Abstract
Aggregation substance (AS), a surface protein encoded on the pheromone-inducible plasmids of Enterococcus faecalis, has been shown to increase adherence and internalization into a number of different cell types, presumably through integrin binding mediated by the N-terminal RGD motif of AS. Here, defined mutations constructed in Asc10, the AS encoded by the plasmid pCF10, are analyzed for their ability to promote increased internalization levels into HT-29 enterocytes. The results clearly show that the previously identified Asc10 functional domain, not the RGD motifs, is critical for Asc10-directed internalization of E. faecalis into HT-29 enterocytes. Also, expression of Asc10 in the nonaggregating E. faecalis strain INY3000 is unable to mediate HT-29 internalization. However, Asc10-expressing E. faecalis cells are not internalized as bacterial aggregates, suggesting bacterial aggregation is not a prerequisite for HT-29 internalization. These data show that Asc10 directs internalization of E. faecalis into HT-29 enterocytes through a non-RGD-dependent mechanism.
Enterococcus faecalis is a growing health concern due to its increased incidence as a causative agent of nosocomial infections, its high levels of antibiotic resistance, and the ability of enterococci to genetically mobilize these resistance elements (for review, see reference 15). A major class of mobilizable DNA in enterococci is the pheromone-inducible plasmid family. Upon exposure to a 7- to 8-amino-acid peptide pheromone secreted by the plasmid-free recipient cell, plasmid-containing donor cells induce expression of conjugation machinery leading to DNA transfer (24). Approximately 20 pheromone plasmids have been identified (40), and they have been found to be more prevalent in clinical enterococcal strains (4). The increased prevalence of pheromone plasmids in clinical strains is likely due to the various antibiotic resistance genes and virulence factors encoded on the plasmids (40). One such factor, a surface protein known as aggregation substance (AS), appears to be encoded on most if not all pheromone plasmids (14) and serves to promote both conjugation of the plasmids and pathogenesis of E. faecalis. Interestingly, a gene encoding AS was recently identified on a pathogenicity island of E. faecalis (31).
With the exception of Asa373 (22), the genes encoding AS proteins are highly conserved (approximately 90% identity). The three most studied AS proteins, Asa1, Asp1, and Asc10, all encode two Arg-Gly-Asp (RGD) integrin binding motifs (28, 40) and are induced in serum without addition of their cognate pheromone (13, 19). These observations initiated studies that found AS promotes adhesion to and invasion of E. faecalis in eukaryotic cells. Asa1 increased adherence to cultured porcine renal tubular cells (19) and adherence to and survival in human macrophages (32). Asc10 was shown to increase internalization into polymorphonuclear leukocytes (PMNs) (34) and led to increased intracellular survival in PMNs (26). AS also increases invasion into the cell lines HT-29, HT-29/1, T84, and Hutu 80 derived from the duodenum and colon, but not the HCT-8 cell line derived from the ileum (23, 29, 38). Importantly, translocation of the intestine by E. faecalis has been hypothesized to be a focal point of more systemic infections (37). Asc10 and Asa1 have both been shown to promote binding of E. faecalis to extracellular matrix molecules (12, 27), and adherence to colon cells mediated by Asa1 has been predicted to occur through fibronectin binding (18). Both Asa1- and Asc10-expressing enterococci have been shown to increase the pathogenicity of E. faecalis in a rabbit infective endocarditis model (3, 13, 30).
The RGD motifs of AS are thought to direct binding of E. faecalis to eukaryotic cell surface integrins, as adherence to renal cells (19), PMNs (34), and macrophages (32) was reduced when the eukaryotic cells were preincubated with RGDS peptide inhibitors. Also, monoclonal antibodies to CR3, CD47, and L-selectin have been shown to reduce AS-mediated adherence to PMNs (34) and macrophages (32). However, no studies have conclusively shown that either RGD motif is necessary for adherence via disruption of these domains using site-directed mutagenesis.
Previously, our investigators described identification of an aggregation domain in Asc10 located between amino acids 473 and 683 by using a collection of 23 31-codon insertion mutants in prgB, the gene encoding Asc10 on the plasmid pCF10 (35, 36). These results were in general agreement with the aggregation domain identified in Asa1 by using in-frame deletion mutations and purified protein fragments (22). In the present study, examination of HT-29 internalization mediated by the Asc10 insertion mutant proteins revealed that the previously identified aggregation functional domain is essential for increased internalization levels. Also, disruption of the RGD motifs by site-directed mutagenesis showed no significant decrease in internalization levels. Expression of Asc10 in the nonaggregating strain INY3000 showed very low levels of internalization. However, the internalization of non-Asc10-expressing E. faecalis is not increased in the presence of Asc10-expressing strains, suggesting that E. faecalis cells were not being internalized as aggregates. These results show that Asc10 promotes internalization of E. faecalis into HT-29 enterocytes through a non-RGD-dependent mechanism.
MATERIALS AND METHODS
Bacterial culture conditions and nisin induction.
E. faecalis was grown at 37°C with gentle shaking in Todd-Hewitt broth (THB; Difco). For DNA isolation and manipulation, Escherichia coli (CC118) was grown at 37°C with shaking in Luria-Bertani medium or brain heart infusion broth (Difco) for erythromycin selection. The antibiotics and concentrations used for E. faecalis were erythromycin (10 μg/ml), rifampin (200 μg/ml), and streptomycin (1 mg/ml), while the antibiotic and concentration used for E. coli was erythromycin (100 μg/ml in brain heart infusion broth) or carbenicillin (50 μg/ml). All antibiotics were obtained from Sigma. prgB and its derivatives were expressed using the nisin-inducible expression plasmid pMSP3535 (2). In this plasmid, transcription of genes is driven from the nisA promoter by using nisin, a small antimicrobial peptide produced by Lactococcus lactis. For induction, nisin was added to a final concentration of 25 ng/ml, which had no observable effects on the growth of E. faecalis.
DNA manipulation and construction of RGD mutations.
Plasmids were isolated with a Qiagen midi or mini kit as recommended by the manufacturer. Restriction enzymes were purchased from Promega, Gibco-BRL, and New England Biolabs. PCR was performed with a Perkin-Elmer Gene Amp PCR system or an Eppendorf Mastercycler using BioXact DNA polymerase (Bioline). The ligation reaction utilized T4 DNA ligase (Gibco-BRL) either overnight at 17°C or for 1 to 2 h at room temperature. All sequencing and primer synthesis were done by the Microchemical Facility at the University of Minnesota. The site-directed RGD mutations in prgB were constructed in the following manner. For the RADS mutation (G607 to A607), two PCR products were generated using BioXact DNA polymerase (Bioline) from the prgB gene in the vector pMSP7517, using the following primer sets: BsrGIf (5′-AGAGATCTACTGATAATGTACAAGC-3′) and RADSr (GTAAAGAGTCGGCACGTTTCAC-3′) and RADSf (5′-GTGAAACGTGCCGACTATTTAC-3′) and BtrI/r (5′-TAGGCTTAAGAAGCAGTCACGTCTTTCGC-3′) with amplification for 20 cycles. The two products were combined and amplified for five PCR cycles using BioXact. The BsrGIf and BtrI/r primers were then added, and amplification was carried out for another 20 cycles. The resulting product was cloned into pGEM-T Easy (Promega). The final nisin-inducible construct, pMSP3607, was generated by digesting and ligating the BsrGI/PshAI fragment from pGEM-T Easy/RADS into a BsrGI-cut PshAI-partial cut pMSP7517 vector backbone to yield a full-length, nisin-inducible prgB gene with the RADS mutation (Table 1). The RADV mutation (G940 to A940) was constructed in the same manner, utilizing the primer sets BsrGIf and RADVr (5′-GAAAGAACATCAGCACGAGCCA-3′) and RADVf (5′-TGGCTCGTGCTGATGTTCTTC-3′) and B3247r (5′-TGACTTTGTTTGTCACC-3′). The RADV mutation was moved to the prgB gene by exchanging the PshAI fragments of pGEM-T Easy/RADV and pMSP7517 to create pMSP3608. Both constructs were sequenced and had the correct G→A mutation. pMSP3607 had no extra additional mutations generated by PCR, while pMSP3608 had a T2112-to-C2112 mutation; however, this mutation is in the wobble position of a valine codon, and the amino acid sequence of the resulting protein is not altered. The double RGD mutant, pMSP3609, was constructed by cloning the PshAI fragment of pMSP3608 into pMSP3607. After electroporation of the plasmids into E. faecalis OG1RF, all of the mutations were again confirmed by sequencing the PCR product derived from the mutant prgB gene.
TABLE 1.
Strains and plasmids used in this study
| Strain or plasmid | Description | Source or reference |
|---|---|---|
| E. coli strain | ||
| CC118 | C. Manoil | |
| E. faecalis strains | ||
| OG1RF | Rifampin, fucidic acid resistant | 6 |
| OG1SSp | Streptomycin, spectinomycin resistant | 6 |
| INY3000 | Streptomycin, spectinomycin resistant, aggregation mutant | 33 |
| Plasmids | ||
| pMSP3535 | Nisin-inducible cloning shuttle vector | 2 |
| pMSP7517 | Nisin-inducible Asc10 in pMSP3535 | 12 |
| pMSP3607 | N-terminal RGD→RAD in prgB | This study |
| pMSP3608 | C-terminal RGD→RAD in prgB | This study |
| pMSP3609 | Double RGD→RAD in prgB | This study |
| pCWΩ258 | 31-amino-acid insertion at base 258 in prgB gene of pMSP7517 | 36 |
| pCWΩ438 | 31-amino-acid insertion at base 438 in prgB gene of pMSP7517 | 36 |
| pCWΩ468 | 31-amino-acid insertion at base 468 in prgB gene of pMSP7517 | 36 |
| pCWΩ1419 | 31-amino-acid insertion at base 1419 in prgB gene of pMSP7517 | 36 |
| pCWΩ1551 | 31-amino-acid insertion at base 1551 in prgB gene of pMSP7517 | 36 |
| pCWΩ1638 | 31-amino-acid insertion at base 1638 in prgB gene of pMSP7517 | 36 |
| pCWΩ2064 | 31-amino-acid insertion at base 2064 in prgB gene of pMSP7517 | 36 |
| pCWΩ2601 | 31-amino-acid insertion at base 2601 in prgB gene of pMSP7517 | 36 |
| pCWΩ2979 | 31-amino-acid insertion at base 2979 in prgB gene of pMSP7517 | 36 |
| pCWΩ3414 | 31-amino-acid insertion at base 3414 in prgB gene of pMSP7517 | 36 |
Quantification of aggregation.
Aggregation of induced cultures was measured as previously described (36). Briefly, 1 ml of a nisin-induced overnight culture was allowed to settle in a plastic cuvette for 1 h, and the optical density at 600 nm was measured using a Beckman DU-70 spectrophotometer.
Western blot analysis.
Proteins expressed on the cell surface of E. faecalis cells were harvested using lysozyme as previously described (8). The lysozyme extraction buffer was slightly modified to include 25 mM EDTA and 5 mg of lysozyme/ml. An equivalent volume (12 μl out of a 200-μl total lysis extraction) was loaded onto a sodium dodecyl sulfate-7.5% polyacrylamide gel electrophoresis (SDS-PAGE). Western blot analysis was performed with an antibody constructed against an N-terminal domain of Asc10 (21) at a dilution of 1/2,500. Detection was performed with the enhanced chemiluminescence protocol (Pierce).
Bacterial internalization into HT-29 enterocytes.
Internalization of E. faecalis strains was determined as previously described (38). Overnight cultures induced with nisin were used for all internalization assays with the exception of the experiment reported below in Fig. 1B. For this experiment, overnight cultures washed twice with 0.9% saline to remove extracellular proteases were inoculated 1/100 in fresh THB with erythromycin and nisin and grown for 3 h with shaking at 37°C. Prior to addition of the bacteria to the HT-29 enterocytes, the cultures were washed twice with 0.9% saline and sonicated at 20 W for 10 s on a 40-W high-intensity ultrasonic processor (Sonics and Materials, Danbury, Conn.) to disperse aggregates. It should be noted that the cultures reaggregated during the assay, as determined by visible observation. The density of each culture was adjusted to 109 CFU/ml by addition of 0.9% saline, using densitometry, and confirmed by dilution plating. One hundred microliters of bacteria was then added to a confluent layer of 105.7 to 106 HT-29 enterocytes (cultured in Dulbecco's modified Eagle's medium supplemented with 15% dialyzed fetal bovine serum, 4 mmol of l-glutamine per liter, and 5 mM galactose for at least 21 days). After 1 h of incubation at 37°C, the enterocytes were washed five times with Hanks' balanced salt solution (HBSS) to remove nonadherent bacteria. The remaining extracellular bacteria were killed by addition of 1 ml of tissue culture medium containing 50 μg of penicillin G (Sigma)/ml and 10 μg of gentamicin/ml and incubating for 2.5 h at 37°C. The effectiveness of the antibiotic treatment was confirmed by plating 50 μl of medium from each well. The enterocytes were then washed three times with HBSS and incubated for 5 min at 37°C with 1 ml of 1% Triton X-100. Invasive bacteria were quantified by serial dilution and plating on THB with erythromycin. In the protocol described above (designated as standard conditions in this report), the HT-29 enterocytes form monolayers with tight junctions. To disrupt the HT-29 monolayers and expose the lateral surface of the enterocytes, calcium-dependent junctional complexes were disrupted by pretreatment of the enterocytes with 1 ml of Krebs Ringer's solution (Sigma) for 1 h at 37°C immediately prior to addition of the bacteria, as previously described (38, 39). When indicated, bacteria were pelleted onto the HT-29 monolayer at 182 × g for 10 min as previously described (5). For the cointernalization experiment described below in Fig. 6, overnight cultures of OG1RF(7517), OG1SSp(3535), and INY3000(3535) were washed and adjusted to 109 CFU/ml as described above. The cultures were then mixed at a ratio of 4:1, 1:1, or 1:4, and 100 μl of the mixed culture was added to each well. Internalization of OG1RF was differentiated from internalized OG1SSp or INY3000 by plating on THB supplemented with rifampin-erythromycin or streptomycin-erythromycin, respectively. For all internalization assays, each strain was examined in three wells for each experiment. The mean of at least three experiments performed on separate days is reported. The number of invasive organisms was converted to a log10 value. The lower limit of detection of this assay is 50 bacteria or 1.7 log10 units, and wells with no detectable viable internalized bacteria were given this value.
FIG. 1.
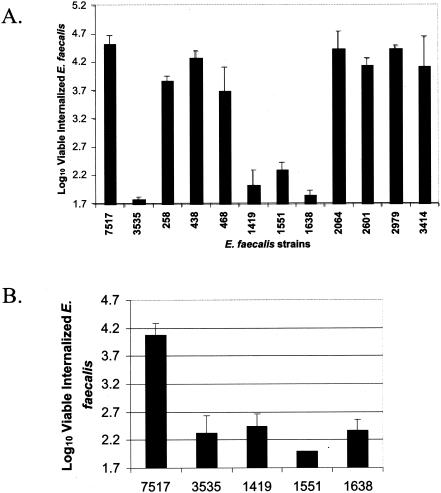
The aggregation domain of Asc10 is required for efficient HT-29 internalization. (A) The numbers of viable, internalized OG1RF cells expressing wild-type Asc10 (7517), the vector control (3535), or 10 different insertion mutants from stationary-phase cultures into HT-29 enterocytes under standard conditions is plotted on a log10 scale. Note that log10 1.7 is the minimum number of bacteria that can be measured in this assay. (B) Internalization of the Asc10 insertion mutants Ω1419, Ω1551, and Ω1638 grown to exponential phase is shown, as described for panel A. Error bars indicate the standard errors of the means.
FIG. 6.
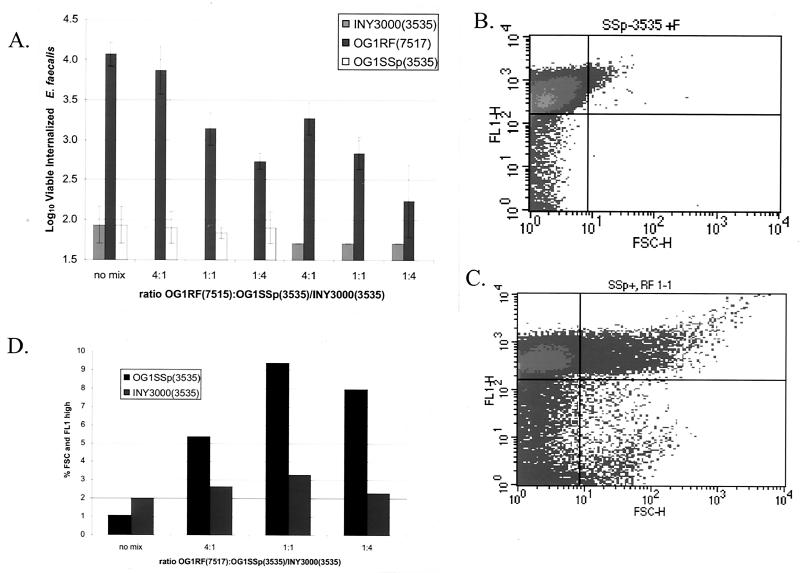
Multicellular bacterial aggregates do not invade HT-29 enterocytes. (A) OG1RF(7517) was mixed with OG1SSp(3535) or INY3000(3535) at ratios of 4:1, 1:1, and 1:4 after adjustment of each culture to 109 CFU/ml. The mixed cultures were then added to HT-29 monolayers, and a standard internalization experiment was performed. The data are plotted as the log10 value of viable internalized E. faecalis organisms. Error bars represent the standard errors of the means. (B and C) An FL-1 (y axis), FSC (x axis) FACS quadrant analysis of OG1SSp(3535) labeled with FITC unmixed (B) or mixed at a 1:1 ratio with unlabeled OG1RF(7517) (C) is shown. (D) The percentage of each mixed population that forms mixed aggregates (high FL-1 and FSC) of FITC-labeled OG1SSp(3535) and INY3000(3535) unmixed or mixed with unlabeled OG1RF(7517) at the ratio of 4:1, 1:1, or 1:4 is indicated. 7517 = Asc10+; 3535 = Asc10−.
Confirmation of mixed aggregates.
To confirm that OG1RF(7517) expressing Asc10 was forming mixed aggregates with OG1SSp(3535), overnight cultures of OG1SSp(3535) and INY3000(3535) were washed twice with 0.9% saline and labeled with 0.1% fluorescein isothiocyanate (FITC) (Sigma) in 50 mM carbonate buffer at pH 9.5 for 30 min at 37°C. This treatment has no effect on cell viability. The labeled bacteria were then washed three times with HBSS to removed unbound FITC and incubated at the indicated ratio with overnight nisin-induced cultures of OG1RF(7517) that had been washed twice with HBSS. After 1 h of incubation, the mixture was examined by fluorescence-activated cell sorter (FACS) analysis on a Becton Dickinson FACScan. The data were analyzed using the software CellQuest, version 3.3 (Becton Dickinson). Mixed aggregates were indicated by high FL-1 and forward scatter (FSC) profiles. The experiment was also duplicated with unlabeled OG1SSp(3535) and INY3000(3535) as controls, and no FL-1 high events were observed (data not shown).
Statistical analysis.
Statistical significance was determined by an analysis of variance performed with the program StatView version 4.5 (Abacus Concepts, Inc.). Differences with P values of <0.05 were considered significant.
RESULTS
The aggregation domain is essential for efficient HT-29 internalization.
In a previous manuscript, we reported the construction of a library of 23 31-codon, nonpolar insertions in Asc10, the AS of pCF10 (36) generated with the transposons TnLacZ/in and TnPhoA/in (20). Analysis of the Asc10 mutant proteins identified an aggregation functional domain from amino acids 473 to 683 (35, 36). The nomenclature used to designate the mutations refers to the base number of prgB preceding the insertion site.
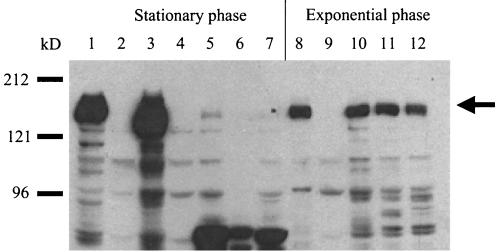
To measure HT-29 internalization, Asc10 and its mutant derivatives were expressed in the E. faecalis strain OG1RF grown to stationary phase using the nisin-inducible construct pMSP3535(3535). pMSP7517(7517) is a pMSP3535 derivative that expresses wild-type Asc10. The log10 value of internalized Asc10-expressing OG1RF(7517) under standard assay conditions was 4.5, while the corresponding levels for OG1RF(3535) were barely detectable at 1.8 (Fig. 1A) (1.7 is the minimum value). Ten Asc10 insertion mutant proteins were examined for the ability to promote increased internalization of OG1RF into HT-29 enterocytes. Seven insertions promoted internalization levels that were not significantly different from that of the wild-type protein, while three insertions, Ω1419, Ω1551, and Ω1638, showed levels similar to the vector control (Fig. 1A). To confirm expression of Ω1419, Ω1551, and Ω1638 on the cell surface from the stationary-phase cultures, a lysozyme surface extract was Western blotted with an anti-Asc10 polyclonal antibody (Fig. 2, lanes 3 to 7). Although Asc10-specific degradation products were visualized, almost no full-length Asc10 was observed. The lack of full-length Asc10 on the cell surface in these cultures is likely due to degradation by GelE, a secreted protease of E. faecalis expressed in high-density cultures (25), as our investigators have shown that some of the 31-amino-acid insertions increase susceptibility to GelE proteolysis (35). Thus, to further examine if Ω1419, Ω1551, and Ω1638 can direct HT-29 internalization, mid-exponential-phase cultures were utilized. Under these conditions, the aggregation insertion mutants were shown to express full-length protein at levels equivalent to that of wild-type Asc10 (Fig. 2, lanes 8 to 12) but were unable to direct efficient internalization of E. faecalis cells into HT-29 enterocytes (Fig. 1B). These results indicate that the previously identified aggregation domain is critical for Asc10-mediated HT-29 internalization.
FIG. 2.
Asc10 expression in strains used in this study. Lysozyme surface extractions were electrophoresed in an SDS-7.5% PAGE gel and Western blotted with an anti-Asc10 polyclonal antibody. Lanes 1 to 7 are from stationary-phase cultures grown overnight, while lanes 8 to 12 are exponential-phase cultures as indicated in Materials and Methods. Lanes: 1 = INY3000(7517); 2 = INY3000(3535); 3 and 8 = OG1RF(7517); 4 and 9 = OG1RF(3535); 5 and 10 = OG1RF(Ω1419); 6 and 11 = OG1RF(Ω1551); 7 and 12 = OG1RF(Ω1638). Molecular mass markers are indicated to the left of the blot. The arrow indicates full-length Asc10.
RGD mutations in Asc10 do not affect aggregation.
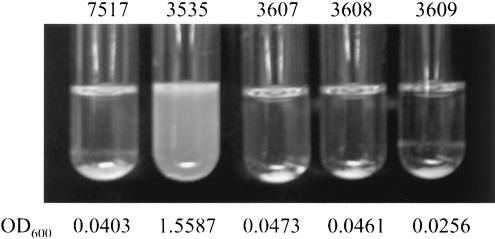
Previous analyses of E. faecalis cell adhesion suggested that AS may mediate increased cell adhesion through integrin binding directed by the RGD motifs (19, 32, 34). To directly examine the role of the RGD motifs, three site-specific RGD mutant prgB genes were constructed: pMSP3607 (3607) has a mutation in the N-terminal RGD motif (G607 to A607), pMSP3608 (3608) has a mutation in the C-terminal RGD motif (G940 to A940), and pMSP3609 (3609) has mutations in both RGD motifs. As RGD motifs serve as cell adhesion domains in eukaryotic systems, it was possible the RGD motifs of AS mediate bacterial aggregation through a similar mechanism. However, analysis of E. faecalis cells expressing the Asc10 RGD mutant proteins clearly showed that aggregation is unaffected by the disruption of these motifs (Fig. 3).
FIG. 3.
Mutation of the RGD motifs had no effect on aggregation. Overnight nisin-induced cultures of OG1RF expressing wild-type Asc10 (7517), the vector control (3535), the N-terminal RGD→RAD (3607) mutation, C-terminal RGD→RAD (3608) mutation, or the double RGD→RAD mutations (3609) allowed to settle for 1 min are shown. The numbers below the pictures indicate the optical density readings at 600 nm of the settled cultures.
The RGD motifs are not required for HT-29 internalization.
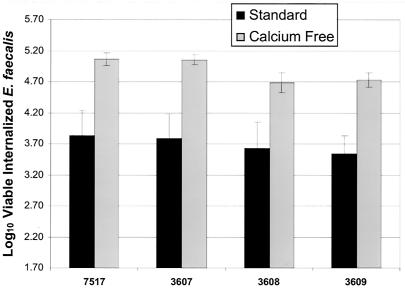
OG1RF strains expressing the Asc10 RGD mutant proteins showed no reduction in internalization levels into HT-29 enterocytes under standard conditions (Fig. 4). Under these conditions, HT-29 cells form monolayers with tight junctions. However, integrin expression is thought to be more prominent on the apical surface of epithelial cells (17). Thus, to expose the lateral surface of the enterocytes, the monolayers were pretreated for 1 h with calcium-free medium. Under these conditions, the calcium-dependent junctions of the HT-29 epithelial cells loosen, exposing more of the basolateral surface of the enterocytes without affecting enterocyte viability (38, 39). However, even under calcium-free conditions, no statistically significant difference (P < 0.05) could be seen in the uptake levels of wild-type Asc10 versus those of the RGD mutant proteins (Fig. 4).
FIG. 4.
Mutations in the RGD motifs of Asc10 did not affect internalization into HT-29 enterocytes. Overnight cultures of OG1RF expressing wild-type Asc10 (7517), the vector control (3535), the N-terminal RGD→RAD (3607) mutation, C-terminal RGD→RAD (3608) mutation, or the double RGD→RAD mutations (3609) were analyzed for the ability to promote internalization into HT-29 enterocytes under both standard and calcium-free conditions. Invasion levels mediated by the RGD mutant proteins did not show any significant reduction in HT-29 internalization under either condition. The data are plotted as the log10 value of internalized E. faecalis (1.7 is the minimum value of the assay). The error bars indicate the standard errors of the means.
INY3000 expressing Asc10 shows low internalization levels.
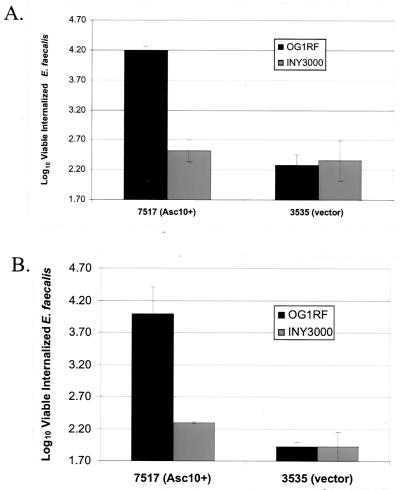
Analysis of the Asc10 insertion and RGD mutations suggested the following possibilities: that bacterial aggregation is required for increased internalization, that the functional domains that promote aggregation and HT-29 internalization overlap, or that one domain mediates both processes. To further examine the role of bacterial aggregation, the internalization of the E. faecalis strain INY3000 expressing wild-type Asc10 was measured. INY3000 contains four Tn916 insertions that abolish its ability to aggregate (33). As shown in Fig. 5A, expression of Asc10 in INY3000 did not lead to efficient internalization of E. faecalis into HT-29 enterocytes. To confirm expression of Asc10 in INY3000(7517), a lysozyme surface extraction of this strain was Western blotted with an anti-Asc10 polyclonal antibody, and it showed high levels of full-length Asc10 on the cell surface (Fig. 2, lane 1). Interestingly, Asc10 purified from the cell surface of INY3000 consistently migrated slower on an SDS-PAGE gel than Asc10 purified from OG1RF (Fig. 2, lane 1 versus lane 3) (see Discussion). These results supported the hypothesis that bacterial aggregation is required for efficient HT-29 internalization. However, it must be noted that the four transposon insertions in INY3000 likely lead to uncharacterized pleiotropic effects outside of a disruption in the Asc10 receptor (see Discussion).
FIG. 5.
Expression of Asc10 in INY3000 did not promote internalization into HT-29 enterocytes. (A) Asc10 was expressed in the wild-type E. faecalis strain OG1RF or the nonaggregating E. faecalis mutant strain INY3000 and analyzed for internalization into HT-29 enterocytes grown under standard conditions. The data are plotted as the log10 value of viable internalized E. faecalis (1.7 is the minimum value of the assay). (B) The same four strains shown in panel A were pelleted onto HT-29 monolayers (182 × g for 10 min), and a standard internalization assay was performed. Error bars for both graphs represent the standard errors of the means.
Increased HT-29 contact does not account for Asc10-mediated internalization.
Visual observation indicated that OG1RF(7517) reaggregates during the course of the internalization assay. Thus, the increased internalization mediated by Asc10 could be due to settling of bacterial aggregates onto the HT-29 monolayers, increasing the contact of the bacteria with the enterocytes. To examine this potential artifact, OG1RF(7517), OG1RF(3535), INY3000(7517), and INY3000(3535) were pelleted onto the monolayer before performing an internalization assay. The centrifugation protocol utilized has been shown to enhance the internalization of Streptococcus pyogenes into different cell lines, presumably through increased contact with the eukaryotic cells (P. P. Cleary, personal communication). However, even with centrifugation, no increase in the internalization levels of any of the four strains examined was observed (Fig. 5B), suggesting that the increased internalization directed by Asc10 is not simply due to increased bacterial contact with the HT-29 enterocytes.
E. faecalis cells were not internalized as aggregates.
If bacterial aggregation is required for efficient internalization of E. faecalis into HT-29 enterocytes, one would predict that Asc10-expressing E. faecalis cells would be internalized as aggregates. To directly test if aggregates are internalized, the Asc10-expressing strain OG1RF(7517) was mixed in an internalization assay with the non-Asc10-expressing strains OG1SSp(3535) and INY3000(3535). OG1RF(7517) can form mixed aggregates with OG1SSp(3535) but not with INY3000(3535) (see below), as this strain is unable to aggregate (33). If Asc10-expressing E. faecalis cells were internalized as aggregates, OG1SSp(3535) would be predicted to have higher internalization levels in the presence of OG1RF(7517) due to the formation of mixed aggregates, while INY3000(3535) internalization would be unaffected by OG1RF(7517). Internalized OG1RF(7517) can be differentiated from internalized OG1SSp(3535) or INY3000(3535) by selection on rifampin or spectinomycin, respectively. As previously described, when assayed alone OG1RF(7517) showed high internalization (log10 of 4.1), while internalization of OG1SSp(3535) or INY3000(3535) alone was barely detectable (both log10 of 1.9) (Fig. 6A). Mixing of OG1RF(7517) with OG1SSp(3535) at any ratio examined (4:1, 1:1, or 1:4) did not increase the number of internalized OG1SSp(3535). As expected, this result was also observed when OG1RF(7517) was mixed with INY3000(3535) (Fig. 6A).
To confirm that mixed aggregates were formed, both OG1SSp(3535) and INY3000(3535) were labeled with FITC prior to mixing with unlabeled OG1RF(7517) at the ratios examined in the internalization assay. After 1 h, the mixed cultures were examined using FACS analysis. Representative FACS quadrant analyses are shown in Fig. 6B [OG1SSp(3535) alone] and 6C [OG1SSp(3535)-OG1RF(7517) mixed 1:1]. The percentage of each mixed population that was high FL-1, FSC (upper right quadrant) for all mixed cultures is graphed in Fig. 6D. As shown in Fig. 6B, FITC labeling increased the intensity of OG1SSp(3535) in the FL-1 spectrum. Unlabeled OG1SSp(3535) had a mean FL-1 value of only 1.06 at these settings (data not shown). However, the FSC profile, which measures particle size, of OG1SSp(3535) was low due to a lack of bacterial aggregation. However, when FITC-labeled OG1SSp(3535) was mixed with OG1RF(7517) at all ratios examined, an increase of FL-1 and FSC high events was observed (Fig. 6C and D), indicating mixed aggregate formation. Also, it is of note that when OG1SSp(3535) and OG1RF(7517) were mixed at a ratio of 1:1 (Fig. 6C), 73.4% of the FSC high events were also FL-1 high, indicating most aggregates consisted of both strains at this ratio. As previously described, this technique only gives a relative level of aggregation, as the aggregates are likely sheared during the analysis (36). As expected, mixing of FITC-labeled INY3000(3535) with OG1RF(7517) only showed modest increases in the FL-1, FSC high population versus FITC-labeled INY3000(3535) (Fig. 6D). In the absence of FITC labeling, no FL-1 high events under any mixing conditions were observed (data not shown). Thus, although OG1RF(7517) reaggregates during the internalization assay, these data indicate that Asc10-expressing E. faecalis cells are not being internalized into HT-29 enterocytes as bacterial aggregates.
DISCUSSION
In this report, examination of a collection of specific mutations in Asc10, the AS protein encoded by the E. faecalis plasmid pCF10, showed that the aggregation domain, not the RGD motifs, is critical for efficient internalization into HT-29 enterocytes. Also, expression of Asc10 in the nonaggregating strain INY3000 resulted in very low internalization levels. However, E. faecalis cells were not internalized as aggregates, suggesting that aggregation itself is not required.
Previous studies in renal cells, PMNs, and macrophages by using RGDS peptide inhibitors and antibodies that bound specific integrin receptors had implicated the RGD motifs of AS as critical functional regions for eukaryotic cell adherence (19, 32, 34). However, in none of these works was the contribution of the RGD motifs directly examined by site-directed mutagenesis. Analyses of the RGD mutations constructed in Asc10 in this study conclusively showed that they play no role in Asc10-mediated internalization into HT-29 enterocytes. It will be interesting to analyze if these RGD mutations affect cell association in neutrophils or macrophages as previously predicted.
Only one other report has attempted to define important regions of AS that mediate eukaryotic cell adherence by analysis of defined mutations (32). In that study, the authors examined the macrophage internalization of E. faecalis cells expressing a collection of Asa1 in-frame deletion mutants and concluded that the N-terminal RGD is necessary for adherence. This conclusion was based on the observation that three deletion mutants that removed the N-terminal RGD motif showed reduced internalization. However, another deletion, located about 33 amino acids to the N terminus of the RGD motif, maintained the motif but also showed reduced internalization levels. The authors hypothesized that this deletion disrupted correct presentation of the motif. However, all four deletion constructs that showed lower macrophage internalization levels also had lost the ability to reproducibly aggregate (22). In light of the data presented here, another interpretation of these data is that the loss of aggregation, and not the loss or disruption of the N-terminal RGD motif, led to decreased internalization.
Even though efficient internalization was only observed when an Asc10 protein with a functional aggregation domain was expressed in an E. faecalis strain capable of aggregation, the data suggest that bacterial aggregation itself is not required. One interpretation of these data is that efficient HT-29 internalization of E. faecalis requires both Asc10 with a functional aggregation domain and an additional factor that is absent or altered in INY3000. Previous characterization of the INY3000 strain found that it had four Tn916 transposon insertions that abolished its ability to act as a receptor for Asc10, and all of these insertions were required for the aggregation-deficient phenotype (33). Biochemical analysis of the cell surface of INY3000 showed alterations in its lipoteichoic acid (LTA), an immunostimulatory, amphiphilic surface molecule ubiquitous in gram-positive bacteria (7), suggesting that LTA my be part of the AS bacterial receptor (33).
Interestingly, the LTA of S. pyogenes has also been implicated as an adhesin (for review, see references 10 and 11). The current model of LTA-mediated adhesion predicts that LTA binds to positively charged surface proteins and is presented with its hydrophobic tails extruding from the cell. These hydrophobic tails then mediate attachment of the bacteria to the eukaryotic cells (11). A similar mechanism could be occurring in Asc10-mediated internalization of E. faecalis into HT-29 enterocytes. Thus, Asc10 mutants defective in LTA binding would be pleiotropic for loss of bacterial aggregation and eukaryotic cell adherence. Thus, one functional domain would mediate both processes. Low internalization levels of INY3000 (Asc10+) would be due to the inability of functional Asc10 to bind the altered LTA of INY3000 on the bacterial cell surface.
Alternatively, LTA and Asc10 could function independently, as alterations in the LTA of Listeria monocytogenes and Staphylococcus aureus disrupted adhesion to different cell lines and artificial surfaces, respectively (1, 9). Also, Hyyrylçäinen et al. described altered folding properties of surface proteins in a Bacillus subtilis strain that had an altered LTA (16). In light of the differential migration pattern of Asc10 purified from INY3000 and OG1RF (Fig. 2, lane 1 versus 3), it is possible that the folding of Asc10 may be altered in INY3000. However, INY3000 expressing Asc10 is proficient as a donor strain for conjugative plasmid transfer (33), indicating that the bacterial binding properties of Asc10 expressed from this strain remain unaltered. Therefore, Asc10 expressed in INY3000 must maintain, at the very least, a partial native folding conformation.
It is also possible that an as-yet-unidentified factor missing or altered in INY3000 is required for high levels of Asc10-mediated internalization into HT-29 cells. For example, a 100-kDa surface protein observed on the surface of OG1SSp was not observed on the surface of INY3000 (33). Also, expression of Asc10 heterologously in Lactococcus lactis did not result in elevated HT-29 internalization levels under standard conditions (38), further supporting that multiple E. faecalis factors may be required for HT-29 internalization.
E. faecalis has become a leading cause of nosocomial infections. Entry into the host through the intestinal mucosal barrier has been shown to occur in mice and is likely in humans (37), suggesting it could be a focal point of infection. In vitro evidence suggests that AS-expressing enterococci would have a higher rate of epithelial cell internalization. The data presented here suggest a novel, non-RGD-dependent pathway of Asc10-mediated eukaryotic cell internalization.
Acknowledgments
We thank Elizabeth Moore and Robb Garnii for excellent technical assistance.
This work was supported by National Institutes of Health (NIH) grant HL-51987 to G.M.D and NIH grant GM-066751 to C.L.W. C.M.W. was supported by NIH training grant 5 T32 AI07421-5.
Editor: J. D. Clements
REFERENCES
- 1.Abachin, E., C. Poyart, E. Pellegrini, E. Milohanic, F. Fiedler, P. Berche, and P. Trieu-Cuot. 2002. Formation of D-alanyl-lipoteichoic acid is required for adhesion and virulence of Listeria monocytogenes. Mol. Microbiol. 43:1-14. [DOI] [PubMed] [Google Scholar]
- 2.Bryan, E. M., T. Bae, H. Kleerebezem, and G. M. Dunny. 2000. Improved vectors for nisin-controlled expression in gram-positive bacteria. Plasmid 44:183-190. [DOI] [PubMed] [Google Scholar]
- 3.Chow, J. W., L. A. Thal, M. B. Perri, J. A. Vazquez, S. M. Donabedian, and D. B. Clewell. 1993. Plasmid-associated hemolysin and aggregation substance production contribute to virulence in experimental enterococcal endocarditis. Antimicrob. Agents Chemother. 37:2474-2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coque, T. M., J. E. Patterson, J. M. Steckelberg, and B. E. Murray. 1995. Incidence of hemolysin, gelatinase, and aggregation substance among enterococci isolated from patients with endocarditis and other infections and from feces of hospitalized and community-based persons. J. Infect. Dis. 171:1223-1229. [DOI] [PubMed] [Google Scholar]
- 5.Cue, D., L. Hong, and P. P. Cleary. 2001. Genetic dissection of the Streptococcus pyogenes M1 protein: regions involved in fibronectin binding and intracellular invasion. Microb. Pathog. 31:231-242. [DOI] [PubMed] [Google Scholar]
- 6.Dunny, G. M., B. L. Brown, and D. B. Clewell. 1978. Induced cell aggregation and mating in Streptococcus faecalis: evidence for a bacterial sex pheromone. Proc. Natl. Acad. Sci. USA 75:3479-3483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fischer, W. 1994. Lipoteichoic acid and lipids in the membrane of Staphylococcus aureus. Med. Microbiol. Immunol. 183:61-76. [DOI] [PubMed] [Google Scholar]
- 8.Galli, D., F. Lottspeich, and R. Wirth. 1990. Sequence analysis of Enterococcus faecalis aggregation substance encoded by the sex pheromone plasmid pAD1. Mol. Microbiol. 4:895-904. [DOI] [PubMed] [Google Scholar]
- 9.Gross, M., S. E. Cramton, F. Gotz, and A. Peschel. 2001. Key role of teichoic acid net charge in Staphylococcus aureus colonization of artificial surfaces. Infect. Immun. 69:3423-3426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hasty, D. L., and H. S. Courtney. 1996. Group A streptococcal adhesion: all the theories are correct. Adv. Exp. Med. Biol. 408:81-94. [PubMed] [Google Scholar]
- 11.Hasty, D. L., I. Ofek, H. S. Courtney, and R. J. Doyle. 1992. Multiple adhesins of streptococci. Infect. Immun. 60:2147-2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hirt, H., S. L. Erlandsen, and G. M. Dunny. 2000. Heterologous inducible expression of Enterococcus faecalis pCF10 aggregation substance Asc10 in Lactococcus lactis and Streptococcus gordonii contributes to cell hydrophobicity and adhesion to fibrin. J. Bacteriol. 182:2299-2306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hirt, H., P. M. Schlievert, and G. M. Dunny. 2002. In vivo induction of virulence and antibiotic resistance transfer in Enterococcus faecalis mediated by the sex pheromone-sensing system of pCF10. Infect. Immun. 70:716-723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hirt, H., G. Wanner, D. Galli, and R. Wirth. 1993. Biochemical, immunological and ultrastructural characterization of aggregation substances encoded by Enterococcus faecalis sex-pheromone plasmids. Eur. J. Biochem. 211:711-716. [DOI] [PubMed] [Google Scholar]
- 15.Huycke, M. M., D. F. Sahm, and M. S. Gilmore. 1998. Multiple-drug resistant enterococci: the nature of the problem and an agenda for the future. Emerg. Infect. Dis. 4:239-249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hyyrylçäinen, H., M. Vitikainen, J. Thwaite, H. Wu, M. Sarvas, C. R. Harwood, V. P. Kontinen, and K. Stephenson. 2000. D-Alanine substitution of teichoic acids as a modulator of protein folding and stability at the cytoplasmic membrane/cell wall interface of Bacillus subtilis. J. Biol. Chem. 275:26696-26703. [DOI] [PubMed] [Google Scholar]
- 17.Isberg, R. R., Z. Hamburger, and P. Dersch. 2000. Signaling and invasin-promoted uptake via integrin receptors. Microbes Infect. 2:793-801. [DOI] [PubMed] [Google Scholar]
- 18.Isenmann, R., M. Schwarz, E. Rozdzinski, C. Christ, E. Schmidt, P. Augat, R. Marre, and H. G. Beger. 2002. Interaction of fibronectin and aggregation substance promotes adherence of Enterococcus faecalis to human colon. Dig. Dis. Sci. 47:462-468. [DOI] [PubMed] [Google Scholar]
- 19.Kreft, B., R. Marre, U. Schramm, and R. Wirth. 1992. Aggregation substance of Enterococcus faecalis mediates adhesion to cultured renal tubular cells. Infect. Immun. 60:25-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Manoil, C., and J. Bailey. 1997. A simple screen for permissive sites in proteins: analysis of Escherichia coli lac permease. J. Mol. Biol. 267:250-263. [DOI] [PubMed] [Google Scholar]
- 21.McCormick, J. K., H. Hirt, C. M. Waters, T. J. Tripp, G. M. Dunny, and P. M. Schlievert. 2000. Antibodies to a surface exposed, N-terminal domain of aggregation substance are not protective in the rabbit model of Enterococcus faecalis infective endocarditis. Infect. Immun. 69:3305-3314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Muscholl-Silberhorn, A. 1999. Cloning and functional analysis of Asa373, a novel adhesin unrelated to the other sex pheromone plasmid-encoded aggregation substances of Enterococcus faecalis. Mol. Microbiol. 34:620-630. [DOI] [PubMed] [Google Scholar]
- 23.Olmsted, S. B., G. M. Dunny, S. L. Erlandsen, and C. L. Wells. 1994. A plasmid-encoded surface protein on Enterococcus faecalis augments its internalization by cultured intestinal epithelial cells. J. Infect. Dis. 170:1549-1556. [DOI] [PubMed] [Google Scholar]
- 24.Olmsted, S. B., S. M. Kao, L. J. van Putte, J. C. Gallo, and G. M. Dunny. 1991. Role of the pheromone-inducible surface protein Asc10 in mating aggregate formation and conjugal transfer of the Enterococcus faecalis plasmid pCF10. J. Bacteriol. 173:7665-7672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Qin, X., K. V. Singh, G. M. Weinstock, and B. M. Murray. 2001. Characterization of fsr, a regulator controlling expression of gelatinase and serine protease in Enterococcus faecalis OG1RF. J. Bacteriol. 183:3372-3382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rakita, R. M., N. N. Vanek, K. Jacques-Palaz, M. Mee, M. M. Mariscalco, G. M. S. Dunny, W. B. Van Winkle, and S. I. Simon. 1999. Enterococcus faecalis bearing aggregation substance is resistant to killing by human neutrophils despite phagocytosis and neutrophil activation. Infect. Immun. 67:6067-6075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rozdzinski, E., R. Marre, M. Susa, R. Wirth, and A. Muscholl-Silberhorn. 2001. Aggregation substance-mediated adherence of Enterococcus faecalis to immobilized extracellular matrix proteins. Microb. Pathog. 30:211-220. [DOI] [PubMed] [Google Scholar]
- 28.Ruoslahti, E. 1996. RGD and other recognition sequences for integrins. Annu. Rev. Cell Dev. Biol. 12:697-715. [DOI] [PubMed] [Google Scholar]
- 29.Sartingen, S., E. Rozdzinski, A. Muscholl-Silberhorn, and R. Marre. 2000. Aggregation substance increases adherence and internalization, but not translocation, of Enterococcus faecalis through different intestinal epithelial cells in vitro. Infect. Immun. 68:6044-6047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schlievert, P. M., P. J. Gahr, A. P. Assimacopoulos, M. M. Dinges, J. A. Stoehr, H. Hirt, and G. M. Dunny. 1998. Aggregation and binding substances enhance pathogenicity in rabbit models of Enterococcus faecalis endocarditis. Infect. Immun. 66:218-223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shankar, N., A. S. Baghdayan, and M. S. Gilmore. 2002. Modulation of virulence within a pathogenicity island in vancomycin-resistant Enterococcus faecalis.. Nature 417:746-750. [DOI] [PubMed] [Google Scholar]
- 32.Sussmuth, S. D., A. Muscholl-Silberhorn, R. Wirth, M. Susa, and R. Marre. 2000. Aggregation substance promotes adherence, phagocytosis, and intracellular survival of Enterococcus faecalis within human macrophages and suppresses respiratory burst. Infect. Immun. 68:4900-4906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Trotter, K. M., and G. M. Dunny. 1990. Mutants of Enterococcus faecalis deficient as recipients in mating with donors carrying pheromone-inducible plasmids. Plasmid 24:57-67. [DOI] [PubMed] [Google Scholar]
- 34.Vanek, N. N., S. I. Simon, K. Jacques-Palaz, M. M. Mariscalco, G. M. Dunny, and R. M. Rakita. 1999. Enterococcus faecalis aggregation substance promotes opsonin-independent binding to human neutrophils via a complement receptor type 3-mediated mechanism. FEMS Immunol. Med. Microbiol. 26:49-60. [DOI] [PubMed] [Google Scholar]
- 35.Waters, C. M., M. H. Antiporta, B. E. Murray, and G. M. Dunny. 2003. Role of the Enterococcus faecalis GelE protease in determination of cellular chain length, supernatant pheromone levels, and the degradation of fibrin and misfolded surface proteins. J. Bacteriol. 185:3613-3623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Waters, C. M., and G. M. Dunny. 2001. Analysis of functional domains of the Enterococcus faecalis pheromone-induced surface protein aggregation substance. J. Bacteriol. 183:5659-5667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wells, C. L., R. P. Jechorek, and S. L. Erlandsen. 1990. Evidence for the translocation of Enterococcus faecalis across the mouse intestinal tract. J. Infect. Dis. 162:82-90. [DOI] [PubMed] [Google Scholar]
- 38.Wells, C. L., E. A. Moore, J. A. Hoag, H. Hirt, G. M. Dunny, and S. L. Erlandsen. 2000. Inducible expression of Enterococcus faecalis aggregation substance surface protein facilitates bacterial internalization by cultured enterocytes. Infect. Immun. 68:7190-7194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wells, C. L., E. M. A. van de Westerlo, R. R. Jechorek, and S. L. Erlandsen. 1995. Exposure of the lateral enterocyte membrane by dissociation of calcium-dependent junctional complex augments endocytosis of enteric bacteria. Shock 4:204-210. [DOI] [PubMed] [Google Scholar]
- 40.Wirth, R. 1994. The sex pheromone system of Enterococcus faecalis. More than just a plasmid-collection mechanism? Eur. J. Biochem. 222:235-246. [DOI] [PubMed] [Google Scholar]