Abstract
In a study of cytokine production ex vivo by Borrelia burgdorferi-stimulated peripheral blood mononuclear cells from 27 patients with culture-positive erythema migrans, production of inflammatory cytokines predominated, particularly gamma interferon and, to a lesser degree, tumor necrosis factor alpha. In contrast, with the exception of interleukin-13, anti-inflammatory cytokine production was negligible. Thus, B. burgdorferi antigens in early Lyme disease often induce a strong inflammatory response.
Lyme disease in the United States is caused by a tick-borne spirochete, Borrelia burgdorferi (10). The infection usually begins with a characteristic expanding skin lesion, erythema migrans (EM), which is often accompanied by nonspecific systemic symptoms (7, 11). Appropriate antibiotic therapy successfully cures the infection (10).
In murine models, both innate and adaptive inflammatory immune responses are critical in the control of early B. burgdorferi infection (2-4, 8). Consistent with the findings in the murine infection, it was recently shown that mRNA expression of inflammatory cytokines, especially gamma interferon (IFN-γ), predominated in EM lesions of 42 human patients with early Lyme disease, and macrophage-derived inflammatory cytokines, interleukin-1β (IL-1β) and tumor necrosis factor alpha (TNF-α), were more frequent in those with systemic symptoms (6). However, cytokine production by circulating lymphocytes had not yet been examined in early Lyme disease. Hence, in the present study, peripheral blood mononuclear cells (PBMC) of patients with EM were tested for proliferative responses and production of pro- and anti-inflammatory cytokines following ex vivo stimulation with B. burgdorferi lysate and recombinant borrelial antigens.
Patients.
During the summer of 2000, 31 patients with EM were recruited for the study at two field sites, one in East Lyme, Conn. (V.S.), and the other in Wakefield, R.I. (N.D.). The Institutional Review Board at New England Medical Center approved the study. The protocols for patient inclusion, collection of specimens, and culturing of skin biopsy samples were the same as in a previous study of cellular and humoral immune responses to B. burgdorferi, carried out in the summers of 1998 and 1999 (13). However, in that study, cytokine determinations were not made. To be certain of the diagnosis, cellular immune responses in the present study were determined only in samples from the 27 patients for whom B. burgdorferi was cultured from EM skin lesions.
The 27 patients had a median age of 45 years; 16 were men and 11 were women. They were initially evaluated during the acute phase of the illness, a median of 4 days (range, 1 to 14 days) after the onset of EM. The lesions were a median of 10 cm in diameter, and six patients (22%) had secondary annular skin lesions. Associated symptoms noted in one- to two-thirds of patients included malaise, fatigue, headache, neck stiffness, fever, chills, and arthralgia or myalgia. All of the patients were treated with 21- to 30-day courses of doxycycline or amoxicillin, and EM and associated symptoms resolved within a median duration of 3 days (range, 1 to 18 days). No patient experienced subsequent manifestations of the illness.
T-cell responses to B. burgdorferi.
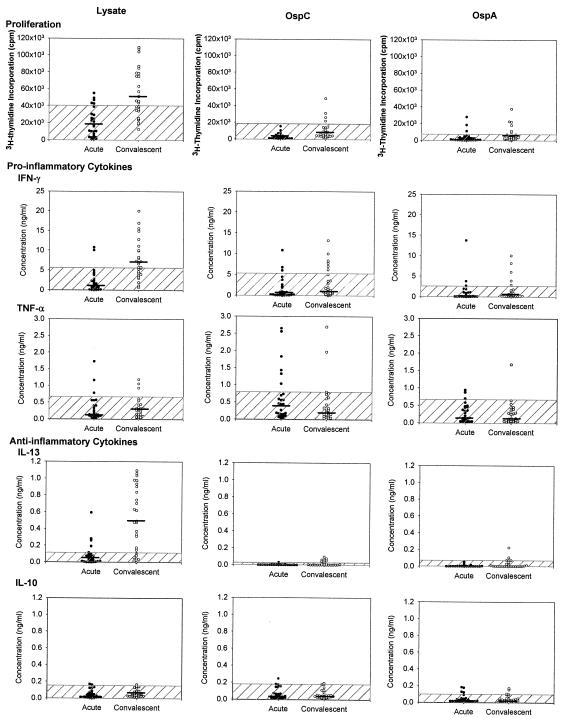
During the acute phase of the illness, PBMC from only a few of the 27 patients had low-level proliferative responses to B. burgdorferi lysate or to the nonlipidated recombinant preparations of OspA or OspC (rOspA and rOspC, respectively), as determined in standard 5-day proliferation assays (Fig. 1). However, 3 to 4 weeks later, at the conclusion of antibiotic treatment, 15 patients (55%) reacted with B. burgdorferi lysate, 6 (22%) responded to rOspA, and 4 (15%) reacted with rOspC. Altogether, 16 patients (59%) had T-cell proliferative responses during convalescence to at least one of the borrelial antigens tested. Similarly, in a previous study (13), 64% of the 39 patients with EM had responses during convalescence to at least one of the borrelial antigens tested. In that study (13), as in the present analysis, we noted that T-cell proliferative responses to OspC and OspA occurred at similar frequencies in early Lyme disease, even though humoral immunity was found only to OspC. The dissociation of the cellular and humoral responses to OspA suggests that spirochetes may still express small amounts of OspA early in the infection, so that processed OspA peptides may trigger T cells. However, the amounts of intact OspA must be insufficient to cross-link immunoglobulin on the surface of B cells, which would be required for B-cell activation.
FIG. 1.
Acute-phase (black circles) and convalescent-phase (open circles) PBMC of 27 patients with culture-positive EM were stimulated for 5 days with B. burgdorferi lysate, rOspC, or rOspA. Proliferative and pro- and anti-inflammatory cytokine responses are shown. The cross-hatched areas are 3 standard deviations above the mean values of 16 healthy control subjects, and median values for each group are indicated (black bars).
After 5 days in culture, supernatant was removed from tissue culture plates and the amounts of IL-2, IL-4, IL-5, IL-10, TNF-α, and IFN-γ were quantified by cytometric bead array (BD Pharmingen, San Diego, Calif.), which was performed according to the manufacturer's instructions. Beads of six discrete fluorescent intensities, each coated with a single anticytokine specificity, were mixed with supernatant. Following subsequent incubation with fluorescently labeled detection antibody, the samples were analyzed by flow cytometry to detect the six cytokines simultaneously. In addition, enzyme-linked immunosorbent assay (BD Pharmingen) was used to measure IL-13 production. The amount of each cytokine in the supernatant was interpolated from a standard curve generated using each recombinant cytokine.
In the 27 patients, PBMC produced primarily the proinflammatory cytokine IFN-γ, particularly during convalescence (Fig. 1). At that time, the cells stimulated with B. burgdorferi lysate from 16 patients (59%), those stimulated with rOspC from 6 patients (22%), and those stimulated with rOspA from 4 patients (15%) produced elevated levels of IFN-γ. In convalescence B. burgdorferi lysate induced a median of 6.6 ng of IFN-γ/ml (range, 0.7 to 20.0 ng/ml), rOspC induced a median of 1.3 ng/ml (range, 0 to 13.2 ng/ml), and rOspA induced a median of 0.4 ng/ml (range, 0 to 10.0 ng/ml). The greater responses during convalescence are consistent with past experience (13). During antibiotic treatment, spirochetal killing and antigen processing continue and may even be amplified, and therefore, adaptive T-cell responses are typically greater during convalescence.
In contrast, PBMC stimulated with rOspC, a prominent early antigen (5, 9), tended to produce more TNF-α (median, 0.4 ng/ml; range, 0 to 2.6 ng/ml) during the acute phase of the illness, although the differences between the acute and convalescent values were not statistically significant. B. burgdorferi may trigger TNF-α production in innate immune cells via the Fc gamma receptor (12), and borrelial lipoproteins may trigger it via the CD14/Toll-like receptor 2 pathway (1, 14). Thus, higher TNF-α levels during the acute illness may be explained by the adjuvant effect of B. burgdorferi lipoproteins on antigen-specific T-cell priming.
During convalescence, B. burgdorferi lysate induced elevated levels of IL-13 (median, 0.5 ng/ml; range, 0 to 1.1 ng/ml), an anti-inflammatory cytokine, in 19 patients (70%). However, very little of this cytokine was induced by rOspC or rOspA. Moreover, patient cultures rarely elaborated IL-10, and levels of IL-2, IL-4, and IL-5 were negligible (data not shown). The elevated IL-13 production in convalescence is consistent with a regulatory role for this cytokine in controlling inflammation.
During acute infection, when a number of patients had negative test results, the strength of proliferative responses induced by B. burgdorferi lysate or recombinant borrelial proteins did not correlate well with cytokine levels. However, during convalescence, proliferative responses to rOspC correlated with levels of both IFN-γ (R = 0.7, P < 0.001) and TNF-α (R = 0.6, P = 0.003), and proliferative responses to rOspA correlated with IFN-γ levels (R = 0.7, P < 0.001).
Clinical correlations.
In the 27 study patients, the symptoms most commonly associated with EM were headache, neck stiffness, fever, chills, arthralgia, myalgia, malaise, or fatigue. During the acute phase of the illness, the five patients whose T cells produced IFN-γ usually had no symptoms (median, 0; range, 0 to 3), whereas the 22 patients whose T cells lacked production of this cytokine had a median of four of these eight symptoms (range, 0 to 8) (Kruskal-Wallis test, P = 0.01). Cytokine production measured during convalescence did not correlate with initial symptoms. In addition, there was no correlation between symptoms and TNF-α or IL-13 production at either time point. Finally, cytokine production did not correlate with clinical evidence of spirochetal dissemination or with the duration of infection prior to treatment. The usual lack of symptoms in those with IFN-γ production suggests that patients whose T cells produce this cytokine during the acute phase of the illness may have more effective spirochetal killing.
In summary, PBMC stimulated ex vivo by B. burgdorferi antigens induced primarily inflammatory cytokines, particularly the T-cell-derived cytokine IFN-γ, and to a lesser degree, the T-cell- and possibly macrophage-derived cytokine TNF-α. In addition, during the acute illness, patients whose T cells produced IFN-γ had significantly fewer symptoms than did those whose T cells lacked IFN-γ production. In contrast, with the exception of IL-13, production of anti-inflammatory cytokines was negligible. Thus, as in murine B. burgdorferi infection, a strong proinflammatory response was often found in human patients with early Lyme disease.
Acknowledgments
We thank Marcia Pelligrino and Norma Grills for help with patient care and the collection of samples, Gail McHugh for culturing of skin biopsy samples, and Colleen Fitzpatrick for help with preparation of the manuscript.
The study was supported by Cooperative Agreement CCU110291 from the Centers for Disease Control and Prevention, the Mathers Foundation, the Lyme/Arthritis Research Foundation, and the Eshe Fund. Brian Moore was the recipient of a summer student fellowship from the Massachusetts Chapter of the Arthritis Foundation.
Editor: W. A. Petri, Jr.
REFERENCES
- 1.Alexopoulou, L., V. Thomas, M. Schnare, Y. Lobet, J. Anguita, R. T. Schoen, R. Medzhitov, E. Fikrig, and R. A. Flavell. 2002. Hyporesponsiveness to vaccination with Borrelia burgdorferi OspA in humans and in TLR1- and TLR2-deficient mice. Nat. Med. 8:878-884. [DOI] [PubMed] [Google Scholar]
- 2.Barthold, S. W., and M. de Souza. 1995. Exacerbation of Lyme arthritis in beige mice. J. Infect. Dis. 172:778-784. [DOI] [PubMed] [Google Scholar]
- 3.Barthold, S. W., C. L. Sidman, and A. L. Smith. 1992. Lyme borreliosis in genetically resistant and susceptible mice with severe combined immunodeficiency. Am. J. Trop. Med. Hyg. 47:605-613. [DOI] [PubMed] [Google Scholar]
- 4.Brown, C. R., and S. L. Reiner. 1999. Genetic control of experimental Lyme arthritis in the absence of specific immunity. Infect. Immun. 67:1967-1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dressler, F., J. A. Whalen, B. N. Reinhardt, and A. C. Steere. 1993. Western blotting in the serodiagnosis of Lyme disease. J. Infect. Dis. 167:392-400. [DOI] [PubMed] [Google Scholar]
- 6.Mullegger, R. R., G. McHugh, R. Ruthazer, B. Binder, H. Kerl, and A. C. Steere. 2000. Differential expression of cytokine mRNA in skin specimens from patients with erythema migrans or acrodermatitis chronica atrophicans. J. Investig. Dermatol. 115:1115-1123. [DOI] [PubMed] [Google Scholar]
- 7.Nadelman, R. B., J. Nowakowski, G. Forseter, N. S. Goldberg, S. Bittker, D. Cooper, M. Aguero-Rosenfeld, and G. P. Wormser. 1996. The clinical spectrum of early Lyme borreliosis in patients with culture-confirmed erythema migrans. Am. J. Med. 100:502-508. [DOI] [PubMed] [Google Scholar]
- 8.Schaible, U. E., S. Gay, C. Museteanu, M. D. Kramer, G. Zimmer, K. Eichmann, U. Museteanu, and M. M. Simon. 1990. Lyme borreliosis in the severe combined immunodeficiency (scid) mouse manifests predominantly in the joints, heart, and liver. Am. J. Pathol. 137:811-820. [PMC free article] [PubMed] [Google Scholar]
- 9.Schwan, T. G., J. Piesman, W. T. Golde, M. C. Dolan, and P. A. Rosa. 1995. Induction of an outer surface protein on Borrelia burgdorferi during tick feeding. Proc. Natl. Acad. Sci. USA 92:2909-2913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Steere, A. C. 2001. Lyme disease. N. Engl. J. Med. 345:115-125. [DOI] [PubMed] [Google Scholar]
- 11.Steere, A. C., N. H. Bartenhagen, J. E. Craft, G. J. Hutchinson, J. H. Newman, D. W. Rahn, L. H. Sigal, P. N. Spieler, K. S. Stenn, and S. E. Malawista. 1983. The early clinical manifestations of Lyme disease. Ann. Intern. Med. 99:76-82. [DOI] [PubMed] [Google Scholar]
- 12.Talkington, J., and S. P. Nickell. 2001. Role of Fc gamma receptors in triggering host cell activation and cytokine release by Borrelia burgdorferi. Infect. Immun. 69:413-419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vaz, A., L. Glickstein, J. A. Field, G. McHugh, V. K. Sikand, N. Damle, and A. C. Steere. 2001. Cellular and humoral immune responses to Borrelia burgdorferi antigens in patients with culture-positive early Lyme disease. Infect Immun. 69:7437-7444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wooten, R. M., Y. Ma, R. A. Yoder, J. P. Brown, J. H. Weis, J. F. Zachary, C. J. Kirschning, and J. J. Weis. 2002. Toll-like receptor 2 is required for innate, but not acquired, host defense to Borrelia burgdorferi. J. Immunol. 168:348-355. [DOI] [PubMed] [Google Scholar]