Abstract
Patients with chronic mucocutaneous candidiasis (CMC) are selectively unable to clear the yeast Candida, which results in persistent debilitating infections affecting the skin, nails, and mucous membranes. The underlying defect is unknown. Recent animal studies highlighted the importance of type 1 cytokines in protection against Candida, and previous work suggested that CMC patients may exhibit altered cytokine production in response to Candida. Based on these findings, in this study we investigated cytokine production in CMC patients by assessing a range of inflammatory, anti-inflammatory, type 1, and type 2 cytokines (interleukin-2 [IL-2], IL-4, IL-5, IL-6, IL-10, IL-12, gamma interferon [IFN-γ], tumor necrosis factor alpha [TNF-α]) in whole-blood cultures in response to five different fractions of Candida albicans (carbohydrate, purified mannan, and protein-rich fractions, etc.), as well as non-Candida antigens. Our results demonstrate that cytokine production is deregulated in a Candida-specific way for some cytokines (IL-2, IL-10), is deregulated more generally for other cytokines (IL-12, IL-6, IFN-γ), and is not markedly altered for still other cytokines (TNF-α, IL-4, IL-5). The most notable finding in CMC patients was the markedly impaired production of IL-12 in parallel with dramatically increased levels of IL-6 and IL-10 that occurred selectively in response to Candida. These results suggest that patients with CMC have impaired production of type 1-inducing cytokines (possibly a macrophage or dendritic cell defect?), which could result in an inability to mount protective cell-mediated responses and a failure to clear Candida. Continued tissue damage and inflammation may trigger production of high levels of inhibitory cytokines, such as the IL-10 production seen in our study, which would further reduce production of type 1-inducing cytokines in a positive feedback loop leading to persistent infection.
Candida albicans is an opportunistic, commensal yeast that inhabits mucous membranes of >80% of adults but causes disease (candidiasis) only if the existing host-pathogen balance is disrupted. A unique form of candidiasis is seen in patients with chronic mucocutaneous candidiasis (CMC), who are selectively unable to clear Candida and as a consequence harbor debilitating, persistent, and refractory infections with this yeast that affect the skin, nails, and mucous membranes. The underlying defect is thought to be a primary immune deficiency, but the nature of the defect(s) has not been defined (3). Patients with CMC may have juvenile or mature onset and familial or sporadic occurrence, and CMC may be present with or without endocrinopathy (14). CMC patients with endocrinopathy and autosomal recessive inheritance are recognized as having the autoimmune polyendocrinopathy candidiasis ectodermal dysplasia syndrome (25), and in these patients a gene defect has been identified that results in various mutations in the AIRE (autoimmune regulator) gene, whose protein product is a DNA transcription factor. The link between the identified gene defect and the inability to clear Candida remains unknown. Other forms of CMC are also recognized (16); these forms include CMC with thyroid disease and dominant inheritance, for which the immune defect has been mapped to chromosome 2p (2), as well as CMC without endocrinopathy, for which no gene defect has been identified yet.
Protective immunity to Candida involves both innate and adaptive cellular and humoral immune responses (3). Current data suggest that protection against disseminated (systemic) diseases is mediated primarily by innate immune mechanisms (neutrophils) and humoral immunity and is often impaired in patients who are receiving immunosuppressive and/or cytotoxic therapy. In contrast, protection against mucocutaneous disease relies on cell-mediated immunity and T cells and is impaired in patients with severe combined immune deficiency, Di George syndrome, AIDS, etc. (13). Interestingly, patients with deficiencies in receptors for gamma interferon (IFN-γ) and interleukin-12 (IL-12) (IFNγR and IL-12R, respectively) do not suffer from serious fungal infections, although a number of them have bouts of refractory candidiasis in addition to infections with atypical mycobacteria (24). In CMC patients, most studies of immune function have revealed normal innate immunity to Candida, as assessed by complement function, neutrophil phagocytosis, and intracellular killing (3). Antibody responses in general and antibody responses specifically to Candida are intact (3, 14, 20). The abnormalities that have been identified were found to affect the cellular arm of the immune response with low or no lymphocyte proliferation, leukocyte and macrophage inhibitory factor production, and delayed-type hypersensitivity responses to Candida (3). However, it is not known whether T cells themselves are impaired or whether the defect(s) affects regulators and/or mediators of T-cell activity, such as cytokines. Recent preliminary data demonstrated that patients with CMC had an altered pattern of cytokine production in response to Candida antigens, with low or no IL-2 production, increased IL-6 production, and markedly high titers of immunoglobulin G1 (IgG1) and IgA Candida-specific antibodies consistent with a low type 1 (Th1) and high type 2 (Th2) cytokine production pattern (16, 17). These preliminary results also demonstrated that the differences in the responses depended on the type of Candida fraction used (e.g., protein- or carbohydrate [CHO]-rich fraction) (17). In this paper we present results of studies of a cohort of CMC patients in which we looked at their ability to respond to stimulation with various CHO- or protein-rich fractions of Candida albicans, as well as non-Candida antigens, by producing a wide range of cytokines that play a role in mediating T-cell activation, including inflammatory cytokines (IL-6, tumor necrosis factor alpha [TNF-α]), anti-inflammatory cytokines (IL-10), and type 1 and type 1-inducing cytokines (IL-2, IFN-γ, IL-12), as well as type 2 cytokines (IL-4, IL-5). Our results revealed that there was increased production of at least one inflammatory cytokine (IL-6) in response to Candida antigens that was paralleled by a significant increase in the production of the anti-inflammatory cytokine IL-10 in most patients. At the same time, the levels of IL-12 and IL-2 were found to be markedly decreased or these cytokines were found to be absent, while IFN-γ production was affected to a lesser degree. Interestingly, the levels of IL-4 and IL-5 production were low and comparable to control production levels. On the whole, alterations in cytokine production were markedly more pronounced upon stimulation with purified CHO Candida fractions and mannan than upon stimulation with protein-rich fractions. Our results also demonstrated that there was normal expression of IFNγR and IL-12R, which indicated that a lack of these cytokine receptors is not a likely underlying immune defect in CMC patients.
MATERIALS AND METHODS
For all patients a CMC questionnaire was filled out and analyzed to confirm that they fulfilled the criteria for diagnosis of CMC (14); this questionnaire included details concerning endocrinopathy and other autoimmune diseases, mode of inheritance, time of onset, other infections, family history, laboratory investigations, etc. The patients included both adults and children. All of the patients had recurring CMC without a known underlying cause and were not on antibiotics or antifungal agents at the time of sampling. Thirteen of the 24 CMC patients had a history of recurring, mild to moderately severe bacterial infections (tonsillitis, sinusitis, otitis media), as well as recurrent viral upper respiratory tract infections. Autoantibodies were detected in five children but in none of the adults, although only one child (a child with parathyroid autoantibodies) had clinical hypoparathyroidism and another child had alopecia. No other patients had evidence of autoimmune disease. Eleven of the 24 patients had a positive family history. Control blood samples were taken from both healthy children and healthy adults to enable sex- and-age matched comparisons. Samples from control children were taken at the time of induction into general anesthesia for surgery that was being done for noninfectious causes (e.g., ingrown toenails, hypospadia), while adult controls were healthy laboratory and hospital staff volunteers. The characteristics and numbers of patients and controls studied are shown in Tables 1 and 2.
TABLE 1.
Patients and controls studied
| Subjects | No. of females | No. of males | Median age (years) | Age range (years) | Total no. |
|---|---|---|---|---|---|
| Patients | |||||
| Children with CMC | 5 | 11 | 6 | 1-11 | 16 |
| Adults with CMC | 7 | 1 | 35 | 18-50 | 8 |
| Total | 12 | 12 | NAa | NA | 24b |
| Controls | |||||
| Children | 11 | 23 | 7.5 | 1-17 | 34 |
| Adults | 7 | 9 | 28 | 21-46 | 16 |
| Total | 18 | 32 | NA | NA | 50 |
NA, not applicable.
Because of limited quantities of blood samples not all cytokines were assessed for each patient in all cases; the smallest number of CMC patients in a group was 13.
TABLE 2.
Patient characteristics
| Condition | No. of patientsa |
|---|---|
| Childhood onsetb | 21 |
| Oral-esophageal Candida | 24 |
| Nail Candida | 12 |
| Skin Candida | 13 |
| Non-Candida infectionsc | 13 |
| Autoantibodiesd | 5 |
| Family history of CMC | 11 |
| AIRE1 mutationse | 2 |
A total of 24 CMC patients were included in the study.
Childhood onset occurred at ages ranging from 3 months to 7 years.
The patients had bacterial, viral, or other fungal infections but not at the time of sampling.
Five patients had parathyroid antibodies, two patients had adrenal antibodies, one patient had a thyroid antibody, one patient had a gastric parietal cell antibody, and one patient had an antinuclear antibody.
All patients were screened for the two AIRE1 mutations most common in the United Kingdom (R257* and 964del13); 1 patient was heterozygous for R257*, and one patient was homozygous for 964del13 (Simon Pearce, University of Newcastle, personal communication).
Ethical approval.
Ethical approval was obtained from the Newcastle and Tyneside Health Authority, and all investigations were conducted according to Declaration of Helsinki principles (33). All individuals received written and/or verbal explanations of the studies involved and gave written informed consent (parents gave consent on behalf of children).
Cytokine production and analysis.
Samples (15 to 50 ml) of heparanized venous blood were drawn from patients and healthy controls and processed identically. The levels of cytokines were determined in whole blood (i.e., in peripheral blood cell cultures). Cultures were stimulated with all the antigens described below, and an unstimulated medium control was always included. Blood was diluted 1:2 in RPMI containing 2 mM glutamine (Sigma) and 2,000 IU of heparin (Sigma) per ml with no additional serum added, and 10 replicate wells per antigen (200 μl/well) were set up in 96-well round-bottom plates (Falcon), which were spun and harvested after incubation for 48 h at 37°C in a humidified incubator containing 5% CO2. Supernatants were stored in aliquots at −70°C until they were tested by an enzyme-linked immunosorbent assay (ELISA) for various cytokines. Before harvest proliferative responses were checked (data not shown); a plasma sample from each individual was also collected and stored at −70°C. Cytokine levels were measured with ELISA OptEIA antibody pair kits (Pharmingen) used in accordance with the manufacturer's protocol. ELISAs specific for the following cytokines were performed: IL-2, IL-4, IL-5, IL-6, IL-10, IL-12 (p70), IFN-γ, and TNF-α. The sensitivities for all cytokines were >5 pg/ml. Because of the high levels of ELISA specificity, additional confirmation of cytokine specificities with neutralizing antibodies was not performed.
Fractionation of C. albicans.
C. albicans was obtained from the Clinical Microbiology Laboratory (University of Newcastle Medical School Newcastle, United Kingdom), where it had been isolated from a patient for diagnostic purposes. Candida growing on solid agar was inoculated into a solution of yeast nitrogen base (Difco) with 1% glucose and grown for fractionation. For all Candida fractions and antigens used the optimal concentrations were predetermined by performing time- and dose-dependent studies. The following Candida and non-Candida stimuli were assessed. For Candida sonicate (CS), freshly grown Candida cultures were washed, resuspended in saline, and autoclaved for 1 h to kill the yeast cells, after which this fraction was briefly sonicated to disperse the yeast in order to obtain single cells. CS was added to cell cultures at a final dilution of 1:10,000 and was included in this study to assess responses to Candida cell surface antigens. For Candida extract (CE), Candida cells prepared as described above were ruptured by subjecting them to Ballotini glass bead treatment (Jencons) with vortexing for two 1-h cycles; the beads were removed by filtration through 100-μm-pore-size cell strainers (Falcon). The CHO content of this fraction was 42.3 mg/ml, and the protein content was 7.3 mg/ml. CE was added to cell cultures at a final dilution of 1:10,000 and was used to assess responses to both surface and intracellular Candida antigens. For the polysaccharide-CHO protein-reduced (PS) fraction, a CE fraction prepared as described above was treated with petroleum ether for 1 h at 40°C to remove the lipids. Subsequently, in order to remove protein, the preparation was centrifuged and resuspended in acetate buffer at a concentration of 5 g/liter, and then it was subjected to chloroform-butanol treatment. Polysaccharide was precipitated by treating the pooled aqueous phase with 95% ethanol (2:1) and dialyzing the preparation against several changes of distilled water. The CHO content of this fraction was 2.2 mg/ml, and the protein content was at the borderline of detection (10 μg/ml) with a Bio-Rad protein assay whose sensitivity was 10 μg/ml. The PS fraction was added to cell cultures at a final dilution of 1:100 and was used to assess responses to enriched CHO-polysaccharide Candida antigens, albeit with low levels of protein present. For the digested (protein-free) polysaccharide-CHO (DPS) fraction, in order to remove the low level of residual protein in the PS fraction prepared as described above, part of the PS fraction was subjected to digestion at 60°C with proteinase K (5 mg/ml) for 1 h before the contents were boiled to destroy the enzyme. The resulting DPS fraction was then dialyzed against distilled water overnight. The CHO content of this fraction remained roughly the same (2.0 mg/ml), while protein was undetectable as assessed by the Bio-Rad protein assay described above. The DPS fraction was added to cell cultures at a final dilution of 1:100 and was used to assess responses to purified CHO-polysaccharide Candida antigens. For the mannan-free (MF) fraction, a preparation of CE was treated with petroleum ether to remove lipids before the resulting pellet was dialyzed overnight against several changes of separation buffer (0.1 M sodium acetate, 0.4 M sodium chloride, 10−3 M calcium chloride-magnesium chloride-manganese chloride [pH 6.5]). Larger pieces of cell debris were removed by centrifugation at a low speed before the supernatant was electrophoresed through a concanavalin A-Sepharose column (Pharmacia), and 5-ml fractions were collected. The fractions were tested for contaminating CHO and electrophoresed again through the column until the carbohydrate content was reduced to below the level of detection with a periodic acid-Schiff stain assay (sensitivity, 10 μg/ml). The protein content was 3.6 mg/ml. The MF fraction was added to cell cultures at a final dilution of 1:10,000 and was used to assess responses to a lipid-free and mannan-free Candida fraction. For the mannan fraction (MAN), a commercial preparation of purified, protein-free mannan from C. albicans NIBSC (batch 77/600; N2 content, 0.02%) was used at a final dilution of 1:100 to assess responses to mannan, which is a major constituent of the Candida cell wall and an immunodominant Candida antigen (9). Pneumovax II (PV) is a commercial vaccine consisting of 23 different pneumococcal polysaccharides at a concentration of 575 μg/0.5 ml (Merieux UK Ltd.) and was used as a control at a final concentration of 1:100 to assess patient responses to non-Candida polysaccharides. Tetanus toxoid (TT), a commercial preparation of purified tetanus protein at a concentration of 4,250 limit of flocculation (Lf)/ml (Pasteur Merieux), was used at a dilution of 1:100 and was included as a control to assess patient responses to a non-Candida protein antigen. Phytohemaglutinin P (PHA) (Sigma) was used as a mitogen at a final concentration of 5 μg/ml to assess responses to nonspecific mitogenic stimulation. In summary, the antigens used were (i) whole Candida fractions (CS and CE), (ii) CHO-rich protein-reduced or protein-free Candida fractions (PS, DPS, and MAN), (iii) a protein-rich CHO-depleted Candida fraction (MF), (iv) a non-Candida protein antigen (TT), and (v) a non-Candida CHO antigen (PV).
IFNγR1 and IL-12Rβ1 expression.
As blood samples were limited, Epstein-Barr virus (EBV) lines were generated from all individuals studied (31) to assess the expression of receptors for IFN-γ and IL-12, which are normally expressed on B cells and EBV-transformed B-cell lines. However, although the IFN-γ signaling pathway was intact, the IL-12 pathway was not expressed, making the EBV cell lines unsuitable for conclusive signaling studies of these cytokines. For detection of IFNγR1, cells were stained with a primary anti-CD119 antibody (Biogenesis) and then with a secondary goat anti-mouse fluorescein isothiocyanate-conjugated antibody (Silenus). IL-12Rβ1 was stained by using a primary anti-IL12Rβ1 antibody (Becton Dickenson), followed by a secondary biotinylated goat anti-mouse antibody (Caltag Laboratories) and then a streptavidin-phycoerythrin conjugate (Southern Biotechnology Association Inc.). Appropriate isotype controls were always included. Receptor expression was assessed with a FACScan flow cytometer (Becton Dickinson).
Statistical analysis and software packages.
Statistical analysis was performed by using MINITAB for Windows, version 9.2. All results were analyzed by using nonparametric tests for nonnormal data distribution. Statistically significant differences (P < 0.05) were analyzed by using the Mann-Whitney test for unpaired data. For graphics GraphPad PRISM (version 2) was used.
RESULTS
The level of IL-2 production was significantly lower in CMC patients than in healthy controls when cells were stimulated with protein-rich Candida fractions (CS, CE, and MF) (Fig. 1). As expected, neither group produced IL-2 upon stimulation with purified CHO fractions (DPS, MAN, and PV) (data not shown). The level of IL-2 production in response to TT was lower in CMC patients, albeit not significantly (data not shown). However, the level of IL-2 production upon mitogen stimulation with PHA was comparable to control levels, suggesting that the overall IL-2-producing capacity was not impaired in CMC patients (Fig. 1). The comparative levels of production of IL-2 in response to various protein-rich Candida fractions and a mitogen are shown in Fig. 2b. These results demonstrate that CMC patients did not produce IL-2 in response to any of the Candida fractions used but produced normal IL-2 levels when they were stimulated with the mitogen. An interesting finding was that decreased IL-2 production was more marked in adults with CMC than in children with CMC when the levels were compared to relevant control levels. Table 3 shows the IL-2 production by children and adults and demonstrates that the lower level of IL-2 production by adult patients than by controls not only was the result of lower actual IL-2 levels but also was due to the fact that healthy control children produced less IL-2 than healthy control adults produced when they were stimulated with Candida fractions (particularly CS), which resulted in smaller differences in IL-2 levels between healthy and sick children. Lower levels of IL-2 production in healthy children than in healthy adults were also seen in response to CE, MF, and PS stimulation but not in response to PHA and TT when both control and sick children produced substantial amounts of IL-2; children with CMC produced even more than control children produced in response to PHA. This suggested that the overall IL-2-producing capacity of both children with CMC and healthy children was intact, while the low levels of IL-2 production seen in both children with CMC and healthy children were specific for Candida. Results are presented for antigens in the cases in which the most marked differences between the patient and control groups were observed.
FIG. 1.
IL-2 production in response to different fractions of C. albicans and other antigens. Whole-blood cultures from patients and controls were stimulated with different C. albicans fractions (CS, CE, MF) or a non-Candida antigen (PHA) for 48 h. Supernatants were collected, and cytokine levels were assessed by performing ELISAs. Each symbol indicates the cytokine level for an individual patient (CMC) (solid symbols) or a control (C) (open symbols). bg, background cutoff level; NS, not significant. Note the log scale.
FIG. 2.
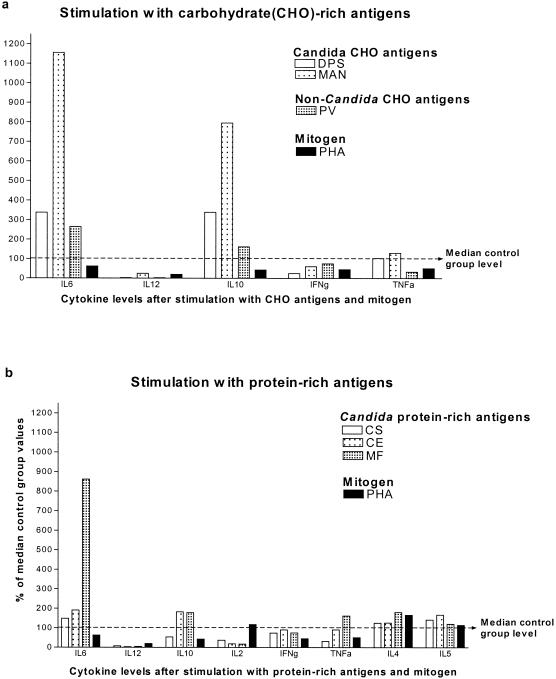
Cytokine production by CMC patients in response to Candida antigens, non-Candida antigens, and mitogen. Whole-blood cultures from CMC patients and controls were stimulated with different C. albicans fractions or non-Candida antigens for 48 h. Supernatants were collected, and cytokine levels were assessed by performing ELISAs. The median level for each cytokine produced by CMC patients was expressed as a percentage of the median control group level (the median control group levels were assigned a value of 100%). Note that various stimuli induced different cytokines.
TABLE 3.
Differences in IL-2 production between children and adults with CMC and controlsa
| Group | Concn of IL-2 (pg/ml) with the following antigens:
|
||||
|---|---|---|---|---|---|
| CS | CE | MF | PS | PHA | |
| Control children | 42 ± 6 | 21 ± 8 | 50 ± 30 | 38 ± 62 | 40 ± 108 |
| Control adults | 275 ± 230b | 45 ± 109c | 45 ± 323d | 74 ± 179e | 51 ± 15 |
| Children with CMC | 57 ± 28 | 17 ± 8 | 52 ± 10 | 33 ± 18 | 96 ± 44 |
| Adults with CMC | 12 ± 3 | 6 ± 1 | <5 | <5 | 10 ± 2 |
The values are means ± standard errors of the means.
The P value for a comparison of control adults and adults with CMC was 0.0061.
The P value for a comparison of control adults and adults with CMC was 0.081.
The P value for a comparison of control adults and adults with CMC was 0.061.
The P value for a comparison of control adults and adults with CMC was 0.02.
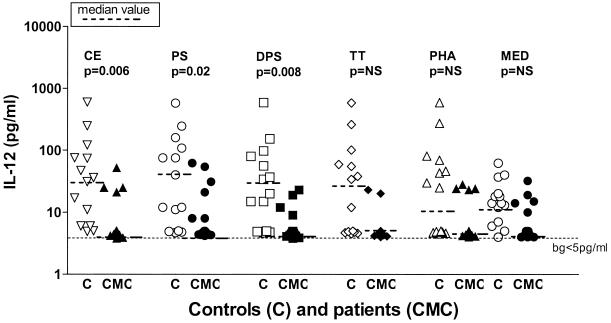
IL-12 production was impaired in both children with CMC and adults with CMC in response to all stimuli tested; the results were most significant for the responses to Candida fractions CE, PS, and DPS, but they were also significant, albeit to a lesser degree, for the responses to the non-Candida stimuli TT and PHA and in unstimulated cultures, as shown in Fig. 3. Figure 2 shows the levels of IL-12 produced in response to various antigens and demonstrates that low levels of IL-12 production were observed in response to both protein-rich and CHO Candida antigens, as well as to non-Candida antigens and mitogen stimulation, suggesting that there was a defect in the overall capacity to produce IL-12. However, as with IL-2, there were notable differences in IL-12 production between children with CMC and adults with CMC. Table 4 shows the responses to antigens with the most marked differences in IL-12 production. Children with CMC produced very little or no IL-12 in response to all stimuli studied, including PHA, while adults with CMC produced markedly more IL-12 than children with CMC produced, although they still produced far less than healthy control adults produced. Healthy children produced lower levels of IL-12 than healthy adults produced, although they still produced significantly more than sick children produced, and the greatest responses were to CE, MF, PS, and DPS. Interestingly, the highest levels of IL-12 production in healthy adults were not in response to the same antigens as the highest levels of IL-12 production in children and were observed in response to MF, PS, and PHA stimulation (Table 4).
FIG. 3.
IL-12 production in response to different fractions of C. albicans and other antigens. Whole-blood cultures from patients and controls were stimulated with different C. albicans fractions (CE, PS, DPS) or non-Candida antigens (TT, PHA) or were not stimulated (MED) for 48 h. Supernatants were collected, and cytokine levels were assessed by performing ELISAs. Each symbol indicates the cytokine level for an individual patient (CMC) (solid symbols) or a control (C) (open symbols). Bg, background cutoff level; NS, not significant. Note the log scale.
TABLE 4.
Differences in IL-12 production between children and adults with CMC and controlsa
| Group | Concn of IL-12 (pg/ml) with the following antigens:
|
||||
|---|---|---|---|---|---|
| CE | MF | PS | DPS | PHA | |
| Control children | 12 ± 10b | 9 ± 8 | 12 ± 13c | 15 ± 10 | <5 |
| Control adults | 126 ± 129 | 216 ± 118d | 202 ± 110d | 126 ± 126d | 261 ± 122d |
| Children with CMC | <5 | <5 | <5 | <5 | <5 |
| Adults with CMC | 35 ± 30 | 46 ± 16 | 42 ± 12 | <5 | 11 ± 7 |
The values are means ± standard errors of the means.
The P value for a comparison of control children and children with CMC was 0.01.
The P value for a comparison of control children and children with CMC was 0.05.
The P value for a comparison of control adults and adults with CMC was 0.03.
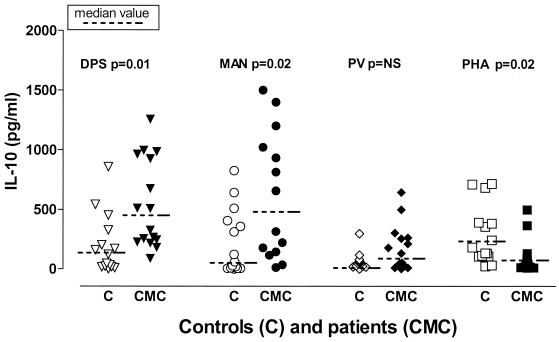
The data for IL-10 production were the most unexpected finding of our study because CMC patients produced extraordinary high IL-10 levels in response to Candida fractions with high CHO contents (DPS and MAN; Fig. 4). They also produced somewhat higher IL-10 levels in response to the non-Candida polysaccharide antigen PV, although they did not produce significantly more IL-10 than the controls produced and they produced far less IL-10 than they produced in response to Candida antigens (Fig. 2a). Interestingly, their IL-10 response to PHA was lower than the response of the controls (Fig. 4). The level of IL-10 production was somewhat higher but was not significantly higher in response to protein-rich Candida fractions (Fig. 2b). The IL-10 responses of children with CMC and adults with CMC did not differ markedly except for the responses to DPS and MAN; children produced significantly higher levels of IL-10 in response to these antigens (data not shown). In contrast to IL-2 and IL-12 production, healthy children were found to produce substantial levels of IL-10 which were comparable to the levels produced by healthy adults, although the stimuli that evoked the greatest responses were not the same (in children the highest levels of IL-10 were induced by MAN and PHA, while in adults the highest levels were induced by DPS).
FIG. 4.
IL-10 production in response to different fractions of C. albicans and other antigens. Whole-blood cultures from patients and controls were stimulated with different C. albicans fractions (DPS, MAN) or non-Candida antigens (PV, PHA) for 48 h. Supernatants were collected, and cytokine levels were assessed by performing ELISAs. Each symbol indicates the cytokine level for an individual patient (CMC) (solid symbols) or a control (C) (open symbols). NS, not significant.
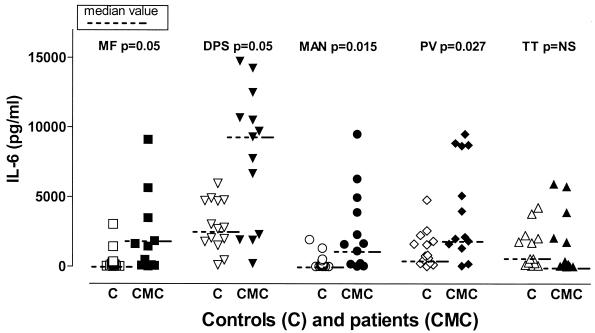
The level of IL-6 production was markedly higher in CMC patients than in controls, particularly in response to Candida and non-Candida fractions with high CHO contents (DPS, MAN, and PV), as well as to the protein-rich fraction MF, albeit to a much lesser degree (Fig. 2 and 5). Healthy individuals and CMC patients produced comparable IL-6 levels in response to TT (Fig. 5), CE, and PS (data not shown). The levels of PHA-stimulated IL-6 production and unstimulated IL-6 production were actually lower than the levels of production of IL-6 by control children and control adults, respectively, although the differences were not significant (data not shown). The individual variations in IL-6 production were marked in all groups and in response to all stimuli used.
FIG. 5.
IL-6 production in response to different fractions of C. albicans and other antigens. Whole-blood cultures from patients and controls were stimulated with different C. albicans fractions (MF, DPS, MAN) or non-Candida antigens (PV, TT) for 48 h. Supernatants were collected, and cytokine levels were assessed by performing ELISAs. Each symbol indicates the cytokine level for an individual patient (CMC) (solid symbols) or a control (C) (open symbols). NS, not significant.
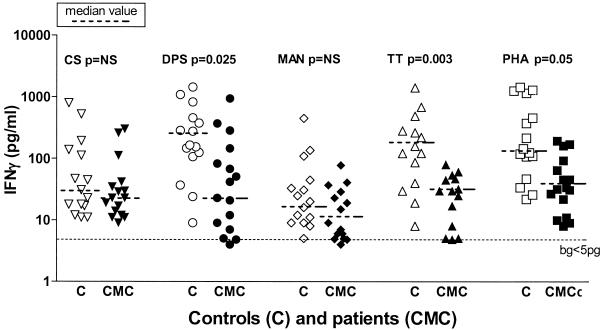
The level of IFN-γ production in CMC patients was not significantly decreased in response to any of the Candida antigen stimuli other than DPS. Interestingly, the level of IFN-γ production was decreased in response to the non-Candida antigens TT and PHA, suggesting that the decrease was not Candida specific and that the overall capacity for IFN-γ production was lower in CMC patients (Fig. 2 and 6). The decrease was seen in both children and adults with CMC. The IFN-γ levels produced by healthy children and adults did not differ significantly irrespective of the stimuli used, although healthy children on average produced less IFN-γ than adults produced, particularly in response to CS and PS (data not shown). In all instances the lowest IFN-γ levels were produced in response to the CHO antigens MAN (Fig. 6) and PV (data not shown).
FIG. 6.
IFN-γ production in response to different fractions of C. albicans and other antigens. Whole-blood cultures from patients and controls were stimulated with different C. albicans fractions (CS, DPS, MAN) or non-Candida antigens (TT, PHA) for 48 h. Supernatants were collected, and cytokine levels were assessed by performing ELISAs. Each symbol indicates the cytokine level for an individual patient (CMC) (solid symbols) or a control (C) (open symbols). bg, background cutoff level; NS, not significant. Note the log scale.
The levels of TNF-α production were not significantly different in CMC patients and controls in response to any of the stimuli tested, although the median levels produced by CMC patients when they were stimulated with polysaccharide fractions of Candida antigens (DPS and MAN) were higher than the levels produced by the controls (Fig. 2), as were the TNF-α levels in unstimulated cultures (data not shown). Also, there were no significant differences in the TNF-α levels produced by adults and children whether they were patients or controls.
The levels of IL-4 production and IL-5 production were low and comparable in all groups. Hardly any production was seen in response to CHO antigens, while the responses to protein-rich antigens and the mitogen were similar in patients and controls, although there was a tendency for CMC patients to produce higher levels of both cytokines. Children with CMC produced on average somewhat higher levels of both cytokines in response to all stimuli, although the difference was never significant when the data were compared to control data (data not shown). No difference was noted in the responses to Candida and non-Candida fractions.
There were not marked differences in IFNγR1 and IL12Rβ1 expression on EBV-transformed cell lines between the groups tested, although the percentage of cells expressing IFNγR1 in children with CMC was on average lower than the percentage of cells expressing IFNγR1 in healthy children, but not significantly lower (Table 5).
TABLE 5.
IFNγR1 and IL12Rβ1 expression on EBV-transformed cell lines in CMC patients and controlsa
| Group | % IFNγR1+ cells | % IL-12Rβ1+ cells |
|---|---|---|
| Control children | 55 ± 4 | 76 ± 5 |
| Control adults | 31 ± 9 | 72 ± 11 |
| Children with CMC | 35 ± 8 | 72 ± 5 |
| Adults with CMC | 29 ± 7 | 73 ± 7 |
The values are means ± standard errors of the means.
DISCUSSION
Taken together, our results demonstrate that cytokine production in CMC patients is deregulated and that this deregulation is Candida specific for some cytokines (IL-2, IL-10), while it is more general for others (IL-12, IL-6, IFN-γ). Production of still other cytokines (TNF-α, IL-4, IL-5) seems to be unaltered.
CMC patients produce unusually high levels of at least one inflammatory cytokine (IL-6) and a potent anti-inflammatory cytokine (IL-10) specifically or most vigorously in response to Candida CHO-rich fractions. Our results also show that CMC patients have impaired production of type 1-inducing and type 1 cytokines, particularly IL-12, as well as IL-2 and IFN-γ; the impairment seems to be of a more general nature, although it is most pronounced in response to Candida antigens. Interestingly, IL-2 production does not seem to be impaired in children with CMC, while all adults with CMC produce markedly lower IL-2 levels, particularly in response to Candida antigens, suggesting that there is progressive deterioration of cytokine production over the years. The findings for IL-12 production are quite the opposite in that children with CMC produce hardly any IL-12 regardless of the stimuli used (including a mitogen), while adults with CMC produce some IL-12, although far less than normal controls produce. When these findings are interpreted, it is noteworthy that the levels of production of both IL-2 and IL-12 are markedly lower in healthy children than in adults, confirming that cytokine production has specificities in children, as previously reported (19), and highlighting the importance of selecting appropriate control groups when children are assessed.
Altered cytokine production was most pronounced in response to CHO antigens, particularly if they were derived from Candida. Indeed, most CMC patients exhibited a pattern of responses to Candida CHO antigens, producing very high levels of IL-6 along with very high levels of IL-10 and little or no IL-12 and IL-2. The underlying reason for this is unclear, although it has previously been reported that CMC patients are frequently susceptible to infections with encapsulated bacteria, such as Streptococcus pneumoniae or Haemophilus influenzae, suggesting that there are impaired or altered responses to polysaccharide antigens (8, 12). This tendency to respond inadequately to CHO antigens may be of crucial importance in impaired protection against Candida (see below).
It has previously been reported that Candida mannan has immunosuppressive properties, and it was suggested that mannan might be responsible for the immunosuppressive effect and impaired immunity in CMC patients with Candida infections (8). In our studies this was not a likely explanation for the altered responses seen, as CMC patients characteristically exhibited increased, not decreased, responses to mannan (IL-6, IL-10), while little or no IL-12 production in response to mannan added to a culture was seen in CMC patients but not in control cultures. Because whole-blood cultures were used, the possibility that there was an immunosuppressive effect of patient serum mediated by substances other than mannan cannot be ruled out, but it is unlikely given that lymphocyte proliferative responses assessed in the same blood samples were intact (data not shown).
Although the results of our study demonstrated that there were cytokine derangements in patients with CMC, the nature of the cell or cells harboring the underlying defect remains elusive. Even though purified cell populations have the advantage of offering a controlled environment and targeted cell analysis, whole-blood cultures are more representative of in vivo circumstances, particularly when total levels of cytokines produced by more than one cell population (e.g., IL-6, IL-10) are assessed. Additionally, whole-blood cultures include cytokines produced by cells other than mononuclear cells that are not as readily isolated, such as neutrophils, and have the advantage of avoiding artifacts caused by in vitro activation during cell separation. Further studies should clarify the role of individual cell populations involved.
Our results confirmed that there was normal type 1 cytokine receptor expression (IFNγR1 and IL-12Rβ1) in all CMC patients, which eliminated the possibility that impaired binding of relevant cytokines is a putative defect in CMC. Importantly, none of our CMC patients completely lacked either one of these receptors, nor was overexpression (as judged by fluorescence intensity) as a marker of a partial autosomal dominant IFNγR1 deficiency found in any of the patients. Normal receptor expression did not eliminate the possibility that there is a defect in the IFN-γ and IL-12 signaling pathways. Nevertheless, a signaling defect does not seem likely because all CMC patients produced IFN-γ in response to at least some antigens and because STAT activation of EBV cell lines following stimulation with recombinant human IFN-γ could be demonstrated in the nine patients tested (data not shown). Workers in other laboratories could not demonstrate IL-12 and IFN-γ signaling defects in CMC either (J.-L. Casanova, personal communication). Thus, although the data for IL-12 and IFN-γ signaling are not conclusive, the studies that have been performed suggest that these signaling pathways are not the likely sites of defects in CMC patients.
We were not able to demonstrate significant alterations in TNF-α or type 2 (IL-4 and IL-5) cytokine production.
The crucial role of T helper type 1 cytokines in protective fungal immunity and particularly in protection against candidiasis was demonstrated recently in a series of well-designed experiments with animal models (reviewed in reference 26), although the role in humans is less well understood. Together, the murine studies demonstrated that IFN-γ and IL-12 are required for survival and clearance of infection (5), while neutralizing IL-4 in susceptible strains improves survival (28). However, the complexity of cytokine networks cannot be overemphasized, particularly regarding the roles of IL-12 and IFN-γ in inducing and mediating Th1 immunity. Consequently, it has been demonstrated that low levels of IL-12 in spite of normal IFN-γ levels result in progressive Candida infection and death in mice (27). Additionally highlighting the complexity of protective responses to fungi in humans, patients with an IFN-γ-IFNγR and/or IL-12-IL-12R deficiency did not have overwhelming or chronic fungal infections (24), even though a number of patients had bouts of candidiasis (Casanova, personal communication). In contrast, patients with the hyper-IgE syndrome who were susceptible to fungal infections were recently shown to exhibit overproduction of IL-12 in parallel with underproduction of IFN-γ (6). As part of this network, the role of inhibitory cytokines, such as IL-10, is crucial in down-regulating type 1 cytokines (IL-12, IL-18, and IFN-γ), as well as inflammatory cytokines (IL-6 and TNF-α) (23). Taken together, these data suggest that although cell-mediated Th1 immunity is essential for protection against fungal infections, the link among type 1-inducing cytokines, induction of Th1 responses, and fungal clearance is more complex than was initially appreciated.
Based on these findings and the results of our study, we speculate that CMC patients overreact to Candida, most likely to CHO moieties such as mannan, glucan, or chitin, by overproducing inflammatory cytokines (e.g., IL-6), which in turn could trigger feedback overproduction of IL-10. High IL-10 levels could then down-regulate IL-12 production, explaining the low levels found in our CMC patients and consequently the impaired induction of protective cell-mediated Th1 antifungal responses. Alternatively, intrinsic impairment of IL-12 production could be the primary defect, leading to poor fungal clearance and persistent infection and inflammation. In both scenarios, the defect could be at the level of accessory cells (macrophages or dendritic cells?) that either overproduce inflammatory cytokines or fail to produce adequate levels of type 1-inducing cytokines such as IL-12 or other type 1-inducing cytokines that have been identified more recently, such as IL-18 (1) and IL-23 (7). Taken together, the data suggest that the immune defect in CMC patients, rather than being at the T-cell level as currently thought, could be at the level of accessory or antigen-presenting cells (i.e., at the level of innate immunity). Given the peculiar susceptibility of CMC patients to Candida, this defect would be more or less selectively expressed in response to Candida rather than other microorganisms. Possible candidates to mediate this defect could be pattern recognition receptors (21), such as the mannose receptor (29), the β glucan receptor (11), or the Toll-like receptors (15), as they could confer the specificity of the defect to Candida and are also directly involved in the regulation and direction of cytokine production patterns (21). It has been shown that binding of Candida mannan to normal monocytes/macrophages directly stimulates TNF-α production by mouse alveolar macrophages (10), while binding of Candida via the mannose and β-glucan receptor stimulates the release of inflammatory eicosanoids from human peripheral blood monocytes (4). Human monocytes stimulated with Candida produce IL-1β, IL-6, and IL-8 (9), while it was recently shown that activation of human Toll-like receptors induces production of IL-1, IL-6, and IL-8 (22), as well as other cytokines (30). Direct involvement of Toll-like receptor 2 in the recognition and response to yeast has also been demonstrated (32). β-Glucan receptors are less likely to be involved as they were recently shown to be intact in at least some patients with CMC that have the AIRE mutation (Siamon Gordon and Gordon Brown, personal communications).
In conclusion, our data suggest that the immune defect underlying susceptibility to Candida infections in patients with CMC is at the level of altered cytokine production, which disrupts protective, antifungal, cell-mediated responses (16, 18). The question of what skews cytokine responses remains unanswered, while the candidate cells that harbor the defect could be dendritic cells or macrophages by virtue of their cytokine-producing function and role in orchestrating Th1 and Th2 responses. These findings have bearing on understanding CMC itself, as well as on understanding the mechanisms involved in protective immunity to fungi in general, which is still poorly understood.
Acknowledgments
This work was funded by registered charity ACTION RESEARCH.
We thank Andrea and David Holroyd for their generous support and all our colleagues who sent patient samples.
Editor: T. R. Kozel
REFERENCES
- 1.Akira, S. 2000. The role of IL-18 in innate immunity. Curr. Opin. Immunol. 12:59-63. [DOI] [PubMed] [Google Scholar]
- 2.Atkinson, T. P., A. A. Schaffer, B. Grimbacher, H. W. Schroeder, Jr., C. Woellner, C. S. Zerbe, and J. M. Puck. 2002. An immune defect causing dominant chronic mucocutaneous candidiasis and thyroid disease maps to chromosome 2p in a single family. Am. J. Hum. Genet. 69:791-803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bodey, G. P (ed.). 1993. Candidiasis. Pathogenesis, diagnosis and treatment, 2nd ed. Raven Press, New York, N.Y.
- 4.Castro, M., J. A. Bjoraker, M. S. Rohrbach, and A. H. Limper. 1996. Candida albicans induces release of inflammatory mediators from human peripheral blood monocytes. Inflammation 20:107-122. [DOI] [PubMed] [Google Scholar]
- 5.Cenci, E., A. Mencacci, G. Del Sero, C. F. d'Ostiani, P. Mosci, A. Bacci, C. Montagnoli, M. Kopf, and L. Romani. 1998. IFNγ is required for IL-12 responsiveness in mice with Candida albicans infection. J. Immunol. 161:3543-3550. [PubMed] [Google Scholar]
- 6.Chehimi, J., M. Elder, J. Greene, L. Noroski, R. Stiehm, J. A. Winkelstein, and K. E. Sullivan. 2001. Cytokine and chemokine dysregulation in hyper-IgE syndrome. Clin. Immunol. 100:49-56. [DOI] [PubMed] [Google Scholar]
- 7.Cua, J. D., J. Sherlock, Y. Chen, C. A. Murphy, B. Joyce, B. Seymore, L. Lucian, W. To, T. Churakova, S. Zurawski, M. Weikowski, S. A. Lira, D. Gormani, R. A. Kasteleini, and J. D. Sedgwick. 2003. IL-23 rather than IL-12 is the critical cytokine for autoimmune inflammation of the brain. Nature 421:744-748. [DOI] [PubMed] [Google Scholar]
- 8.Cuneyt, V., R. L. Roberts, and R. Stiehm. 2003. The syndrome of chronic mucocutaneous candidiasis with selective antibody deficiency. Ann. Allergy Asthma Immunol. 90:259-264. [DOI] [PubMed] [Google Scholar]
- 9.Fisher, A., J. J. Ballet, and C. Griscelli. 1978. Specific inhibition of in-vitro Candida-induced lymphocyte proliferation by polysaccharide antigens present in serum of patients with chronic mucocutaneous candidiasis. J. Clin. Investig. 62:1005-1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Garner, R. E., K. Rubanowice, R. T. Sawyer, and J. A. Hudson. 1994. Secretion of TNFα by alveolar macrophages in response to Candida albicans mannan. J. Leukoc. Biol. 55:161-168. [DOI] [PubMed] [Google Scholar]
- 11.Gordon, S. 1998. The role of the macrophage in immune regulation. Res. Immunol. 149:685-688. [DOI] [PubMed] [Google Scholar]
- 12.Herrod, H. G. 1990. Chronic mucocutaneous candidiasis in childhood and complications of non-Candida infection: report of the paediatric immunodeficiency collaborative study group. J. Pediatr. 116:377-382. [DOI] [PubMed] [Google Scholar]
- 13.IUIS Scientific Group. 1999. Primary immunodeficiency diseases. Report of an IUIS Scientific Group. Clin. Exp. Immunol. 118(Suppl. 1):17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kirkpatrick, C. H. 2001. Chronic mucocutaneous candidiasis. Pediatr. Infect. Dis. J. 20:197-206. [DOI] [PubMed] [Google Scholar]
- 15.Krutzik, S. R., P. A. Sieling, and R. L. Modlin. 2001. The role of Toll-like receptors in host defense against microbial infection. Curr. Opin. Immunol. 13:104-108. [DOI] [PubMed] [Google Scholar]
- 16.Lilic, D. 2002. New perspectives on the immunology of CMC. Curr. Opin. Infect. Dis. 15:143-147. [DOI] [PubMed] [Google Scholar]
- 17.Lilic, D., A. J. Cant, M. Abinun, J. E. Calvert, and G. P. Spickett. 1996. Chronic mucocutaneous candidiasis. I. Altered-antigen stimulated IL2, IL4, IL6 and IFNγ production. Clin. Exp. Immunol. 105:205-212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lilic, D., and I. Gravenor. 2001. Immunology of CMC. J. Clin. Pathol. 54:81-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lilic, D., A. J. Cant, M. Abinun, J. E. Calvert, and G. P. Spickett. 1997. Cytokine production differs in children and adults. Pediatr. Res. 42:237-240. [DOI] [PubMed] [Google Scholar]
- 20.Lilic, D., J. E. Calvert, A. J. Cant, M. Abinun, and G. P. Spickett. 1996. Chronic mucocutaneous candidiasis. II. Class and subclass of specific antibody responses in vivo and in vitro. Clin. Exp. Immunol. 105:213-219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Medzhitov, R., and C. Janeway. 2000. Innate immunity. N. Engl. J. Med. 343:338-344. [DOI] [PubMed] [Google Scholar]
- 22.Medzhitov, R., P. Preston-Hurlbut, and C. Janeway. 1997. A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature 338:394-397. [DOI] [PubMed] [Google Scholar]
- 23.Moore, K. W., R. de Waal Malefyt, R. L. Coffman, and A. O'Garra. 2001. Interleukin-10 and the interleukin 10 receptor. Annu. Rev. Immunol. 19:683-765. [DOI] [PubMed] [Google Scholar]
- 24.Ottenhoff, H. M., D. Kumararatne, and J.-L. Casanova. 1998. Novel human immunodeficiencies reveal the essential role of type 1 cytokines in immunity to intracellular bacteria. Immunol. Today 19:491-494. [DOI] [PubMed] [Google Scholar]
- 25.Peterson, P., K. Nagamine, H. Scott, M. Heino, J. Kudoh, N. Shimizu, S. E. Antonarakis, and K. J. Krohn. 1998. APECED: a monogenic autoimmune disease providing new clues to self-tolerance. Immunol. Today 19:384-386. [DOI] [PubMed] [Google Scholar]
- 26.Romani, L. 1999. The T cell response against fungal infections. Curr. Opin. Microbiol. 2:363-367.10458979 [Google Scholar]
- 27.Romani, L., A. Mencacci, L. Tonnetti, R. Spaccapelo, E. Cenci, S. Wolf, P. Puccetti, and F. Bistoni. 1994. Interleukin-12 but not interferon-γ production correlates with the induction of T helper type 1 phenotype in murine candidiasis. Eur. J. Immunol. 24:909-915. [DOI] [PubMed] [Google Scholar]
- 28.Romani, L., A. Mencacci, U. Grohmann, S. Mocci, P. Mosci, P. Puccetti, and F. Bistoni. 1992. Neutralizing antibody to interleukin 4 induces systemic protection and T helper type 1-associated immunity in murine candidiasis. J. Exp. Med. 176:19-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stahl, P. D., and R. A. B. Ezekowitz. 1998. The mannose receptor is a pattern recognition receptor involved in host defense. Curr. Opin. Immunol. 10:50-55. [DOI] [PubMed] [Google Scholar]
- 30.Takeuchi, O., K. Hoshino, T. Kawai, H. Sanjo, H. Takada, T. Ogawa, K. Takeda, and S. Akira. 1999. Differential roles of TLR2 and TLR4 in recognition of Gram-negative and Gram-positive bacterial cell wall components. Immunity 11:443-451. [DOI] [PubMed] [Google Scholar]
- 31.Tosato, G. 1991. Generation of Epstein-Barr virus (EBV)-immortalized B cell lines, p. 7.22.1-7.22.3. In J. E. Coligan, A. M. Kruisbeek, D. H. Marguklies, E. M. Shevach, and W. Strober (ed.), Current protocols in immunology. National Institutes of Health, Bethesda, Md. [DOI] [PubMed]
- 32.Underhill, D. M., A. Ozinsky, A. M. Hajjar, A. Stevens, C. B. Wilson, M. Bassetti, and A. Aderem. 1999. The Toll-like receptor 2 is recruited to the macrophage phagosomes and discriminates between pathogens. Nature 401:811-815. [DOI] [PubMed] [Google Scholar]
- 33.World Medical Assembly. 1964. The Declaration of Helsinki. World Medical Assembly, Ferney-Voltaire, France. (Amended in 1975, 1983, 1989, and 1996.)