol. 132:2483-2490. [DOI] [PubMed] [Google Scholar]
- 52.Unkmeir, A., U. Kammerer, A. Stade, C. Hubner, S. Haller, A. Kolb-Maurer, M. Frosch, and G. Dietrich. 2002. Lipooligosaccharide and polysaccharide capsule: virulence factors of Neisseria meningitidis that determine meningococcal interaction with human dendritic cells. Infect. Immun. 70:2454-2462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Uronen, H., A. J. Williams, G. Dixon, S. R. Andersen, P. van der Ley, M. van Deuren, R. E. Callard, and N. Klein. 2000. Gram-negative bacteria induce proinflammatory cytokine production by monocytes in the absence of lipopolysaccharide (LPS). Clin. Exp. Immunol. 122:312-315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.van Deuren, M., P. Brandtzaeg, and J. van der Meer. 2000. Update on meningococcal disease with emphasis on pathogenesis and clinical management. Clin. Microbiol. Rev. 13:144-166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wetzler, L. M. 1994. Induction of B-lymphocyte costimulatory ligand B7-2 by neisserial porins—possible mechanism behind their adjuvant activity. Clin. Res. 42:287. [Google Scholar]
- 56.Whelan, M., M. M. Harnett, K. M. Houston, V. Patel, W. Harnett, and K. P. Rigley. 2000. A filarial nematode-secreted product signals dendritic cells to acquire a phenotype that drives development of Th2 cells. J. Immunol. 164:6453-6460. [DOI] [PubMed] [Google Scholar]